Back to Journals » Cancer Management and Research » Volume 14
Utility of Matrix Metalloproteinases in the Diagnosis, Monitoring and Prognosis of Ovarian Cancer Patients
Authors Kicman A, Niczyporuk M , Kulesza M, Motyka J , Ławicki S
Received 9 August 2022
Accepted for publication 8 November 2022
Published 30 November 2022 Volume 2022:14 Pages 3359—3382
DOI https://doi.org/10.2147/CMAR.S385658
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Aleksandra Kicman,1 Marek Niczyporuk,1 Monika Kulesza,2 Joanna Motyka,2 Sławomir Ławicki2
1Department of Aesthetic Medicine, Medical University of Bialystok, Bialystok, Poland; 2Department of Population Medicine and Lifestyle Diseases Prevention, Medical University of Bialystok, Bialystok, Poland
Correspondence: Aleksandra Kicman, Department of Aesthetic Medicine, Medical University of Bialystok, Akademicka 3, Białystok, 15-267, Poland, Tel +48 857 485 827, Email [email protected]
Abstract: Ovarian cancer is one of the most common gynecologic malignancies. It is characterized by a high mortality rate, which is mainly due to the asymptomatic course of the disease. In light of the high mortality rate and increasing morbidity, new diagnostic methods are being explored to enable earlier detection, better monitoring, and improved prognosis. Such diagnostic methods include the assessment of tumor markers in various biological samples. Among the markers currently being investigated, extracellular matrix metalloproteinases (MMPs) are of particular interest. The objective of this article was to compile the existing knowledge of MMPs in ovarian cancer patients and to describe their potential diagnostic utility. Additionally, this article provides an overview of the symptoms, complications, and risk factors associated with ovarian cancer and the role of MMPs in physiology and pathology. Preliminary results indicate that tissue expression and blood and body fluid levels of MMPs may be different in ovarian cancer patients than in healthy women. The expression and concentration of individual MMPs have been shown to be correlated with cancer stage and disease severity. In addition, the preliminary value of some of these enzymes in predicting prognosis is discussed. However, as the amount of data is limited, more studies are needed to fully evaluate the potential function of individual MMPs in ovarian cancer patients. Based on the knowledge gathered for this article, it seems that MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-13, are tentatively the most useful. A thorough evaluation of their utility as modern biomarkers in ovarian cancer requires further investigation.
Keywords: carcinogenesis, extracellular matrix metalloproteinases, ovarian cancer, tumor markers
Introduction
The number of women developing gynecologic cancers, including ovarian cancer, is steadily increasing. In 90% of cases, ovarian cancer originates from epithelial cells; ovarian cancers of epithelial origin are referred to as ovarian carcinomas or ovarian epithelial.1–3 Ovarian cancer is one of the most common gynecologic malignancies, ranking third in incidence after cervical and endometrial cancers, accounting for 3.4% of all cancers types diagnosed in women. Ovarian cancer has a poor prognosis and the highest mortality rate of all gynecologic cancers. According to World Health Organization reports, less than 30% of patients survive more than five years after diagnosis. This unfavorable prognosis results not only from a lack of effective screening tests but also from the usually asymptomatic or minimally symptomatic disease progression—most patients are diagnosed at an advanced stage, and 60% have metastatic foci at the time of diagnosis.2–5
Considering the high mortality rate, asymptomatic course, lack of effective screening tests, and increasing number of diagnosed cases, novel methods for early diagnosis of ovarian cancer are constantly being sought. Modern diagnostic methods would enable earlier detection of ovarian cancer at a less advanced stage, thereby improving prognosis. Such investigations could include the assessment of tumor markers in biological samples. For example, the preliminary utility of tumor markers (evaluated in peripheral blood) has been demonstrated in the diagnosis of breast6,7 and gynecologic cancers,8–10 including ovarian cancer.11,12 In addition to early detection of cancer, tumor markers can also be used to assess prognosis and monitor the clinical course of the disease.13–16 Among the groups of molecules considered to be potential markers for ovarian cancer, matrix metalloproteinases (MMPs) are of particular interest.8,17–19
Ovarian Cancer: An Overview
Classification and Symptoms of Ovarian Cancer
Ovarian cancers originate from three types of cells— epithelial cells, germ cells or from sex chord and stromal cells. As mentioned in the introduction, epithelial malignancies (ovarian carcinoma or ovarian epithelial cancer) account for the majority of lesions diagnosed in patients. It is important to note that non-epithelial malignancies have a much better prognosis and are less invasive than carcinomas. Ovarian epithelial cancer is a heterogeneous disease. It can be subdivided into high-and low-differentiated serous, endometrioid, clear cell, and mucinous carcinomas based on histopathologic, molecular, and immunologic findings. The different types of ovarian carcinomas differ in pathogenesis, prognosis, and response to chemotherapy. The most commonly diagnosed type, which also has the highest mortality rate, is high-grade serous ovarian carcinoma, which accounts for 70–80% of ovarian cancer-related deaths. The second most common type is endometrioid carcinoma, which has a better prognosis than the high-grade serous type.1–3,17,20–22
Because of its asymptomatic course, ovarian cancer is often called the “silent killer”. Most patients are in stage III or IV at the time of diagnosis according to the International Federation of Gynecology and Obstetrics (FIGO) classification (see Table 1). Due to the lack of anatomical barriers around the ovary, exfoliated cancer cells easily enter the peritoneum, where they are distributed through the peritoneal fluid to the abdominal organs and then further to distant organs. This results in the formation of numerous metastatic foci—mainly in the liver, lymph nodes, bones, brain, and lungs. Metastasis to the intestines, resulting in their obstruction, is particularly life threatening. It is estimated that bowel obstruction is the most common cause of death in the course of ovarian cancer.2,17,20,23–26
 |
Table 1 FIGO’s Staging Classification for Ovarian Cancer |
Nonspecific and minimal symptoms appear in the advanced stages of ovarian cancer and are usually not directly related to the reproductive system. Patients mainly report abdominal and pelvic pain or discomfort, which is most often confused with other complaints, such as indigestion, irritable bowel syndrome, or menstrual pain. Other gastrointestinal symptoms include bloating, early satiety, increased abdominal girth, and loss of appetite. Some of the other commonly reported symptoms include back pain, general fatigue, and urinary symptoms, such as frequent or excessive urination. Typical gynecological symptoms include menstrual disorders, postmenopausal bleeding, and pain or bleeding during intercourse.4,29,30 As the disease progresses, some patients may experience intestinal obstruction (as previously mentioned) or ureteral obstruction, which can result in renal failure. The development of ascites is associated with a poor prognosis.2,24,31 It should also be mentioned that the presence of cancer cells can induce paraneoplastic syndromes, such as subacute cerebellar degeneration, dermatomyositis, migratory thrombophlebitis (Trousseau syndrome), disseminated intravascular coagulation, and hemolytic anemia.4,32 The symptoms and complications of ovarian cancer, as well as the most common locations of metastases, are presented in Figure 1.
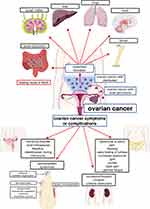 |
Figure 1 Symptoms and complications of ovarian cancer and the most common sites of metastatic foci. |
Selected Risk and Protective Factors for Ovarian Cancer
Age
Age is one of the most important risk factors for ovarian cancer. In general, the risk of ovarian cancer increases with age. The disease mainly affects postmenopausal women; 50% of cases are diagnosed after the age of 65, while ovarian cancer is rare before 40 years of age. Among all types of ovarian cancer in the postmenopausal period, high-grade serous ovarian carcinoma is the most common and, as discussed earlier, has the poorest prognosis. It is also notable that ovarian cancer has a worse prognosis in older patients compared to younger patients, mainly due to less aggressive treatment approaches and higher disease stage at the time of diagnosis.2,5,33,34
Genetic Factors
Besides age, genetics are considered the second most important risk factor for ovarian cancer. Genetic predisposition to ovarian cancer manifests as hereditary ovarian cancer, hereditary ovarian and breast cancer syndrome, and hereditary nonpolyposis colorectal cancer (Lynch syndrome). Hereditary ovarian and breast cancer syndrome is the most common.27,34 Patients whose mother and/or sister(s) also suffer from ovarian cancer are particularly vulnerable. Interestingly, a family history of breast and uterine cancer in a mother or sister also increases a woman’s likelihood of developing ovarian cancer. According to the literature, over 50% of genetically determined ovarian cancers are caused by BRCA1 and BRCA2 gene mutations.1,5,35,36 The BRCA1 and BRCA2 genes are classified as tumor suppressor genes; functional BRCA1/BRCA2 proteins are responsible for repairing DNA double helix breaks through the process of homologous recombination. BRCA1/BRCA2 mutations are inherited in an autosomal dominant manner; however, they function as recessive genes at the cellular level. Loss of function of these genes results in genome instability and, consequently, neoplastic cellular transformation.37–40 The estimated average risk of developing ovarian cancer is 20–50% in patients with a BRCA1 mutation and 5–25% in patients with a BRCA2 mutation. According to the literature, patients with BRCA1/BRCA2 mutations most often develop high-grade serous carcinoma. However, it should be noted that ovarian cancer patients with mutations in the BRCA genes have a better prognosis than patients with other types of mutations. In addition, BRCA2 gene mutation patients have a better prognosis than BRCA1 gene mutation patients, mainly because the former respond better to cisplatin therapy. A protective method in patients with BRCA1/BRCA2 gene mutations is prophylactic salpingo-oophorectomy, which has been shown to reduce the risk of ovarian cancer by 75%.1,5,41,42
Lynch syndrome is caused by an autosomal dominant mutation of the genes responsible for DNA repair, including MHL1, MSH2, MSH6, and PMS2. This syndrome is usually associated with an increased risk of developing colorectal cancer; however, patients also tend to develop ovarian cancer.33,35,43–45 It is estimated that 10–15% of genetically determined ovarian cancer cases are associated with Lynch syndrome,5,20,46 and the lifetime risk of ovarian cancer in Lynch syndrome patients is estimated to be 8%.46 The most common histological subtype of cancer in these patients is endometrioid carcinoma.47 However, patients have a relatively good prognosis, which is related to earlier detection—usually stage I or II—according to FIGO (see Table 1). For the patients with Lynch syndrome, salpingo-oophorectomy is a prophylactic approach.44,46,48
Many other mutations may increase the risk of developing ovarian cancer. These include mutations in the TP53 (Li-Fraumeni syndrome),20,49,50 STK11 (Peutz‑Jeghers syndrome), BRIP1,51 RAD51C,52 and PALB2 genes.53 These mutations have not been studied as thoroughly as mutations in the BRCA1/BRCA2 and DNA repair genes.
