Back to Journals » Journal of Pain Research » Volume 13
Vagus Nerve Stimulation Transiently Mitigates Chemotherapy-Induced Peripheral Neuropathy in Rats
Authors Zhang R, Gan Y, Li J, Feng Y
Received 9 September 2020
Accepted for publication 10 December 2020
Published 22 December 2020 Volume 2020:13 Pages 3457—3465
DOI https://doi.org/10.2147/JPR.S281190
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Ran Zhang,1 Yu Gan,1 Jun Li,2 Yi Feng1,2
1Department of Anesthesiology, Peking University People’s Hospital, Beijing, People’s Republic of China; 2Department of Pain Medicine, Peking University People’s Hospital, Beijing, People’s Republic of China
Correspondence: Jun Li; Yi Feng No. 11 Xi Zhi Men Nan Da Jie, Xicheng District, Beijing, People’s Republic of China
Tel +86-1088325581
Email [email protected]
Background: Chemotherapy-induced peripheral neuropathy is a severe side effect of chemotherapeutic agents. Vagus nerve stimulation attenuates neuroinflammation by activating the cholinergic anti-inflammatory pathway and thus may attenuate CIPN.
Methods: Adult male Sprague-Dawley rats received intraperitoneal paclitaxel injection (2 mg/kg) every other day for a total of 4 injections. Three weeks later, the left cervical vagus nerve was exposed under general anesthesia, and the rats randomly received 20-min stimulation (1 V, 2 ms, 5 Hz, 30 s ON/5 min OFF) or sham stimulation. Heat and mechanical pain sensitivity was evaluated using Hargreaves and von Frey tests before and after treatment (n=12 per group per time point). Additionally, rats receiving paclitaxel or saline but no surgery were included. Expression of representative pro- and anti-inflammatory cytokines in dorsal root ganglia was assessed by Western blotting assays and immunohistochemistry.
Results: Paclitaxel significantly reduced the sensitivity for heat (withdrawal latency: paclitaxel 6.16 ± 0.54 s vs saline 9.93 ± 0.78 s, p< 0.001) and mechanical pain (withdrawal frequency: paclitaxel 32.22 ± 15.51% vs saline 3.33 ± 4.92%, p< 0.001). Compared with sham-stimulated rats, rats receiving vagus nerve stimulation had significantly higher sensitivity for heat (withdrawal latency: VNS 10.28 ± 1.15 s vs sham 6.27 ± 0.56 s, p< 0.001) and mechanical pain (withdrawal frequency: VNS 10.00 ± 9.54% vs Sham 31.67 ± 18.99%, p=0.003) on +1 day, but not 7 days later (withdrawal latency: VNS 6.97 ± 1.13 s vs Sham 6.23 ± 0.79 s, p=0.080; withdrawal frequency: VNS 21.67 ± 11.93% vs Sham 23.33 ± 7.79%, p=0.689). Western blotting assays and immunohistochemistry revealed that interleukin-10 level was elevated in the dorsal root ganglia of rats receiving vagus nerve stimulation while no apparent changes in NF-κB or TNF-α levels were observed.
Conclusion: Vagus nerve stimulation could transiently attenuate paclitaxel-induced hyperalgesia in rats. Future studies are needed to investigate whether stimulation with different protocols could achieve durable effects.
Keywords: chemotherapy-induced peripheral neuropathy, neuroinflammation, vagus nerve stimulation, cholinergic anti-inflammatory pathway, interleukin-10
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN), which is a severe side effect of chemotherapeutic agents, manifests pain, paresthesia, numbness, and tingling sensation. Approximately 70% of the patients receiving chemotherapy develop CIPN within the first month of treatment.1,2 Dose reduction or discontinuation of treatment results in dissipation of CIPN in majority of the cases, but is incompatible with the overall treatment goal.3 Additionally, CIPN persists for over 6 months after discontinuation of chemotherapy in approximately 30% of the patients. Antidepressants and photobiomodulation are also commonly used, but typically with only limited effects.4
Neuroinflammation is a key contributor to CIPN. Chemotherapeutic agents increase production and release of pro-inflammatory cytokines by glial cells, which in turn cause a cascade of signaling effects to sensitize neurons to incoming stimuli.5 Treatments that inhibit neuroinflammation have been shown to be effective in attenuating CIPN. Vagus nerve stimulation (VNS) activates the cholinergic anti-inflammatory pathway, down-regulates pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α),6,7 and inhibits neuroinflammation.8,9 These findings led us to hypothesize that VNS might mitigate CIPN.