Gynecologic and Gynecologic-Related Factors
In addition to genetic determinants, there are many other risk factors for ovarian cancer that have been studied to varying degrees. The vast majority are associated with pathologies within the reproductive system. The impact of endometriosis on ovarian cancer risk is particularly well understood. Because endometrial lesions tend to become malignant, patients with endometriosis, especially those located within the ovaries, have a higher risk of developing ovarian cancer; this risk is 50% higher than in the general population.34,54 Ovarian cancer in patients with endometriosis is known as endometriosis-associated ovarian cancer (EAOC). Histologically, the most common EAOC tumor types are clear cell, endometrioid, and low-grade serous carcinoma. The pathological mechanism of the formation of a neoplastic lesion from an endometriosis focus is complex and includes oxidative stress, inflammatory processes, estrogen effects (hyperestrogenism), hemorrhages, and somatic mutations in the PIK3CA, PTEN, and ARID1A genes.5,55–57 In general, patients with EAOC have a good prognosis as long as they are diagnosed early enough.58
According to a few scientific reports, benign ovarian cysts can be precursors to malignant lesions. Although most cysts disappear spontaneously, there is a small risk of developing ovarian cancer from benign lesions. In postmenopausal patients, the likelihood of a malignant lesion developing from a simple cyst is estimated at 0.3%. With complex cysts, this risk increases to 36%.59,60 Other documented risk factors for ovarian cancer include pelvic inflammatory disease. Patients with recurrent pelvic inflammatory disease have been shown to have a higher risk of developing ovarian cancer.61–63 One cause of pelvic inflammatory disease is Chlamydia trachomatis infection; patients with a history of C. trachomatis infection have been shown to have a higher risk of developing ovarian cancer.64,65
The role of polycystic ovary syndrome (PCOS) in the development of ovarian cancer is controversial. Since PCOS patients have ovulatory-free cycles, some researchers assert that they should have a lower risk of ovarian cancer (the effect of ovulation on ovarian cancer risk will be described later).66,67 Conversely, a study by Schildkraut et al68 found a 2.5-fold increased risk of ovarian cancer in PCOS patients, especially in those with elevated body mass index (BMI) and no oral contraceptive use. However, it should be noted that the study was conducted on a small group of patients. Far more studies suggest that PCOS does not increase the risk of ovarian cancer.69,70
Other debatable factors associated with ovarian cancer risk include the use of talc, which is an ingredient in baby powder, and feminine hygiene products. A study by Cramer et al71 determined that the regular application of talc to the genital area was associated with an increase in overall ovarian cancer risk. However, according to O’Brien et al,72 there is no association between talc use and increased ovarian cancer risk. Because talc can be contaminated with carcinogens, such as asbestos and quartz, more research on the potential links between talc and the development of ovarian cancer is warranted.34,73
Besides the factors that increase the risk of ovarian cancer, there are numerous protective factors, including tubal ligation, oral contraceptive use, and pregnancy.5,34 Large studies have determined that tubal ligation reduces the risk of ovarian cancer. The greatest decrease in risk was observed for endometrioid ovarian carcinoma. It is likely that ligated fallopian tubes provide a mechanical barrier to carcinogens.33,74,75
According to the “Incessant Ovulation Theory”, uninterrupted ovulation may contribute to increased ovarian cancer risk. During ovulation, the ovarian epithelium is damaged and then undergoes regeneration. Repeated damage to the ovarian epithelium translates into the possibility of errors during the replication process and resulting DNA damage, which in turn increases the risk of cancerous transformation.5,22,76,77 Therefore, any factor that inhibits ovulation might contribute to a decrease in ovarian cancer risk; one such factor is oral contraceptive use. According to the literature, oral contraceptive use reduces the risk of ovarian cancer regardless of its type.5,33,78–82 The greatest protective effect of oral contraceptive use is observed in women taking the medication for a longer period of time—the risk of getting the disease decreased with the duration of pill use.78,80 It is estimated that in women taking the pill for 15 years, the risk of developing ovarian cancer decreases by 70%. Interestingly, a protective effect was already observed with lower doses of the drug.78 Positive effects of hormonal contraception were also found in BRCA1/BRCA2 mutation carriers. In female carriers, long-term use translated into a reduced risk of ovarian cancer.33,83
Pregnancy is another confirmed protective factor. Both term and non-delivered pregnancies reduce the risk of ovarian cancer. It has been shown that an increase in the number of pregnancies translated into a further decrease in the risk of ovarian cancer.5,22,33,34,84 However, the exact mechanism of the protective effect of pregnancy on ovarian cancer risk is not well understood. In addition to inhibition of ovulation during pregnancy, one of the postulated reasons is the high concentration of progesterone that occurs physiologically in the tissues of pregnant women to maintain pregnancy.22,84 This theory is supported by in vitro studies by Yu et al85 and Lima et al86 in which progesterone inhibited proliferation, migration, and invasion of ovarian cancer cells and also induced apoptosis.
Lactation is directly related to pregnancy. Breastfeeding is one of the better documented factors in reducing the risk of ovarian cancer. In fact, a stronger protective effect was observed with longer duration of breastfeeding.5,33,87–89 According to a study by Babic et al,90 breastfeeding for 12 months or longer reduced the risk of ovarian cancer by 34%; this relationship was demonstrated mainly for high-grade serous and endometrioid carcinomas. As in the case of pregnancy, the protective mechanism of breastfeeding consists mainly of the induction of ovulatory-free cycles and probably the inhibition of luteinizing hormone release, which has been postulated to be involved in the pathogenesis of ovarian cancer.34
Lifestyle Factors
Numerous lifestyle-related factors are associated with decreased or increased risks in the development of ovarian cancer. An association between obesity and increased ovarian cancer risk has been demonstrated. Women with a higher BMI were more likely to develop ovarian cancer than women in the healthy weight range.5,34,91,92 Interestingly, women who had a high BMI in early adulthood also had an increased risk of ovarian cancer in later life.91 Furthermore, obesity translated into a worse prognosis in the course of ovarian cancer.34,93,94 The pathogenesis of obesity-related ovarian cancer most likely results from changes in the bioavailability of active compounds in female tissues. Obese women have an increased availability of compounds with potentially procarcinogenic properties, including leptin, inflammatory mediators, androgens, and estrogens, while a decrease in progesterone (as mentioned earlier) who had a protective effect on the development of ovarian cancer.5,34,91,94
Although cigarette smoking is one of the most important factors that increases the risk of several types of cancer, no significant association has been found between smoking and the overall risk of ovarian cancer.95,96 However, it should be noted that female smokers have an increased risk of mucinous carcinoma.34,96–98 Furthermore, smoking patients with ovarian cancer had a worse prognosis compared to non-smoking ovarian cancer patients.34,94
Alcohol is another factor linked to an increased risk of various types of cancer. In the case of ovarian cancer, studies have not confirmed a link between alcohol consumption and an increased likelihood of developing this type of cancer.5,33,99,100
The effect of diet on increasing or decreasing cancer risk has been known for many years; individual nutrients also affect ovarian cancer risk. The main foods with a protective effect include fresh fruits and vegetables, which is mostly due to their antioxidant properties.34,101–103 At the same time, consumption of salted and canned vegetables has been shown to increase the likelihood of developing ovarian cancer.101,102 The effect of dairy products depends on their type—consumption of milk, sour milk products, and yogurt increased the risk of ovarian cancer, while an inverse relationship was observed with cheese.104 It has also been shown that patients who have a diet high in fat (especially animal fat or saturated fat and cholesterol) were more likely to develop ovarian cancer.34,103 A similar relationship was demonstrated for smoked and fried foods.101
Matrix Metalloproteinases—Physiological Role and Involvement in Pathological States
Matrix metalloproteinases (MMPs) comprise a group of proteolytic enzymes that are similar in structure whose enzymatic activity depends on zinc ions.17,105–114 Twenty-eight enzymes of the MMP family have been identified in vertebrates. However, only 23 MMPs are expressed in humans.107,113,115 Interestingly, MMP-23 exists in two isoforms that are encoded by two separate genes—MMP-23A and MMP-23B.105,114,116 Based on their specificity to the degraded substrate, MMPs can be divided into six groups: (1) gelatinases; (2) collagenases; (3) stromelysins; (4) matrilysins; (5) membrane-type MMPs; and (6) other MMPs.110,113,116–118 The division of MMPs with examples is shown in Figure 2.
MMPs are produced by various cell types, including smooth muscle cells, leukocytes, platelets, fibroblasts, and endothelial cells.105–113 The primary function of MMPs is to maintain physiological tissue homeostasis by digesting components of the extracellular matrix. In addition to the degeneration of extracellular matrix components, MMPs are also involved in the degradation of other proteolytic enzymes (including other MMPs), protease inhibitors, blood clotting factors, cytokines, antimicrobial peptides, growth factors, adhesion molecules, and membrane-bound receptors.17,106–113,118,119 They also mediate the formation of chemokines, growth factors, and other biologically active peptides from their inactive precursors.109
MMPs are secreted into the environment as inactive zymogens known as proMMPs. ProMMPs are maintained in an inactive form by binding between a conserved cysteine in the propeptide domain of the molecule and a zinc ion in the catalytic center. ProMMPs are activated by breaking the chemical bond between the cysteine amino acid and the zinc ion, which can occur through three different mechanisms. The first involves limited proteolysis, resulting in the removal of the prodomain. This process occurs through the activity of other proteolytic enzymes, such as plasmin, furin, chymase, or other MMPs, including MMP-3, MMP-10, and MMP-14.105,107,111,113,115,119 In the second mechanism, proMMPs are activated by reactions between the cysteine amino acid in the propeptide domain and alkylating agents, heavy metal ions, or reactive oxygen species. The third mechanism of MMPs activation occurs through allosteric reconformation of the prodomain.105,106,119,120
The proteolytic activity of MMPs is regulated by tissue inhibitors of metalloproteinases (TIMPs). TIMPs bind covalently to a given MMP or its precursor form, thereby inhibiting its activity. Four types of TIMPs have been identified: TIMP-1, TIMP-2, TIMP-3, and TIMP-4. The different types of TIMPs differ in their affinity for MMPs.105,106,111,113,114,118,121 Interestingly, MMPs activity can also be controlled by non-specific inhibitors, such as α2-macroglobulin, α1-antitrypsin, β-amyloid precursor protein, tissue factor pathway inhibitor-2, and serine proteinase inhibitor.105,113,117
MMPs have multiple physiological roles, including regulation of cellular processes related to differentiation and proliferation, apoptosis, and induction of inflammatory or immune responses. These enzymes are involved in wound healing, tissue remodeling, ovulation, and restoration of the endometrium during the menstrual cycle. During fetal development, MMPs are involved in embryogenesis and organogenesis, with particular emphasis on the development of the cardiovascular, respiratory, and musculoskeletal systems. They are also essential during the final stages of pregnancy and childbirth.105–107,109,110,113,117,118,122
Physiologically, the activity of MMPs is maintained in a state of equilibrium; if their activity becomes dysregulated, these enzymes can contribute to the onset and progression of various pathological conditions. Dysregulation of MMPs has been shown to be associated with the progression of several cardiovascular (aortic and intracranial aneurysms, arteriosclerosis, coronary artery disease, pathological myocardial remodeling, and hypertension),107,111,113,117,123,124 nervous (Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, amyotrophic lateral sclerosis and multiple sclerosis),107,111,113,125,126 excretory (renal fibrosis, chronic kidney disease, and diabetic nephropathy),111,127,128 respiratory,107,111,113,129,130 musculoskeletal (osteoarthritis and osteoporosis),107,110,118,131 and liver diseases (hepatic fibrosis, cirrhosis, and portal hypertension).107,111,132
MMPs also play an important role in all stages of carcinogenesis.122 Among other functions, MMPs affect tumor cell proliferation, migration, and invasion, stimulate angiogenesis, and induce epithelial–mesenchymal transition within the cancerous lesion.111,112,114,118,121,122,133 In the early stages of cancer, MMPs induce DNA damage and resultant genomic instability.134,135 For example, MMP-2 localizes in the cell nucleus and degrades proteins responsible for repairing DNA damage.136 At the stage of tumor progression, a particularly important role of MMPs is the aforementioned stimulation of angiogenesis and lymphangiogenesis, which has been shown to enable further growth of the tumor mass.112,116,135,137 In the later stages of the disease, MMPs activity has been associated with the formation of metastatic foci, mainly due to their proteolytic properties that enable digestion of the extracellular matrix.111,112,135,138 The role of MMPs in the process of carcinogenesis is shown in Figure 3.
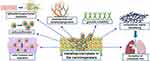 |
Figure 3 Multidirectional role of MMPs in carcinogenesis. |
Metalloproteinases in Ovarian Cancer
Gelatinases—MMP-2 and MMP-9
MMP-2 expression is found in physiological ovarian tissue,14,16 whereas MMP-9 expression is low14,139 or undetectable.139,140 Expression of MMP-215,141–147 and MMP-914,140,147,148 is found in ovarian cancer samples. Expression of MMP-2 and MMP-9 is found directly in cancer epithelial cells,14,15,141–148 within the stroma of the tumor lesion,15,141–143,145,147 and in metastatic foci.141 Expression of MMP-9 is higher in cancer compared to benign lesions.139,142 Expression of MMP-2145–147 and MMP-9 is found in all histological types of ovarian carcinoma.146–148 According to most reports, there are no differences in MMP-2 and MMP-9 expression between the different histological types of ovarian carcinoma.14,15,139 However, according to Jeleniewicz et al146 the highest expression of MMP-2 was found in the serous ovarian carcinoma type and chemotherapy-sensitive tumors. Additionally, a single study determined that the highest expression of MMP-9 was in the high-grade serous ovarian carcinoma.147 MMP-2 expression levels were independent of disease stage, tumor size, and treatment effects. However, there was a correlation between MMP-2 expression and the tendency of ovarian cancer to recur, as well as the presence of metastatic lesions.15 In contrast to MMP-2, MMP-9 expression was closely related to the ovarian cancer stage according to the FIGO classification; it was higher in stages III and IV than in less advanced stages.14,139,140 Furthermore, MMP-9 levels were higher in patients with established metastatic foci than in patients without metastasis.142,148 Levels of MMP-9 expression have also been shown to be positively correlated with the number of vasculogenic-like networks formed in cancerous tissue.139 The formation of vasculogenic-like networks is associated with the so-called “vascular mimicry phenomenon”, which involves the transformation of cancer cells into endothelial-like cells and the formation of vascular-like structures. In addition to providing for the metabolic needs of the growing tumor lesion, these structures have been shown to provide an alternative pathway for cancer cell extravasation and subsequent metastasis.149–151
The relationship between MMP-2 expression and patient prognosis is controversial. MMP-2 expression in cancer epithelial cells,141,147 stroma, and metastatic foci141 was associated with poorer patient prognosis. However, as reported by Ekinci et al,15 Vos et al,145 and Jeleniewicz et al,146 Maneti et al,152 there was no association between MMP-2 expression in cancer epithelial cells and patient prognosis. The presence of MMP-2 in the stroma of the lesion is another matter of debate. Morales-Vásquez et al147 and Maneti et al152 stated that this MMP has a protective effect in ovarian cancer patients, while Ekinci et al15 reported that the survival time of patients expressing stromal MMP-2 was shorter. The relationship between MMP-9 expression and patient survival is unclear. According to Hu et al,14 the mean survival time of ovarian cancer patients expressing MMP-9 was significantly shorter than that of patients with negative MMP-9 expression, while a study by Sillanpää et al148 described an inverse relationship.
Although the preliminary usefulness of gelatinases determined from peripheral blood has been determined in patients with different types of cancers,153,154 studies of MMP-2 and MMP-9 in patients with ovarian cancer are incomplete and inconclusive. Serum MMP-2 levels were lower in ovarian cancer patients than in healthy women. However, there was no difference in the levels of this MMP between patients with ovarian cancer and women with benign lesions.155 In contrast to MMP-2, the serum and plasma levels of MMP-9 were higher in women with ovarian cancer than in healthy patients or those with benign lesions.14,152,156,157 Serum MMP-2 levels were not dependent on ovarian cancer stage according to FIGO,155 while MMP-9 levels were higher in patients with stage III and IV disease according to FIGO than in women with stage I and II disease.156,157 However, there was no correlation between MMP-9 levels and the histological types of ovarian carcinoma.14,156 Additionally, elevated serum MMP-9 levels were found in women who were insensitive to chemotherapy or had ascites or metastatic foci.14,156 Importantly, Ławicki et al157–159 investigated plasma levels of MMP-9 and the most common marker in ovarian cancer (CA-125) in two independent studies and reported that the highest diagnostic sensitivity values for ovarian cancer were obtained when these two markers were evaluated together. In addition, MMP-9 levels decreased in patients after surgery, indicating the potential use of this enzyme to assess the effectiveness of surgical procedures. The potential of MMP-2 and MMP-9 to predict the prognosis of ovarian cancer patients has not yet been thoroughly researched. A single study demonstrated that there was no relationship between plasma MMP-2 and MMP-9 levels and prognosis.152
Interestingly, there is a single report of elevated MMP-2 and MMP-9 concentrations in the urine of women with ovarian cancer in whom the routine marker CA-125 remained within reference norms.160 This indicates that MMP expression levels and blood concentrations may not be the only parameters that potentially have diagnostic value.
Collagenases—MMP-1, MMP-8, MMP-13, and MMP-18
The expression or activity of collagenases in healthy ovarian tissue varies by type. MMP-1 activity161 and MMP-18 expression162 have been identified in ovarian tissue collected from healthy women. MMP-13 expression was not found in physiological ovarian tissue,16 and MMP-8 expression has not been studied. The expression of MMP-1, MMP-8, and MMP-13 was found in ovarian cancer samples, while the presence of MMP-18 has not been investigated.16,163–166 According to Behrens et al,163 MMP-1 expression was higher in ovarian cancers compared to benign lesions. The expression of the other collagenases in benign lesions has not yet been studied. The relationship between collagenase expression levels and prognosis has been studied using MMP-8 and MMP-13 as examples—higher expression of these collagenases was associated with poorer prognosis.16,165 Patients who were at higher stages of ovarian cancer exhibited higher MMP-8 tissue levels. Moreover, higher expression of MMP-8 was associated with higher expression of MMP-9, whose diagnostic significance was discussed in the previous section.165 MMP-13 expression was not related to disease stage or whether the cancer occupied one or two ovaries.166
The activity of individual collagenases was also examined in fluids collected from ovarian cysts. MMP-1 and MMP-13 activity in the ovarian cysts was determined to be low, and there was no difference in MMP-1 activity between benign and malignant cysts. MMP-8 activity was higher in malignant cysts than in benign cysts.164
A single study reported on MMP-13 levels in peritoneal fluid from patients with advanced forms of ovarian cancer (FIGO stage III or IV); patients with higher MMP-13 levels had a worse prognosis than patients with lower MMP-13 levels.167 There are currently no studies on the diagnostic utility of collagenases measured in peripheral blood in ovarian cancer patients, but preliminary studies indicate that they potentially have diagnostic value in other types of cancer, such as gastric cancer168 and skin cancer.169
Stromelysins—MMP-3, MMP-10, and MMP-11
As with collagenases, the expression of stromelysins in physiological ovarian tissue depends on their type. MMP-3 is found in physiological ovarian tissue, but MMP-10 is not expressed.16,170 To date, MMP-11 expression in physiological ovarian tissue has not been studied.Patients with ovarian cancer express all three of these stromelysins.16,166,171,172 Interestingly, high MMP-11 expression was associated with higher tissue expression of other MMPs, including MMP-2 and MMP-13.166 The potential utility of these two enzymes in ovarian cancer was described in earlier sections of this article. In the cases of MMP-3 and MMP-11, higher expression was found at higher stages according to the FIGO classification,166,171,173 while there was no correlation between the levels of these stromelysins in cancerous tissue and patient prognosis.16,172 In contrast, high MMP-10 expression was associated with a better prognosis for patients at stages III and IV.16
There is very limited research on the potential utility of stromelysins as tumor markers in peripheral blood. A single study reported that patients with ovarian cancer had higher MMP-3 levels compared to women with benign lesions. Higher MMP-3 levels were found in women in more advanced stages according to the FIGO classification. Furthermore, patients with high baseline MMP-3 levels had a worse prognosis than patients with lower levels of this enzyme.174
Matrilysins—MMP-7 and MMP-26
Low expression of MMP-7 was found in physiological ovarian tissue.175–177 High or low expression of MMP-26 was also observed, depending on the structure of the ovary.178 Samples from ovarian cancer patients exhibited the expression of both of these matrilysins.16,143,144,166,175,177–179 MMP-7 was detected in the stroma of a cancerous lesion143 and in metastatic foci of ovarian cancer.177 MMP-7 expression was the same in the metastatic foci as in the primary lesion.177 Interestingly, MMP-7 was identified in the mucin of mucinous ovarian carcinoma, indicating that MMP-7 is produced by cancer gland cells.177 Data on MMP-7 expression in ovarian cancer compared to benign lesions are scarce and contradictory. According to Wang et al,176 higher MMP-7 expression was found in patients with serous ovarian carcinoma compared to benign lesions; however, Brun et al143 documented higher MMP-7 expression in benign lesions compared to serous ovarian carcinoma. To the best of our knowledge, no studies have compared MMP-26 expression between benign and malignant ovarian lesions. No correlation was found between MMP-7 expression and ovarian cancer stage according to the FIGO classification,166 while MMP-26 expression was dependent on FIGO stage, with higher expression levels observed in stages III and IV compared to stage I.178