In the current study, we investigated whether VNS could reduce pain hypersensitivity in a rat model of paclitaxel-induced CIPN.10 We also examined the potential effects of VNS on the levels of representative pro- and anti-inflammatory cytokines in the dorsal root ganglion (DRG) in this model.11
Materials and Methods
Animals and Experimental Design
The study protocol was approved by the Ethics Committee of Peking University People’s Hospital, and the study was carried out in accordance with the Guidelines for the Ethical Review of Laboratory Animal Welfare (People’s Republic of China National Standard GB/T 35892–2018).
Adult male Sprague-Dawley rats (220–250 g; Vital River Laboratory, Beijing, China) were maintained in a specific-pathogen-free small animal facility at 22 ± 3°C under a 12/12 h light/dark cycle, with ad libitum access to standard rodent chow and water.
Rats were randomly divided into the following four groups: control, CIPN, sham, and VNS. The CIPN, sham, and VNS groups received 2 mg/kg paclitaxel intraperitoneally on alternative days for a total of 4 days. The control group received saline. On day 0, VNS was performed in the VNS group, mock surgery was performed in the sham group, and no procedure was carried out in the CIPN or control groups. Behavioral tests were performed on days −28, 0, +1, and +7 (n=12 per group per time point) (Figure 1).
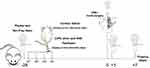 |
Figure 1 Experimental design. Abbreviation: VNS, vagus nerve stimulation. |
VNS
Rats were anesthetized with 1.5%-3.0% isoflurane (Abbott Laboratories, Chicago, IL, USA). The left cervical vagus nerve was isolated as described previously.12 Briefly, VNS was delivered using an electronic stimulator (YLS-9A; Zhongshidichuang Technology, Beijing, China). The vagus nerve was stimulated for 20 min using the following parameters: 1 V, 2 ms, 5 Hz, and 30 s ON/5 min OFF.13,14 Rats in the sham stimulation group received identical surgery but without actual VNS. Heart rate and blood pressure were monitored throughout the procedure using a tail-cuff system (BP2010A, Softron, Beijing, China).
Behavioral Tests
The person who performed the behavioral tests was blinded to the study design. Heat pain sensitivity in the plantar test was measured as described previously.15 Briefly, with the rats in a plastic compartment (24*20*10 cm), the plantar surface of the hindlimb was targeted using a radiant heat source (BME-410C fully automatic heat-pain stimulator, Institute of Biomedical Engineering, Chinese Academy of Medical Sciences, Beijing, China). Heat intensity was adjusted such that mean baseline withdrawal latencies were between 7 and 12 s. Heat hyperalgesia was defined as a hindpaw withdrawal latency shorter than at baseline. Maximal latency was set at 15 s to avoid tissue damage. Each rat was tested 3 times for each hindpaw, with 5-min intervals. The average of six measurements was used for analysis (n=12 per group per time point).
Mechanical pain was examined using a von Frey method as described previously.16 Briefly, rats were placed in a plastic cage on an elevated wire mesh platform. Pressure (4 or 15 g) was directed to the mid-plantar surface of the hindpaw for 5 s for 5 times at each hindpaw. For each rat, the percentage of withdrawal response in 10 tests (5 tests for each hindpaw, both sides) was used to reflect pain sensitivity (n=12 per group per time point). Allodynia was defined as increased frequency of withdrawal to 4-g von Frey hair. Hyperalgesia was defined as increased frequency of withdrawal to 15-g von Frey hair.