A study by Sillanpää et al177 suggests a potentially protective role for MMP-7 in ovarian cancer. Patients with high expression of this MMP in cancerous tissue had a better prognosis in terms of 10-year disease-related survival rate and recurrence-free survival time. The protective properties of MMP-7 seem to confirm the results presented in the same study, which state that low expression of MMP-7 was associated with advanced tumor stage, high histological tumor grade, and large primary residual tumor. The study by Sillanpää et al117 is not supported by the results of Zeng et al,16 who found no relationship between MMP-7 expression and prognosis. At present, no relationship has been demonstrated between MMP-26 expression levels and prognosis.16
Some studies have noted the preliminary potential of MMP-7 as a biomarker in peripheral blood samples. In ovarian cancer patients, plasma or serum MMP-7 levels were higher than in healthy subjects and those with benign lesions.155,179–181 No relationship was found between serum MMP-7 levels and tumor stage, tumor grade, and presence of metastasis or ascites,155,179 but a relationship was shown between MMP-7 levels and primary tumor size.179 Notably, MMP-7 levels after surgery and chemotherapy were reduced, which suggests the possibility of using this MMP to evaluate the efficacy of ovarian cancer treatment in the future.155,179 According to Będkowska et al,181 MMP-7 had comparable diagnostic sensitivity and specificity values and negative and positive predictive values as two routine ovarian cancer markers (CA-125 and HE4). In addition, preliminary analyses indicate the possibility of detecting ovarian cancer at earlier stages using simultaneous determination of MMP-7 and CA-125.180 To the best of our knowledge, no studies have evaluated the levels or diagnostic utility of MMP-26 in the serum or plasma of ovarian cancer patients. However, elevated levels of this MMP are found in other types of cancer, including breast cancer18 and prostate cancer.182
Membrane-Type MMPs—MMP-14, MMP-15, MMP-16, MMP-17, MMP-24, and MMP-25
Research on the expression of membrane-type MMPs in physiological ovarian tissue is inconsistent. On one hand, some scientific reports have confirmed the expression of MMP-14, MMP-15, MMP-16, MMP-24, and MMP-25 in normal ovarian tissue.183,184 On the other hand, a study by Testuri et al185 failed to determine whether membrane MMPs were expressed in physiological ovarian tissue. Similar observations for MMP-25 were made by Zeng et al,16 but the study was conducted on a small number of samples (n = 3). In ovarian cancer, the expression of MMP-14,13,16,142,144,166,184–187 MMP-15,16,185 MMP-16,16,166,185,188 MMP-17, MMP-24,16,166,185 and MMP-2516,166 was confirmed by numerous studies. Both epithelial and stromal expression was demonstrated for MMP-14.13,142,145,186 Epithelial and stromal MMP-14 expression was associated with ascites and lymph node involvement, whereas high mRNA levels in the epithelium, in particular, translated to the development of distant metastatic foci.13 Interestingly, an inverse relationship with CA-125 was observed for MMP-14 expression. Tumors with high MMP-14 expression simultaneously had low CA-125.189 A study by Sakata et al142 determined that benign lesions had lower MMP-14 expression than cancer; however, according to Testuri et al,185 there was no expression of MMP-14 or other membrane MMPs in nonmalignant ovarian lesions. Therefore, further studies are needed to clearly ascertain the potential of membrane MMPs as auxiliary markers for differentiating between benign and malignant lesions. The relationship between FIGO stage and expression of membrane-type MMPs is a matter of debate. According to Escalona et al,173 mRNA levels of MMP-14 increased with higher FIGO classification stages. On the contrary, Wang et al166 found no relationship between MMP-14 expression and ovarian cancer stage. According to a single study, there was no relationship between MMP-17 and MMP-24 expression and FIGO stage. However, MMP-16 and MMP-25 expression was higher in FIGO stages III and IV than in less advanced stages.166
A few studies have reported an association between membrane MMP expression and prognosis. According to Kamat et al,13 patients with high epithelial and stromal MMP-14 expression had low disease-related survival rate values; the lowest disease-related survival rate values were among women with high MMP-14 expression found only in cancerous epithelium. Patients with moderate MMP-14 expression in the epithelium accompanied by low stromal expression had the best prognoses.13 In addition, Wang et al166 determined that high levels of mRNA for MMP-14 in ovarian cancer were associated with a poorer prognosis. However, it should be noted that according to Zeng et al,16 MMP-14 expression was not associated with patient survival. A similar relationship was found for MMP-15,166 MMP-16,16 MMP-17,16,166 and MMP-24.166 Interestingly, high expression of MMP-25 was associated with longer overall survival.16
To the best of our knowledge, only a single study to date has suggested that MMP-14 can be used as a blood-based marker for ovarian cancer patient. MMP-14 concentrations were higher in women with ovarian cancer compared to healthy patients and those with benign lesions.190 However, the concentrations and potential utility of other membrane MMPs as markers in peripheral blood in patients with ovarian cancer have not yet been investigated.
Other Types of MMPs—MMP-12, MMP-19, MMP-20, and MMP-21
There is limited research on the other MMPs. No MMP-12 expression was found in normal ovarian tissue.16 Expression of MMP-19, MMP-20, and MMP-21 has not yet been studied. MMP-12, MMP 19, MMP-20, and MMP-21 mRNA was found in serous and endometrioid ovarian carcinoma.16,166 According to Wang et al,166 there was no correlation between MMP-12, MMP-19, MMP-20, and MMP-21 mRNA levels and FIGO stage. Differences were found between the expression of individual MMPs and prognosis. High levels of MMP-12 expression in stage III or IV patients were associated with better overall survival. The same study simultaneously found that MMP-19, MMP-20, and MMP-21 expression was not associated with prognosis.16 However, it should be noted that Wang et al166 determined that high MMP-19 and MMP-20 expression was associated with poor overall survival and that these two MMPs could serve as independent factors to predict poor prognosis in ovarian cancer patients. The poor prognosis of female patients has been shown to be due to a complex mechanism of action, studied in vitro, in which MMP-19 and MMP-20 induced resistance to anti-cancer drugs and stimulated cancer cell invasion.166
To the best of our knowledge, no studies have established the potential of other MMPs as peripheral blood markers in ovarian cancer patients. A single study of colon cancer patients determined that MMP-12 has potential as a novel tumor marker.191 It is unfortunate that the potential of MMP-19, MMP-20, and MMP-21 as tumor markers has not been determined by any oncology studies. Therefore, future investigations of this group of enzymes should be conducted to evaluate their potential utility, not only in ovarian cancer patients, but also in other types of cancer. A representation of the most significant characteristics of MMPs is presented in Table 2
 |
Table 2 The Most Significant Properties of MMPs Groups Found in Tissue Studies and Body Fluids |
Conclusion
Ovarian cancer is one of the most common gynecologic malignancies, and many interrelated factors contribute to its prevalence. Due to its usually asymptomatic or minimally symptomatic course, it is most often detected at an advanced stage, which translates into an unfavorable prognosis. Considering the increasing number of cases and its insidious nature, it is vital to search for new ways to enable earlier diagnosis of ovarian cancer. Additionally, besides early detection, it is necessary to properly control its progress and assess its prognosis. It might be possible to measure these tumor markers in different biological samples as diagnostic parameters. This article summarized the potential role of extracellular MMPs in the diagnosis, monitoring, and prognosis assessment of ovarian cancer patients.
MMPs are involved in all stages of carcinogenesis, and their role and potential utility in ovarian cancer remains controversial. Most currently known MMPs are expressed in ovarian cancer, and high expression of some of them (eg, MMP-8, MMP-9, and MMP-14) has been associated with a more unfavorable disease course. Conversely, the potentially protective properties of some MMPs, including MMP-7 and MMP-25, have been demonstrated. In addition to tissue expression, some studies have preliminarily established the utility of MMPs as markers in peripheral blood, urine, and other body fluids. However, as with tissue expression assays, these data are incomplete and often conflicting. Nevertheless, according to initial studies, individual enzymes from the MMP family such as MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-13, and MMP-14 may be suitable for early diagnosis, monitoring, and assessing prognosis of patients with ovarian cancer. A clear determination of the diagnostic utility of these enzymes requires further research. A summary of the diagnostic potential of individual MMPs is presented in Figure 4.
Funding
This work was supported by the Medical University of Białystok (Poland) grant number SUB/1/DN/22/002/1201.
Disclosure
The authors declare no conflicts of interest in relation to this work. The funders had no role in the writing of the manuscript.
References
1. Hirst J, Crow J, Godwin A. Ovarian cancer genetics: subtypes and risk factors. In: Devaja O, Papadopoulos A, editors. Ovarian Cancer - From Pathogenesis to Treatment. London: IntechOpen; 2018:1–37.
2. Ravindran F, Choudhary B. Ovarian cancer: molecular classification and targeted therapy. In: Ho G, Webber K, editors. Ovarian Cancer - Updates in Tumour Biology and Therapeutics. London: IntechOpen; 2021:1–21.
3. De Leo A, Santini D, Ceccarelli C, et al. What is new on ovarian carcinoma: integrated morphologic and molecular analysis following the new 2020 World Health Organization classification of female genital tumors. Diagnostics. 2021;11(4):697. doi:10.3390/diagnostics11040697
4. Doubeni CA, Doubeni AR, Myers AE. Diagnosis and management of ovarian cancer. Am Fam Physician. 2016;93(11):937–944.
5. Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health. 2019;11:287–299. doi:10.2147/IJWH.S197604
6. Dąbrowska E, Przylipiak A, Zajkowska M, Piskór BM, Borowik-Zaręba A, Ławicki S. C-C motif chemokine ligand 5 and C-C chemokine receptor type 5: possible diagnostic application in breast cancer patients. Acta Biochim Pol. 2020;67(4):539–449. doi:10.18388/abp.2020_5402
7. Będkowska GE, Gacuta E, Zbucka-Krętowska M, et al. Plasma levels and diagnostic utility of VEGF in a three-year follow-up of patients with breast cancer. J Clin Med. 2021;10(22):5452. doi:10.3390/jcm10225452
8. Lubowicka E, Zbucka-Kretowska M, Sidorkiewicz I, et al. Diagnostic power of cytokine M-CSF, metalloproteinase 2 (MMP-2) and tissue inhibitor-2 (TIMP-2) in cervical cancer patients based on ROC analysis. Pathol Oncol Res. 2020;26(2):791–800. doi:10.1007/s12253-019-00626-z
9. Sidorkiewicz I, Piskór B, Dąbrowska E, et al. Plasma levels and tissue expression of selected cytokines, metalloproteinases and tissue inhibitors in patients with cervical cancer. Anticancer Res. 2019;39(11):6403–6412. doi:10.21873/anticanres.13854
10. Zajkowska M, Zbucka-Krętowska M, Sidorkiewicz I, et al. Plasma levels and diagnostic utility of macrophage-colony stimulating factor, matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 as tumor markers in cervical cancer patients. Tumour Biol. 2018;40(7):1010428318790363. doi:10.1177/1010428318790363
11. Będkowska GE, Ławicki S, Gacuta E, Pawłowski P, Szmitkowski M. M-CSF in a new biomarker panel with HE4 and CA 125 in the diagnostics of epithelial ovarian cancer patients. J Ovarian Res. 2015;8(1):27. doi:10.1186/s13048-015-0153-3
12. Ławicki S, Będkowska GE, Gacuta-Szumarska E, Szmitkowski M. The plasma concentration of VEGF, HE4 and CA125 as a new biomarkers panel in different stages and sub-types of epithelial ovarian tumors. J Ovarian Res. 2013;6(1):45. doi:10.1186/1757-2215-6-45
13. Kamat AA, Fletcher M, Gruman LM, et al. The clinical relevance of stromal matrix metalloproteinase expression in ovarian cancer. Clin Cancer Res. 2006;12(6):1707–1714. doi:10.1158/1078-0432.CCR-05-2338
14. Hu X, Li D, Zhang W, Zhou J, Tang B, Li L. Matrix metalloproteinase-9 expression correlates with prognosis and involved in ovarian cancer cell invasion. Arch Gynecol Obstet. 2012;286(6):1537–1543. doi:10.1007/s00404-012-2456-6
15. Ekinci T, Ozbay PO, Yiğit S, Yavuzcan A, Uysal S, Soylu F. The correlation between immunohistochemical expression of MMP-2 and the prognosis of epithelial ovarian cancer. Polish Gynaecology. 2014;85(2):121–130. doi:10.17772/gp/1702
16. Zeng L, Qian J, Zhu F, Wu F, Zhao H, Zhu H. The prognostic values of matrix metalloproteinases in ovarian cancer. J Int Med Res. 2020;48(1):300060519825983. doi:10.1177/0300060519825983
17. Al-Alem L, Curry TE. Ovarian cancer: involvement of the matrix metalloproteinases. Reproduction. 2015;150(2):R55–R64. doi:10.1530/REP-14-0546
18. Piskór BM, Przylipiak A, Dąbrowska E, et al. Plasma concentrations of matrilysins MMP-7 and MMP-26 as diagnostic biomarkers in breast cancer. J Clin Med. 2021;10(7):1436. doi:10.3390/jcm10071436
19. Będkowska GE, Piskór B, Gacuta E, et al. Diagnostic power of selected cytokines, MMPs and TIMPs in ovarian cancer patients – ROC analysis. Anticancer Res. 2019;39(5):2575–2582. doi:10.21873/anticanres.13380
20. Toss A, Tomasello C, Razzaboni E, et al. Hereditary ovarian cancer: not only BRCA 1 and 2 genes. Biomed Res Int. 2015;2015:341723. doi:10.1155/2015/341723
21. Javadi S, Ganeshan DM, Qayyum A, Iyer RB, Bhosale P. Ovarian cancer, the revised FIGO staging system, and the role of imaging. AJR Am J Roentgenol. 2016;206(6):1351–1360. doi:10.2214/AJR.15.15199
22. Troisi R, Bjørge T, Gissler M, et al. The role of pregnancy, perinatal factors and hormones in maternal cancer risk: a review of the evidence. J Intern Med. 2018;283(5):430–445. doi:10.1111/joim.12747
23. Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177(3):1053–1064. doi:10.2353/ajpath.2010.100105
24. Daniele A, Ferrero A, Fuso L, et al. Palliative care in patients with ovarian cancer and bowel obstruction. Support Care Cancer. 2015;23(11):3157–3163. doi:10.1007/s00520-015-2694-9
25. Lee YC, Jivraj N, O’Brien C, et al. Malignant bowel obstruction in advanced gynecologic cancers: an updated review from a multidisciplinary perspective. Obstet Gynecol Int. 2018;2018:1867238. doi:10.1155/2018/1867238
26. Deng K, Yang C, Tan Q, et al. Sites of distant metastases and overall survival in ovarian cancer: a study of 1481 patients. Gynecol Oncol. 2018;150(3):460–465. doi:10.1016/j.ygyno.2018.06.022
27. Prat J, Ribé A, Gallardo A. Hereditary ovarian cancer. Hum Pathol. 2005;36(8):861–870. doi:10.1016/j.humpath.2005.06.006
28. Berek JS, Renz M, Kehoe S, Kumar L, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl S1):61–85. doi:10.1002/ijgo.13878
29. Goff BA. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291(22):2705–2712. doi:10.1001/jama.291.22.2705
30. Ebell MH, Culp MB, Radke TJ. A systematic review of symptoms for the diagnosis of ovarian cancer. Am J Prev Med. 2016;50(3):384–394. doi:10.1016/j.amepre.2015.09.023
31. Ahmed N, Stenvers KL. Getting to know ovarian cancer ascites: opportunities for targeted therapy-based translational research. Front Oncol. 2013;3:256. doi:10.3389/fonc.2013.00256
32. Shanbhogue AKP, Shanbhogue DKP, Prasad SR, Surabhi VR, Fasih N, Menias CO. Clinical syndromes associated with ovarian neoplasms: a comprehensive review. Radiographics. 2010;30(4):903–919. doi:10.1148/rg.304095745
33. Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14. doi:10.1016/j.bpobgyn.2016.08.006
34. Zayyan MS. Risk factors for ovarian cancer. In: Lasfar A, Cohen-Solal K, editors. Tumor Progression and Metastasis. London: IntechOpen; 2020:1–27.
35. Ueki A, Hirasawa A. Molecular features and clinical management of hereditary gynecological cancers. Int J Mol Sci. 2020;21(24):9504. doi:10.3390/ijms21249504
36. Sekine M, Nishino K, Enomoto T. Differences in ovarian and other cancers risks by population and BRCA mutation location. Genes. 2021;12(7):1050. doi:10.3390/genes12071050
37. Stratton MR, Rahman N. The emerging landscape of breast cancer susceptibility. Nat Genet. 2008;40(1):17–22. doi:10.1038/ng.2007.53
38. Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol. 2015;26(7):1291–1299. doi:10.1093/annonc/mdv022
39. Stoppa-Lyonnet D. The biological effects and clinical implications of BRCA mutations: where do we go from here? Eur J Hum Genet. 2016;24(Suppl1):S3–S9. doi:10.1038/ejhg.2016.93
40. Gorodetska I, Kozeretska I, Dubrovska A. BRCA genes: the role in genome stability, cancer stemness and therapy resistance. J Cancer. 2019;10(9):2109–2127. doi:10.7150/jca.30410
41. Neff RT, Senter L, Salani R. BRCA mutation in ovarian cancer: testing, implications and treatment considerations. Ther Adv Med Oncol. 2017;9(8):519–531. doi:10.1177/1758834017714993
42. Liu YL, Breen K, Catchings A, et al. Risk-reducing bilateral salpingo-oophorectomy for ovarian cancer: a review and clinical guide for hereditary predisposition genes. JCO Oncol Pract. 2022;18(3):201–209. doi:10.1200/OP.21.00382
43. Duraturo F, Liccardo R, De Rosa M, Izzo P. Genetics, diagnosis and treatment of Lynch syndrome: old lessons and current challenges. Oncol Lett. 2019;17(3):3048–3054. doi:10.3892/ol.2019.9945
44. Biller LH, Syngal S, Yurgelun MB. Recent advances in Lynch syndrome. Fam Cancer. 2019;18(2):211–219. doi:10.1007/s10689-018-00117-1
45. Lepore Signorile M, Disciglio V, Di Carlo G, Pisani A, Simone C, Ingravallo G. From genetics to histomolecular characterization: an insight into colorectal carcinogenesis in lynch syndrome. Int J Mol Sci. 2021;22(13):6767. doi:10.3390/ijms22136767
46. Nakamura K, Banno K, Yanokura M, et al. Features of ovarian cancer in Lynch syndrome (Review). Mol Clin Oncol. 2014;2(6):909–916. doi:10.3892/mco.2014.397
47. Helder-Woolderink JM, Blok EA, Vasen HF, Hollema H, Mourits MJ, De Bock GH. Ovarian cancer in Lynch syndrome; a systematic review. Eur J Cancer. 2016;55:65–73. doi:10.1016/j.ejca.2015.12.005
48. Crispens MA. Endometrial and ovarian cancer in lynch syndrome. Clin Colon Rectal Surg. 2012;25(2):97–102. doi:10.1055/s-0032-1313780
49. Neto N, Cunha TM. Do hereditary syndrome-related gynecologic cancers have any specific features? Insights Imaging. 2015;6(5):545–552. doi:10.1007/s13244-015-0425-x
50. Angeli D, Salvi S, Tedaldi G. Genetic predisposition to breast and ovarian cancers: how many and which genes to test? Int J Mol Sci. 2020;21(3):1128. doi:10.3390/ijms21031128
51. Weber-Lassalle N, Hauke J, Ramser J, et al. BRIP1 loss-of-function mutations confer high risk for familial ovarian cancer, but not familial breast cancer. Breast Cancer Res. 2018;20(1):7. doi:10.1186/s13058-018-0935-9
52. Clague J, Wilhoite G, Adamson A, Bailis A, Weitzel JN, Neuhausen SL. RAD51C germline mutations in breast and ovarian cancer cases from high-risk families. PLoS One. 2011;6(9):e25632. doi:10.1371/journal.pone.0025632
53. Yang X, Leslie G, Doroszuk A, et al. Cancer risks associated with germline PALB2 pathogenic variants: an international study of 524 families. J Clin Oncol. 2020;38(7):674–685. doi:10.1200/JCO.19.01907
54. Samartzis EP, Labidi-Galy SI, Moschetta M, et al. Endometriosis-associated ovarian carcinomas: insights into pathogenesis, diagnostics, and therapeutic targets-a narrative review. Ann Transl Med. 2020;8(24):1712. doi:10.21037/atm-20-3022a
55. Brilhante AV, Augusto KL, Portela MC, et al. Endometriosis and ovarian cancer: an integrative review (endometriosis and ovarian cancer). Asian Pac J Cancer Prev. 2017;18(1):11–16. doi:10.22034/APJCP.2017.18.1.11
56. Králíčková M, Laganà AS, Ghezzi F, Vetvicka V. Endometriosis and risk of ovarian cancer: what do we know? Arch Gynecol Obstet. 2020;301(1):1–10. doi:10.1007/s00404-019-05358-8
57. Kornovski Y, Atanasova Y, Kostov S, Slavchev S, Yordanov AD. Endometriosis and risk of ovarian cancer. Oncol Clin Pract. 2021;17(3):125–127. doi:10.5603/OCP.2021.0012
58. Bounous VE, Ferrero A, Fuso L, et al. Endometriosis-associated ovarian cancer: a distinct clinical entity? Anticancer Res. 2016;36(7):3445–3449.