Western Blotting Assays
Rats were euthanized with CO2 inhalation after behavioral tests (n=7 per group per time point). Bilateral DRGs at L4-6 were removed, snap-frozen in liquid nitrogen, and stored at −80°C. Tissues were homogenized with an electric homogenizer in radioimmunoprecipitation assay buffer (Thermo Fisher Scientific, Waltham, MA, USA) containing protease and phosphatase inhibitors (Applygen Technologies, Beijing, China). Proteins were separated by 10% SDS-PAGE, transferred to PVDF membranes (Millipore, USA), blocked with 5% low-fat milk for 1 h at room temperature, and then incubated overnight at 4°C in Tris-buffered saline (TBS). Primary antibodies (all at 1:1000 dilution) against the following proteins were used: TNF-α (catalog No. YT4689; ImmunoWay, Plano, TX, USA), nuclear factor kappa B (NF-κB) (8242; Cell Signaling Technology, Beverly, MA, USA), interleukin-10 (20850-1-AP; Proteintech, Rosemont, IL, USA), and β-actin (TA-09; Zhongshan Golden Bridge, Beijing, China). After washing, membranes were incubated at room temperature for 1 h with an appropriate secondary antibody (1:3000 dilution; Zhongshan Golden Bridge). Protein bands were visualized using the ECL method (Thermo Fisher Scientific, Waltham, MA, USA), and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Immunohistochemistry
Rats were deeply anesthetized with sodium pentobarbital (40 mg/kg), and then perfused with 0.9% saline (pH 7.4) at 37°C followed by ice-cold 4% paraformaldehyde through the ascending aorta (n=5 per group per time point). Bilateral DRGs at L4-6 were harvested, fixed in formaldehyde, and paraffin-embedded. Sections (4 μm) were stained with hematoxylin and eosin using a standard protocol.17
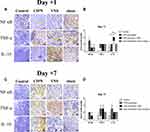
Immunohistochemistry was performed as described previously.18 Briefly, after antigen retrieval at 95°C, slices were incubated at 4°C overnight with primary antibodies (diluted 1:100) against the following proteins: NF-κB subunit p65 (Abcam, Cambridge, UK), TNF-α (Abcam) and IL-10 (Proteintech). The tissue sections were then incubated with a biotinylated goat anti-rabbit secondary antibody conjugated to streptavidin-horseradish peroxidase. Antibody binding was visualized using 3ʹ-diaminobenzidine, and slices were counterstained with hematoxylin. For negative controls, primary antibody was omitted. DRGs were identified within tissue sections and selected for image capture using confocal laser scanning microscopy (Leica, Germany). The images were quantitatively evaluated using the IHC Profiler plugin in ImageJ as described.19 Staining was scored automatically based on the average gray value (staining intensity) and percentage of total surface area showing positive staining (staining area) as following: 4 = high positive, 3 = positive, 2 = low positive, and 1 = negative.
Statistical Analysis
Normally distributed data were expressed as mean ± standard deviation (SD). Differences in normally distributed data with heterogeneous variance were assessed for significance using one-way ANOVA followed by the Tukey post hoc test, while Welch’s ANOVA followed by Dunnett T3 post hoc test was applied to data that violated the homogeneity of variance. Skewed data were presented as median and interquartile range, and differences were assessed for significance using Kruskal–Wallis tests. Immunohistochemistry scores were assessed using Mann–Whitney tests. GraphPad Prism 8 (GraphPad Software, San Diego, CA) was used for statistical analyses. P < 0.05 was considered statistically significant.
Results
Behavioral Tests
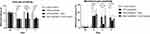
The heat or mechanical pain sensitivity at the baseline did not differ significantly among the four groups (Figure 2). Paclitaxel treatment significantly reduced withdrawal latency in the Hargreaves test (withdrawal latency: paclitaxel 6.16 ± 0.54 s vs saline 9.93 ± 0.78 s, p<0.001) (Figure 2A). Compared to sham-stimulated rats, rats receiving VNS showed significantly longer withdrawal latency on +1 day (withdrawal latency: VNS 10.28 ± 1.15 s vs Sham 6.27 ± 0.56 s, p<0.001), but not on +7 day (withdrawal latency: VNS 6.97 ± 1.13 s vs Sham 6.23 ± 0.79 s, p=0.080) (Figure 2A).
A similar response pattern was observed in mechanical pain. Paclitaxel treatment significantly increased withdrawal frequency in the von Frey test (withdrawal frequency: paclitaxel 32.22 ± 15.51% vs saline 3.33 ± 4.92%, p<0.001) (Figure 2B). Compared with sham-stimulated rats, withdrawal frequency declined at 1 day after the surgery (withdrawal frequency: VNS 10.00 ± 9.54% vs Sham 31.67 ± 18.99%, p=0.003), but not 7 days later (withdrawal frequency: VNS 21.67 ± 11.93% vs Sham 23.33 ± 7.79%, p=0.689) (Figure 2B).
Representative Pro- and Anti-Inflammatory Cytokines in DRG
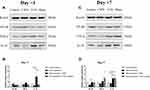
Compared with untreated rats, no changes in NF-κB or TNF-α expression were observed in rats receiving VNS while IL-10 levels were significantly upregulated (Figure 3A and B). Additionally, compared to sham-stimulated rats, Western blot analysis demonstrated there was a nearly 5-fold increase in IL-10 levels in the DRG of rats receiving VNS on +1 day (p = 0.008), but there was no statistical difference between the two groups on +7 day (p=0.843). These results were confirmed by immunohistochemistry (Figure 4). Interestingly, Western blot analysis showed slightly higher IL-10 levels in rats receiving anesthesia and surgery (VNS and sham stimulation) than that without the former intervention (Control and CIPN) on +7 day. However, immunohistochemistry did not reveal statistical difference in DRG neurons.
Discussion
Therapeutic management of CIPN is a long-standing challenge in cancer treatment, and the US Food and Drug Administration has not licensed any medication for its management.20 CIPN involves the infiltration of immune cells into the peripheral nervous system, which triggers inflammation of peripheral sensory neurons in the DRG. For this reason, attenuating neuroinflammation is a popular goal for therapeutic management.5,21 In the current study, we found that VNS alleviated heat and mechanical hyperalgesia in a rat model of CIPN. This effect appeared on +1 but not on +7 day. Furthermore, Western blotting assays and immunohistochemistry revealed that IL-10 expression was significantly up-regulated on +1 day but declined over time. Surprisingly, VNS did not significantly alter expression of pro-inflammatory effectors NF-κB and TNF-α at any time point tested.
The vagus nerve is composed of 80% sensory afferent fibers and 20% motor efferent fibers. It provides a considerable interface between the brain and the whole body, including heart, lung, and gastrointestinal system. The vagus nerve regulates acetylcholine release from the efferent cholinergic nerve, a parasympathetic element of the autonomic nervous system.22 VNS can modulate the autonomic nervous system by activating the cholinergic anti-inflammatory pathway, suppressing inflammation.23 To our knowledge, different pulse widths activated special nerves. For instance, pulse width of 0.2 ~ 0.5 ms typically produces central effects through the vagal afferents. And 2 ms exerts anti-inflammatory effects via the vagal efferents. In clinical practice, 0.5 ms is a commonly used therapeutic stimulation pulse width for epilepsy and depression.24 And Borovikova et al reported a stimulation pulse width of 2 ms in their study on cholinergic anti-inflammatory activities of VNS.6
VNS has shown preclinical efficacy in animal models of some inflammation-related disorders, such as sepsis, inflammatory bowel disease, myocardial ischemia reperfusion injury, rheumatoid arthritis, and kidney ischemia-reperfusion injury.25,26 Moreover, VNS has been approved by the Food and Drug Administration for treatment of certain brain-related conditions.27,28 We found that a single treatment of VNS effectively alleviated hyperalgesia after 24 h: paclitaxel shortened withdrawal latency and increased withdrawal frequency in rats, indicating increased sensitivity to heat and pain, which was reversed by VNS. Nevertheless, the relief was short-lived: heat sensitivity and pain returned to pre-treatment levels by day +7. Similarly, another study showed that VNS mitigated kidney ischemia-reperfusion injury transiently, with effects peaking around 24 h and lasting for 2 days.25 Our results provide evidence supporting VNS as a potential novel treatment of CIPN, especially since the anatomy and function of the vagus nerve are comparable between rats and humans.29