59. Rossing MA, Cushing-Haugen KL, Wicklund KG, Doherty JA, Weiss NS. Risk of epithelial ovarian cancer in relation to benign ovarian conditions and ovarian surgery. Cancer Causes Control. 2008;19(10):1357–1364. doi:10.1007/s10552-008-9207-9
60. Al Zahidy Z. Causes and management of ovarian cysts. Egypt J Hosp Med. 2018;70(10):1818–1822. doi:10.12816/0044759
61. Rasmussen CB, Jensen A, Albieri V, Andersen KK, Kjaer SK. Is pelvic inflammatory disease a risk factor for ovarian cancer? Cancer Epidemiol Biomarkers Prev. 2017;26(1):104–109. doi:10.1158/1055-9965.EPI-16-0459
62. Zhou Z, Zeng F, Yuan J, et al. Pelvic inflammatory disease and the risk of ovarian cancer: a meta-analysis. Cancer Causes Control. 2017;28(5):415–428. doi:10.1007/s10552-017-0873-3
63. Piao J, Lee EJ, Lee M. Association between pelvic inflammatory disease and risk of ovarian cancer: an updated meta-analysis. Gynecol Oncol. 2020;157(2):542–548. doi:10.1016/j.ygyno.2020.02.002
64. Trabert B, Waterboer T, Idahl A, et al. Antibodies against chlamydia trachomatis and ovarian cancer risk in two independent populations. J Natl Cancer Inst. 2019;111(2):129–136. doi:10.1093/jnci/djy084
65. Fortner RT, Terry KL, Bender N, et al. Sexually transmitted infections and risk of epithelial ovarian cancer: results from the Nurses’ health studies. Br J Cancer. 2019;120(8):855–860. doi:10.1038/s41416-019-0422-9
66. Daniilidis A, Dinas K. Long term health consequences of polycystic ovarian syndrome: a review analysis. Hippokratia. 2009;13(2):90–92.
67. Matevossian K, Carpinello O. Polycystic ovary syndrome: menopause and malignancy. Clin Obstet Gynecol. 2021;64(1):102–109. doi:10.1097/GRF.0000000000000560
68. Schildkraut JM, Schwingl PJ, Bastos E, Evanoff A, Hughes C. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet Gynecol. 1996;88(4 Pt 1):554–559. doi:10.1016/0029-7844(96)00226-8
69. Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(5):748–758. doi:10.1093/humupd/dmu012
70. Gottschau M, Kjaer SK, Jensen A, Munk C, Mellemkjaer L. Risk of cancer among women with polycystic ovary syndrome: a Danish cohort study. Gynecol Oncol. 2015;136(1):99–103. doi:10.1016/j.ygyno.2014.11.012
71. Cramer DW, Vitonis AF, Terry KL, Welch WR, Titus LJ. The association between talc use and ovarian cancer: a retrospective case-control study in two US states. Epidemiology. 2016;27(3):334–346. doi:10.1097/EDE.0000000000000434
72. O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323(1):49–59. doi:10.1001/jama.2019.20079
73. Wentzensen N, O’Brien KM. Talc, body powder, and ovarian cancer: a summary of the epidemiologic evidence. Gynecol Oncol. 2021;163(1):199–208. doi:10.1016/j.ygyno.2021.07.032
74. Madsen C, Baandrup L, Dehlendorff C, Kjaer SK. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: a nationwide case-control study. Acta Obstet Gynecol Scand. 2015;94(1):86–94. doi:10.1111/aogs.12516
75. Gaitskell K, Green J, Pirie K, Reeves G, Beral V; Million Women Study Collaborators. Tubal ligation and ovarian cancer risk in a large cohort: substantial variation by histological type. Int J Cancer. 2016;138(5):1076–1084. doi:10.1002/ijc.29856
76. Fleming JS, Beaugié CR, Haviv I, Chenevix-Trench G, Tan OL. Incessant ovulation, inflammation and epithelial ovarian carcinogenesis: revisiting old hypotheses. Mol Cell Endocrinol. 2006;247(1–2):4–21. doi:10.1016/j.mce.2005.09.014
77. Budiana ING, Angelina M, Pemayun TGA. Ovarian cancer: pathogenesis and current recommendations for prophylactic surgery. J Turk Ger Gynecol Assoc. 2019;20(1):47–54. doi:10.4274/jtgga.galenos.2018.2018.0119
78. Royar J, Becher H, Chang-Claude J. Low-dose oral contraceptives: protective effect on ovarian cancer risk. Int J Cancer. 2001;95(6):370–374. doi:10.1002/1097-0215(20011120)95:6<370::aid-ijc1065>3.0.co;2-t
79. Havrilesky LJ, Moorman PG, Lowery WJ, et al. Oral contraceptive pills as primary prevention for ovarian cancer: a systematic review and meta-analysis. Obstet Gynecol. 2013;122(1):139–147. doi:10.1097/AOG.0b013e318291c235
80. Huang Z, Gao Y, Wen W, et al. Contraceptive methods and ovarian cancer risk among Chinese women: a report from the Shanghai Women’s Health Study. Int J Cancer. 2015;137(3):607–614. doi:10.1002/ijc.29412
81. Stewart C, Ralyea C, Lockwood S. Ovarian cancer: an integrated review. Semin Oncol Nurs. 2019;35(2):151–156. doi:10.1016/j.soncn.2019.02.001
82. Karlsson T, Johansson T, Höglund J, Ek WE, Johansson Å. Time-dependent effects of oral contraceptive use on breast, ovarian, and endometrial cancers. Cancer Res. 2021;81(4):1153–1162. doi:10.1158/0008-5472.CAN-20-2476
83. Schrijver LH, Antoniou AC, Olsson H, et al. Oral contraceptive use and ovarian cancer risk for BRCA1/2 mutation carriers: an international cohort study. Am J Obstet Gynecol. 2021;225(1):51.e1–51.e17. doi:10.1016/j.ajog.2021.01.014
84. Han KH, Kim MK, Kim HS, Chung HH, Song YS. Protective effect of progesterone during pregnancy against ovarian cancer. J Cancer Prev. 2013;18(2):113–122. doi:10.15430/jcp.2013.18.2.113
85. Yu S, Lee M, Shin S, Park J. Apoptosis induced by progesterone in human ovarian cancer cell line SNU-840. J Cell Biochem. 2001;82(3):445–451. doi:10.1002/jcb.1171
86. Lima MA, Silva SV, Jaeger RG, Freitas VM. Progesterone decreases ovarian cancer cells migration and invasion. Steroids. 2020;161:108680. doi:10.1016/j.steroids.2020.108680
87. Su D, Pasalich M, Lee AH, Binns CW. Ovarian cancer risk is reduced by prolonged lactation: a case-control study in southern China. Am J Clin Nutr. 2013;97(2):354–359. doi:10.3945/ajcn.112.044719
88. Luan NN, Wu QJ, Gong TT, Vogtmann E, Wang YL, Lin B. Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies. Am J Clin Nutr. 2013;98(4):1020–1031. doi:10.3945/ajcn.113.062794
89. Li DP, Du C, Zhang ZM, et al. Breastfeeding and ovarian cancer risk: a systematic review and meta-analysis of 40 epidemiological studies. Asian Pac J Cancer Prev. 2014;15(12):4829–4837. doi:10.7314/apjcp.2014.15.12.4829
90. Babic A, Sasamoto N, Rosner BA, et al. Association between breastfeeding and ovarian cancer risk. JAMA Oncol. 2020;6(6):e200421. doi:10.1001/jamaoncol.2020.0421
91. Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: a systematic review and meta-analysis. Eur J Cancer. 2007;43(4):690–709. doi:10.1016/j.ejca.2006.11.010
92. Olsen CM, Nagle CM, Whiteman DC, et al. Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocr Relat Cancer. 2013;20(2):251–262. doi:10.1530/ERC-12-0395
93. Bae HS, Kim HJ, Hong JH, Lee JK, Lee NW, Song JY. Obesity and epithelial ovarian cancer survival: a systematic review and meta-analysis. J Ovarian Res. 2014;7:41. doi:10.1186/1757-2215-7-41
94. Nagle CM, Bain CJ, Webb PM. Cigarette smoking and survival after ovarian cancer diagnosis. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2557–2560. doi:10.1158/1055-9965.EPI-06-0592
95. Zhou A, Minlikeeva AN, Khan S, Moysich KB. Association between cigarette smoking and histotype-specific epithelial ovarian cancer: a review of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2019;28(7):1103–1116. doi:10.1158/1055-9965.EPI-18-1214
96. Santucci C, Bosetti C, Peveri G, et al. Dose-risk relationships between cigarette smoking and ovarian cancer histotypes: a comprehensive meta-analysis. Cancer Causes Control. 2019;30(9):1023–1032. doi:10.1007/s10552-019-01198-8
97. Faber MT, Kjær SK, Dehlendorff C, et al. Cigarette smoking and risk of ovarian cancer: a pooled analysis of 21 case-control studies. Cancer Causes Control. 2013;24(5):989–1004. doi:10.1007/s10552-013-0174-4
98. Wentzensen N, Poole EM, Trabert B, et al. Ovarian cancer risk factors by histologic subtype: an analysis from the ovarian cancer cohort consortium. J Clin Oncol. 2016;34(24):2888–2898. doi:10.1200/JCO.2016.66.8178
99. Genkinger JM, Hunter DJ, Spiegelman D, et al. Alcohol intake and ovarian cancer risk: a pooled analysis of 10 cohort studies. Br J Cancer. 2006;94(5):757–762. doi:10.1038/sj.bjc.6603020