In a rat model of paclitaxel-induced neuropathy similar to ours, increased expression of pro-inflammatory cytokines (TNF-α, IL-1β) was accompanied by decreased expression of anti-inflammatory cytokines (IL-10, IL-4). The overall effect was neuroinflammation and neurotoxicity in the DRG.30,31 In fact, paclitaxel augments expression of NF-κB in DRG, up-regulating TNF-α.32 Additionally, TNF-α serves as an activator of NF-κB in neurons.33 Accordingly, the interaction between NF-κB and TNF-α plays a vital role in the development and maintenance of CIPN. The role of IL-10 in CIPN is less clear due to conflicting findings.6,34–36 However, it is clear that IL-10 is required for recovery from CIPN.37,38 Moreover, IL-10 inhibits NF-κB activity and suppresses TNF-α production by activating the JAK/STAT signaling pathway, which VNS also activates.39,40 Our results confirm up-regulation of IL-10 after VNS which may be involved in the protective effect. An interesting finding is that Western blot analysis showed higher IL-10 levels in rats receiving anesthesia and surgery (VNS and sham stimulation) on +7 day, which seems to be in contradiction to the results of behavioral tests consistent with IHC. Given the evidence, isoflurane and surgery alone induced a significant elevation of IL-10 expression in DRG.41 Besides, IL-10 level was elevated in DRG from sham-operated rats of another model of neuropathic pain from 3 to 14 days after the surgery.42 There are many types of cells in DRG, including immune cells (neutrophils, macrophages), glial cells (microglia and astrocytes), and neurons. Isoflurane was reported to have anti-inflammatory effects in all cells except neuronal cell lines.43 It is possible that IL-10 level was upregulated in DRG of rats receiving VNS and sham surgery but not in neurons. However, the potential mechanism is still not clear. A better understanding of the molecular basis for agonist and antagonist mechanism of IL-10 receptor remains to be explored.
The expression of NF-κB and TNF-α was comparable between rats treated with paclitaxel and untreated rats. A possible explanation is that NF-κB and TNF-α production are transiently up-regulated early under neurotoxic conditions in the DRG. In favor of this hypothesis, one study reported that paclitaxel treatment enhanced pro-inflammatory cytokine production in the spinal cord in the first 8 days, but this effect disappeared by day 29.44 Further studies should clarify the roles of IL-10 and TNF-α in CIPN and identify the specific pathway(s) stimulated by VNS.
Withdrawal response of rats to von Frey hair ranges from 5.7 to 15 g after paclitaxel treatment.10 In the present study, mechanical allodynia, defined as higher withdrawal frequency in response to a 4-g von Frey hair, was not detected in any group. Nevertheless, we were able to detect significant differences in mechanical hyperalgesia (defined as higher withdrawal frequency in response to a 15-g von Frey hair) between rats treated with VNS or not.
The study has some limitations. First, we failed to identify exactly when the protective effect of VNS first appeared and exactly how long it lasted in our CIPN model. Our work should be repeated with continuous measurements. Second, CIPN is a chronic disorder, this study was performed with acute but not chronic VNS. It is of interest that chronic VNS would be a translational therapeutic approach, as demonstrated for VNS in epilepsy and depression. Third, the scope of our study was limited to the peripheral nervous system, but the effects of VNS on the central nervous system should also be examined. Fourth, our study just selected IL-10 and TNF-α as the representative anti-inflammatory and pro-inflammatory cytokines, other pain-related cytokines were not examined, such as IL-4, IL-6, and IL-1β. Lastly, many complex signaling pathways participate in the development and maintenance of CIPN, such as the ERK, p38, and JAK/STAT signaling pathways. Regrettably, this study fails to explore the orle of these signaling pathways in VNS-mediated activities and thus reveal the potential underlying mechanism. Further study is needed to reveal the molecular mechanisms.
This study demonstrated that acute VNS could transiently attenuate CIPN. Further studies are required to reveal whether chronic VNS will generate durable protective effect in CIPN. Recently, non-invasive VNS procedure has been developed in rats and human.45,46 An ongoing clinical trial (NCT04367480) is testing the effects of transcutaneous electrical nerve stimulation on CIPN. It is expected that VNS may be used for the treatment of CIPN in the future.
Conclusions
VNS could transiently attenuate paclitaxel-induced hyperalgesia in rats. Future studies are needed to investigate whether stimulation with different protocols could achieve durable effects.
Abbreviations
CIPN, chemotherapy-induced peripheral neuropathy; VNS, vagus nerve stimulation.