100. Chang ET, Canchola AJ, Lee VS, et al. Wine and other alcohol consumption and risk of ovarian cancer in the California Teachers Study cohort. Cancer Causes Control. 2007;18(1):91–103. doi:10.1007/s10552-006-0083-x
101. Zhang M, Yang ZY, Binns CW, Lee AH. Diet and ovarian cancer risk: a case–control study in China. Br J Cancer. 2002;86(5):712–717. doi:10.1038/sj.bjc.6600085
102. Pan SY, Ugnat A-M, Mao Y, Wen SW, Johnson KC; Canadian Cancer Registries Epidemiology Research Group. A case-control study of diet and the risk of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2004;13(9):1521–152. doi:10.1158/1055-9965.1521.13.9
103. Plagens-Rotman K, Chmaj-Wierzchowska K, Pięta B, Bojar I. Modifiable lifestyle factors and ovarian cancer incidence in women. Ann Agric Environ Med. 2018;25(1):36–40. doi:10.5604/12321966.1233565
104. Faber MT, Jensen A, Søgaard M, et al. Use of dairy products, lactose, and calcium and risk of ovarian cancer – results from a Danish case-control study. Acta Oncol. 2012;51(4):454–464. doi:10.3109/0284186X.2011.636754
105. Löffek S, Schilling O, Franzke C-W. Series “matrix metalloproteinases in lung health and disease”: biological role of matrix metalloproteinases: a critical balance. Eur Respir J. 2011;38(1):191–208. doi:10.1183/09031936.00146510
106. Liu J, Khalil RA. Matrix metalloproteinase inhibitors as investigational and therapeutic tools in unrestrained tissue remodeling and pathological disorders. Prog Mol Biol Transl Sci. 2017;148:355–420. doi:10.1016/bs.pmbts.2017.04.003
107. Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci. 2017;147:1–73. doi:10.1016/bs.pmbts.2017.02.005
108. Xie Y, Mustafa A, Yerzhan A, et al. Nuclear matrix metalloproteinases: functions resemble the evolution from the intracellular to the extracellular compartment. Cell Death Discov. 2017;3(1):17036. doi:10.1038/cddiscovery.2017.36
109. Cerofolini L, Fragai M, Luchinat C. Mechanism and inhibition of matrix metalloproteinases. Curr Med Chem. 2019;26(15):2609–2633. doi:10.2174/0929867325666180326163523
110. Rangasamy R, Geronimo G, Ortín O, et al. Molecular imaging probes based on matrix metalloproteinase inhibitors (MMPIs). Molecules. 2019;24(16):2982. doi:10.3390/molecules24162982
111. Raeeszadeh-Sarmazdeh M, Do LD, Hritz BG. Metalloproteinases and their inhibitors: potential for the development of new therapeutics. Cells. 2020;9(5):1313. doi:10.3390/cells9051313
112. Roy R, Morad G, Jedinak A, Moses MA. Metalloproteinases and their roles in human cancer. Anat Rec. 2020;303(6):1557–1572. doi:10.1002/ar.24188
113. Laronha H, Caldeira J. Structure and function of human matrix metalloproteinases. Cells. 2020;9(5):1076. doi:10.3390/cells9051076
114. Niland S, Riscanevo AX, Eble JA. Matrix metalloproteinases shape the tumor microenvironment in cancer progression. Int J Mol Sci. 2021;23(1):146. doi:10.3390/ijms23010146
115. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–573. doi:10.1016/j.cardiores.2005.12.002
116. Quintero-Fabián S, Arreola R, Becerril-Villanueva E, et al. Role of matrix metalloproteinases in angiogenesis and cancer. Front Oncol. 2019;9:1370. doi:10.3389/fonc.2019.01370
117. Benjamin MM, Khalil RA. Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. Exp Suppl. 2012;103:209–279. doi:10.1007/978-3-0348-0364-9_7
118. Tokuhara CK, Santesso MR, Oliveira GS, et al. Updating the role of matrix metalloproteinases in mineralized tissue and related diseases. J Appl Oral Sci. 2019;27:e20180596. doi:10.1590/1678-7757-2018-0596
119. Ra H-J, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26(8):587–596. doi:10.1016/j.matbio.2007.07.001
120. Hadler-Olsen E, Fadnes B, Sylte I, Uhlin-Hansen L, Winberg J-O. Regulation of matrix metalloproteinase activity in health and disease. FEBS J. 2011;278(1):28–45. doi:10.1111/j.1742-4658.2010.07920.x
121. Shimoda M, Ohtsuka T, Okada Y, Kanai Y. Stromal metalloproteinases: crucial contributors to the tumor microenvironment. Pathol Int. 2021;71(1):1–14. doi:10.1111/pin.13033
122. Cabral-Pacheco GA, Garza-Veloz I, Castruita-de la Rosa C, et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int J Mol Sci. 2020;21(24):9739. doi:10.3390/ijms21249739
123. Azevedo A, Prado AF, Antonio RC, Issa JP, Gerlach RF. Matrix metalloproteinases are involved in cardiovascular diseases. Basic Clin Pharmacol Toxicol. 2014;115(4):301–314. doi:10.1111/bcpt.12282
124. Zhang X, Ares WJ, Taussky P, Ducruet AF, Grandhi R. Role of matrix metalloproteinases in the pathogenesis of intracranial aneurysms. Neurosurg Focus. 2019;47(1):E4. doi:10.3171/2019.4.FOCUS19214
125. Brkic M, Balusu S, Libert C, Vandenbroucke RE. Friends or foes: matrix metalloproteinases and their multifaceted roles in neurodegenerative diseases. Mediators Inflamm. 2015;2015:620581. doi:10.1155/2015/620581
126. Behl T, Kaur G, Sehgal A, et al. Multifaceted role of matrix metalloproteinases in neurodegenerative diseases: pathophysiological and therapeutic perspectives. Int J Mol Sci. 2021;22(3):1413. doi:10.3390/ijms22031413
127. Parrish AR. Matrix metalloproteinases in kidney disease: role in pathogenesis and potential as a therapeutic target. Prog Mol Biol Transl Sci. 2017;148:31–65. doi:10.1016/bs.pmbts.2017.03.001
128. Zakiyanov O, Kalousová M, Zima T, Tesař V. Matrix metalloproteinases in renal diseases: a critical appraisal. Kidney Blood Press Res. 2019;44(3):298–330. doi:10.1159/000499876
129. Gueders MM, Foidart J-M, Noel A, Cataldo DD. Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in the respiratory tract: potential implications in asthma and other lung diseases. Eur J Pharmacol. 2006;533(1–3):133–144. doi:10.1016/j.ejphar.2005.12.082
130. Houghton AM. Matrix metalloproteinases in destructive lung disease. Matrix Biol. 2015;44–46:167–174. doi:10.1016/j.matbio.2015.02.002
131. Paiva KBS, Granjeiro JM. Matrix metalloproteinases in bone resorption, remodeling, and repair. Prog Mol Biol Transl Sci. 2017;148:203–303. doi:10.1016/bs.pmbts.2017.05.001
132. Naim A, Pan Q, Baig MS. Matrix Metalloproteinases (MMPs) in liver diseases. J Clin Exp Hepatol. 2017;7(4):367–372. doi:10.1016/j.jceh.2017.09.004
133. Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278(1):16–27. doi:10.1111/j.1742-4658.2010.07919.x
134. Radisky DC, Bissell MJ. Matrix metalloproteinase-induced genomic instability. Curr Opin Genet Dev. 2006;16(1):45–50. doi:10.1016/j.gde.2005.12.011
135. Farina AR, Mackay AR. Gelatinase B/MMP-9 in tumour pathogenesis and progression. Cancers. 2014;6(1):240–296. doi:10.3390/cancers6010240
136. Kwan JA, Schulze CJ, Wang W, et al. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J. 2004;18(6):690–692. doi:10.1096/fj.02-1202fje
137. Rajabi M, Mousa SA. The role of angiogenesis in cancer treatment. BioMedicines. 2017;5(4):34. doi:10.3390/biomedicines5020034
138. Gonzalez-Avila G, Sommer B, Mendoza-Posada DA, Ramos C, Garcia-Hernandez AA, Falfan-Valencia R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit Rev Oncol Hematol. 2019;137:57–83. doi:10.1016/j.critrevonc.2019.02.010
139. Czekierdowski A, Czekierdowska S, Daniłoś J, Rogala E, Nowicka A. Vasculogenic mimicry and matrix metalloproteinase MMP-9 expression in women with ovarian tumors. Prz Menopauzalny. 2012;11(2):108–114.
140. Wei S, Juan C, Xiurong L, Jie Y. Study on the expression of MMP-9 and NF-κB proteins in epithelial ovarian cancer tissue and their clinical value. BIO Web Conf. 2017;8:1059. doi:10.1051/bioconf/20170801059
141. Davidson B, Goldberg I, Gotlieb WH, et al. High levels of MMP-2, MMP-9, MT1-MMP and TIMP-2 mRNA correlate with poor survival in ovarian carcinoma. Clin Exp Metastasis. 1999;17(10):799–808. doi:10.1023/a:1006723011835
142. Sakata K, Shigemasa K, Nagai N, Ohama K. Expression of matrix metalloproteinases (MMP-2, MMP-9, MT1-MMP) and their inhibitors (TIMP-1, TIMP-2) in common epithelial tumors of the ovary. Int J Oncol. 2000;17(4):673–681.
143. Brun J-L, Cortez A, Commo F, Uzan S, Rouzier R, Daraï E. Serous and mucinous ovarian tumors express different profiles of MMP-2, −7, −9, MT1-MMP, and TIMP-1 and -2. Int J Oncol. 2008;33(6):1239–1246.