Acknowledgments
The authors thank Mr. Kai Liu and Ms. Yujing Chi for technical assistance with experiments. This research was supported by the Peking University People’s Hospital Research and Development Fund (grant number RDY2016-27).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155(12):2461–2470. doi:10.1016/j.pain.2014.09.020
2. McCormick B, Lowes DA, Colvin L, et al. MitoVitE, a mitochondria-targeted antioxidant, limits paclitaxel-induced oxidative stress and mitochondrial damage in vitro, and paclitaxel-induced mechanical hypersensitivity in a rat pain model. Br J Anaesth. 2016;117(5):659–666. doi:10.1093/bja/aew309
3. Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: american Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941–1967.
4. Hou S, Huh B, Kim HK, et al. Treatment of Chemotherapy-Induced Peripheral Neuropathy: systematic Review and Recommendations. Pain Physician. 2018;21(6):571–592.
5. Lees JG, Makker PG, Tonkin RS, et al. Immune-mediated processes implicated in chemotherapy-induced peripheral neuropathy. Eur J Cancer. 2017;73:22–29.
6. Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462.
7. Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–388.
8. Meneses G, Bautista M, Florentino A, et al. Electric stimulation of the vagus nerve reduced mouse neuroinflammation induced by lipopolysaccharide. J Inflamm. 2016;13:33.
9. Huffman WJ, Subramaniyan S, Rodriguiz RM, et al. Modulation of neuroinflammation and memory dysfunction using percutaneous vagus nerve stimulation in mice. Brain Stimul. 2019;12(1):19–29.
10. Polomano RC, Mannes AJ, Clark US, et al. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94(3):293–304. doi:10.1016/S0304-3959(01)00363-3
11. Brandolini L, Angelo M, Antonosante A, et al. Chemokine Signaling in Chemotherapy-Induced Neuropathic Pain. Int J Mol Sci. 2019;20(12):12. doi:10.3390/ijms20122904
12. Krahl SE, Senanayake SS, Pekary AE, et al. Vagus nerve stimulation (VNS) is effective in a rat model of antidepressant action. J Psychiatr Res. 2004;38(3):237–240. doi:10.1016/j.jpsychires.2003.11.005
13. Bernik TR, Friedman SG, Ochani M, et al. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg. 2002;36(6):1231–1236. doi:10.1067/mva.2002.129643
14. Bernik TR, Friedman SG, Ochani M, et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195(6):781–788. doi:10.1084/jem.20011714
15. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi:10.1016/0304-3959(88)90209-6
16. Siau C, Bennett GJ. Dysregulation of cellular calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth Analg. 2006;102(5):1485–1490. doi:10.1213/01.ane.0000204318.35194.ed
17. Fischer AH, Jacobson KA, Rose J, et al. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008;2008:4986.
18. Shi S-R, Liu C, Taylor CR. Standardization of immunohistochemistry for formalin-fixed, paraffin-embedded tissue sections based on the antigen-retrieval technique: from experiments to hypothesis. J Histochemistry Cytochemistry. 2007;55(2):105–109. doi:10.1369/jhc.6P7080.2006
19. Varghese F, Bukhari AB, Malhotra R, et al. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. 2014;9(5):e96801. doi:10.1371/journal.pone.0096801
20. Ma J, Kavelaars A, Dougherty PM, et al. Beyond symptomatic relief for chemotherapy-induced peripheral neuropathy: targeting the source. Cancer. 2018;124(11):2289–2298. doi:10.1002/cncr.31248
21. Tonello R, Lee SH, Berta T. Monoclonal Antibody Targeting the Matrix Metalloproteinase 9 Prevents and Reverses Paclitaxel-Induced Peripheral Neuropathy in Mice. j pain. 2019;20(5):515–527. doi:10.1016/j.jpain.2018.11.003
22. Ruffoli R, Giorgi FS, Pizzanelli C, et al. The chemical neuroanatomy of vagus nerve stimulation. J Chem Neuroanat. 2011;42(4):288–296. doi:10.1016/j.jchemneu.2010.12.002
23. Rosas-Ballina M, Olofsson PS, Ochani M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334(6052):98–101. doi:10.1126/science.1209985
24. Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev. 2005;29(3):493–500. doi:10.1016/j.neubiorev.2005.01.004
25. Inoue T, Abe C, Sung SS, et al. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through alpha7nAChR+ splenocytes. J Clin Invest. 2016;126(5):1939–1952. doi:10.1172/JCI83658
26. Andersson U, Tracey KJ. Reflex principles of immunological homeostasis. Annu Rev Immunol. 2012;30(1):313–335. doi:10.1146/annurev-immunol-020711-075015
27. Stacey WC, Litt B. Technology insight: neuroengineering and epilepsy-designing devices for seizure control. Nat Clin Pract Neurol. 2008;4(4):190–201. doi:10.1038/ncpneuro0750
28. Cai PY, Bodhit A, Derequito R, et al. Vagus nerve stimulation in ischemic stroke: old wine in a new bottle. Front Neurol. 2014;5:107.
29. Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85(1–3):1–17.
30. Janes K, Wahlman C, Little JW, et al. Spinal neuroimmune activation is independent of T-cell infiltration and attenuated by A3 adenosine receptor agonists in a model of oxaliplatin-induced peripheral neuropathy. Brain Behav Immun. 2015;44:91–99.
31. Doyle T, Chen Z, Muscoli C, et al. Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain. J Neurosci. 2012;32(18):6149–6160.
32. Kim HK, Hwang SH, Lee SO, et al. Pentoxifylline Ameliorates Mechanical Hyperalgesia in a Rat Model of Chemotherapy-Induced Neuropathic Pain. Pain Physician. 2016;19(4):E589.
33. Snow WM, Albensi BC. Neuronal Gene Targets of NF-kappaB and Their Dysregulation in Alzheimer’s Disease. Front Mol Neurosci. 2016;9:118.
34. Pavlov VA, Ochani M, Yang LH, et al. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med. 2007;35(4):1139–1144.
35. Rezende-Neto JB, Alves RL, Carvalho MJ, et al. Vagus nerve stimulation improves coagulopathy in hemorrhagic shock: a thromboelastometric animal model study. J Trauma Manag Outcomes. 2014;8:15.
36. Hilderman M, Qureshi AR, Al-Abed Y, et al. Cholinergic anti-inflammatory pathway activity in dialysis patients: a role for neuroimmunomodulation? Clin Kidney J. 2015;8(5):599–605.
37. Ledeboer A, Jekich BM, Sloane EM, et al. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun. 2007;21(5):686–698.
38. Krukowski K, Eijkelkamp N, Laumet G, et al. CD8+ T Cells and Endogenous IL-10 Are Required for Resolution of Chemotherapy-Induced Neuropathic Pain. J Neurosci. 2016;36(43):11074–11083.
39. Hutchins AP, Diez D, The M-SD. IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief Funct Genomics. 2013;12(6):489–498.
40. de Jonge WJ, van der Zanden EP, The FO, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6(8):844–851.
41. Cuellar JM, Borges PM, Cuellar VG, et al. Cytokine expression in the epidural space: a model of noncompressive disc herniation-induced inflammation. Spine. 2013;38(1):17–23.
42. Jancalek R, Dubovy P, Svizenska I, et al. Bilateral changes of TNF-alpha and IL-10 protein in the lumbar and cervical dorsal root ganglia following a unilateral chronic constriction injury of the sciatic nerve. J Neuroinflammation. 2010;7:11.
43. Lee YM, Song BC, Yeum KJ. Impact of volatile anesthetics on oxidative stress and inflammation. Biomed Res Int. 2015;2015:242709.
44. Burgos E, Gomez-Nicola D, Pascual D, et al. Cannabinoid agonist WIN 55212-2 prevents the development of paclitaxel-induced peripheral neuropathy in rats. Possible involvement of spinal glial cells. Eur J Pharmacol. 2012;682(1–3):62–72.
45. Subramanian M, Edwards L, Melton A, et al. Non-invasive vagus nerve stimulation attenuates proinflammatory cytokines and augments antioxidant levels in the brainstem and forebrain regions of Dahl salt sensitive rats. Sci Rep. 2020;10(1):17576.
46. Badran BW, Yu AB, Adair D, et al. Laboratory administration of transcutaneous auricular vagus nerve stimulation (taVNS): technique, targeting, and considerations. J Vis Exp. 2019;143.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.