144. Brun J-L, Cortez A, Lesieur B, Uzan S, Rouzier R, Daraï E. Expression of MMP-2, −7, −9, MT1-MMP and TIMP-1 and −2 has no prognostic relevance in patients with advanced epithelial ovarian cancer. Oncol Rep. 2012;27(4):1049–1057. doi:10.3892/or.2011.1608
145. Vos MC, van der Wurff AAM, Bulten J, et al. Limited independent prognostic value of MMP-14 and MMP-2 expression in ovarian cancer. Diagn Pathol. 2016;11(1):34. doi:10.1186/s13000-016-0485-3
146. Jeleniewicz W, Cybulski M, Nowakowski A, et al. MMP-2 mRNA expression in ovarian cancer tissues predicts patients‘response to platinum-taxane chemotherapy. Anticancer Res. 2019;39(4):1821–1827. doi:10.21873/anticanres.13289
147. Morales-Vásquez F, Castillo-Sánchez R, Gómora MJ, et al. Expression of metalloproteinases MMP-2 and MMP-9 is associated to the presence of androgen receptor in epithelial ovarian tumors. J Ovarian Res. 2020;13(1):86. doi:10.1186/s13048-020-00676-x
148. Sillanpää S, Anttila M, Voutilainen K, et al. Prognostic significance of matrix metalloproteinase-9 (MMP-9) in epithelial ovarian cancer. Gynecol Oncol. 2007;104(2):296–303. doi:10.1016/j.ygyno.2006.09.004
149. Ge H, Luo H. Overview of advances in vasculogenic mimicry – a potential target for tumor therapy. Cancer Manag Res. 2018;10:2429–2437. doi:10.2147/CMAR.S164675
150. Wechman SL, Emdad L, Sarkar D, Das SK, Fisher PB. Vascular mimicry: triggers, molecular interactions and in vivo models. Adv Cancer Res. 2020;148:27–67. doi:10.1016/bs.acr.2020.06.001
151. Hujanen R, Almahmoudi R, Salo T, Salem A. Comparative analysis of vascular mimicry in head and neck squamous cell carcinoma: in vitro and in vivo approaches. Cancers. 2021;13(19):4747. doi:10.3390/cancers13194747
152. Manenti L, Paganoni P, Floriani I, et al. Expression levels of vascular endothelial growth factor, matrix metalloproteinases 2 and 9 and tissue inhibitor of metalloproteinases 1 and 2 in the plasma of patients with ovarian carcinoma. Eur J Cancer. 2003;39(13):1948–1956. doi:10.1016/s0959-8049(03)00427-1
153. Xu D, Su C, Guo L, et al. Predictive significance of serum MMP-9 in papillary thyroid carcinoma. Open Life Sci. 2019;14(1):275–287. doi:10.1515/biol-2019-0031
154. Sheen-Chen S-M, Chen H-S, Eng H-L, Sheen -C-C, Chen W-J. Serum levels of matrix metalloproteinase 2 in patients with breast cancer. Cancer Lett. 2001;173(1):79–82. doi:10.1016/s0304-3835(01)00657-7
155. Acar A, Onan A, Coskun U, et al. Clinical significance of serum MMP-2 and MMP-7 in patients with ovarian cancer. Med Oncol. 2008;25(3):279–283. doi:10.1007/s12032-007-9031-1
156. Li W, Cui Z, Kong Y, Liu X, Wang X. Serum levels of S100A11 and MMP-9 in patients with epithelial ovarian cancer and their clinical significance. Biomed Res Int. 2021;2021:7341247. doi:10.1155/2021/7341247
157. Zhang W, Yang H-C, Wang Q, et al. Clinical value of combined detection of serum matrix metalloproteinase-9, heparanase, and cathepsin for determining ovarian cancer invasion and metastasis. Anticancer Res. 2011;31(10):3423–3428.
158. Ławicki S, Będkowska E, Szumarska-Gacuta E, et al. Concentration and diagnostic utility of metalloproteinase-9 (MMP-9) in patients with ovarian cancer. Diagn Lab. 2013;49(3):335.
159. Ławicki S, Głażewska EK, Sobolewska M, Będkowska GE, Szmitkowski M. Plasma levels and diagnostic utility of macrophage colony-stimulating factor, matrix metalloproteinase-9, and tissue inhibitor of metalloproteinases-1 as new biomarkers of breast cancer. Ann Lab Med. 2016;36(3):223–229. doi:10.3343/alm.2016.36.3.223
160. Coticchia CM, Curatolo AS, Zurakowski D, et al. Urinary MMP-2 and MMP-9 predict the presence of ovarian cancer in women with normal CA125 levels. Gynecol Oncol. 2011;123(2):295–300. doi:10.1016/j.ygyno.2011.07.034
161. Postawski K, Rechberger T, Jakimiuk AJ, Skorupski P, Bogusiewicz M, Jakowicki JA. Interstitial collagenase (MMP-1) activity in human ovarian tissue. Gynecol Endocrinol. 1999;13(4):273–278. doi:10.3109/09513599909167566
162. Cossins J, Dudgeon TJ, Catlin G, Gearing AJ, Clements JM. Identification of MMP-18, a putative novel human matrix metalloproteinase. Biochem Biophys Res Commun. 1996;228(2):494–498. doi:10.1006/bbrc.1996.1688
163. Behrens P, Rothe M, Florin A, Wellmann A, Wernert N. Invasive properties of serous human epithelial ovarian tumors are related to Ets-1, MMP-1 and MMP-9 expression. Int J Mol Med. 2001;8(2):149–154. doi:10.3892/ijmm.8.2.149
164. Stenman M, Paju A, Hanemaaijer R, et al. Collagenases (MMP-1, −8 and −13) and trypsinogen-2 in fluid from benign and malignant ovarian cysts. Tumour Biol. 2003;24(1):9–12. doi:10.1159/000070655
165. Stadlmann S, Pollheimer J, Moser PL, et al. Cytokine-regulated expression of collagenase-2 (MMP-8) is involved in the progression of ovarian cancer. Eur J Cancer. 2003;39(17):2499–2505. doi:10.1016/j.ejca.2003.08.011
166. Wang S, Jia J, Liu D, et al. Matrix metalloproteinase expressions play important role in prediction of ovarian cancer outcome. Sci Rep. 2019;9(1):11677. doi:10.1038/s41598-019-47871-5
167. Hantke B, Harbeck N, Schmalfeldt B, et al. Clinical relevance of matrix metalloproteinase-13 determined with a new highly specific and sensitive ELISA in ascitic fluid of advanced ovarian carcinoma patients. Biol Chem. 2003;384(8):1247–1251. doi:10.1515/BC.2003.137
168. Laitinen A, Hagström J, Mustonen H, et al. Serum MMP-8 and TIMP-1 as prognostic biomarkers in gastric cancer. Tumour Biol. 2018;40(9):1010428318799266. doi:10.1177/1010428318799266
169. Wang H, Li H, Yan Q, et al. Serum matrix metalloproteinase-13 as a diagnostic biomarker for cutaneous squamous cell carcinoma. BMC Cancer. 2021;21(1):816. doi:10.1186/s12885-021-08566-1
170. Bogusiewicz M, Rechberger T, Jakimiuk AJ, Skorupski P, Jakowicki JA, Postawski K. Evaluation of matrix metalloproteinases-1 and −3 concentrations in the tunica albuginea, the apical wall of atretic follicles and the corpus luteum of normal human ovaries. Gynecol Endocrinol. 2000;14(1):25–31. doi:10.3109/09513590009167656
171. Mueller J, Brebeck B, Schmalfeldt B, Kuhn W, Graeff H, Höfler H. Stromelysin-3 expression in invasive ovarian carcinomas and tumours of low malignant potential. Virchows Arch. 2000;437(6):618–624. doi:10.1007/s004280000261
172. Périgny M, Bairati I, Harvey I, et al. Role of immunohistochemical overexpression of matrix metalloproteinases MMP-2 and MMP-11 in the prognosis of death by ovarian cancer. Am J Clin Pathol. 2008;129(2):226–231. doi:10.1309/49LA9XCBGWJ8F2KM
173. Escalona RM, Kannourakis G, Findlay JK, Ahmed N. Expression of TIMPs and MMPs in ovarian tumors, ascites, ascites-derived cells, and cancer cell lines: characteristic modulatory response before and after chemotherapy treatment. Front Oncol. 2022;11:796588. doi:10.3389/fonc.2021.796588
174. Cymbaluk-Płoska A, Chudecka-Głaz A, Pius-Sadowska E, Machaliński B, Menkiszak J, Sompolska-Rzechuła A. Suitability assessment of baseline concentration of MMP3, TIMP3, HE4 and CA125 in the serum of patients with ovarian cancer. J Ovarian Res. 2018;11(1):1. doi:10.1186/s13048-017-0373-9
175. Tanimoto H, Underwood LJ, Shigemasa K, et al. The matrix metalloprotease pump-1 (MMP-7, Matrilysin): a candidate marker/target for ovarian cancer detection and treatment. Tumour Biol. 1999;20(2):88–98. doi:10.1159/000030051
176. Wang F-Q, So J, Reierstad S, Fishman DA. Matrilysin (MMP-7) promotes invasion of ovarian cancer cells by activation of progelatinase. Int J Cancer. 2005;114(1):19–31. doi:10.1002/ijc.20697
177. Sillanpää SM, Anttila MA, Voutilainen KA, et al. Prognostic significance of matrix metalloproteinase-7 in epithelial ovarian cancer and its relation to β-catenin expression. Int J Cancer. 2006;119(8):1792–1799. doi:10.1002/ijc.22067
178. Ripley D, Tunuguntla R, Susi L, Chegini N. Expression of matrix metalloproteinase-26 and tissue inhibitors of metalloproteinase-3 and −4 in normal ovary and ovarian carcinoma. Int J Gynecol Cancer. 2006;16(5):1794–1800. doi:10.1111/j.1525-1438.2006.00714.x
179. Gershtein ES, Levkina NV, Digayeva MA, Laktionov KP, Tereshkina IV, Kushlinsky NE. Matrix metalloproteinases 2, 7, and 9 and tissue inhibitor of metalloproteinases-1 in tumors and serum of patients with ovarian neoplasms. Bull Exp Biol Med. 2010;149(5):628–631. doi:10.1007/s10517-010-1010-4
180. Meinhold-Heerlein I, Bauerschlag D, Zhou Y, et al. An integrated clinical-genomics approach identifies a candidate multi-analyte blood test for serous ovarian carcinoma. Clin Cancer Res. 2007;13(2):458–466. doi:10.1158/1078-0432.CCR-06-0691
181. Będkowska GE, Gacuta E, Zajkowska M, et al. Plasma levels of MMP-7 and TIMP-1 in laboratory diagnostics and differentiation of selected histological types of epithelial ovarian cancers. J Ovarian Res. 2017;10(1):39. doi:10.1186/s13048-017-0338-z
182. Cheng T, Li F, Wei R, et al. MMP26: a potential biomarker for prostate cancer. J Huazhong Univ Sci Technolog Med Sci. 2017;37(6):891–894. doi:10.1007/s11596-017-1823-8
183. Puttabyatappa M, Jacot TA, Al-Alem LF, et al. Ovarian membrane-type matrix metalloproteinases: induction of MMP14 and MMP16 during the periovulatory period in the rat, macaque, and human. Biol Reprod. 2014;91(2):34. doi:10.1095/biolreprod.113.115717
184. Vos MC, van der Wurff AA, Last JT, et al. Immunohistochemical expression of MMP-14 and MMP-2, and MMP-2 activity during human ovarian follicular development. Reprod Biol Endocrinol. 2014;12(1):12. doi:10.1186/1477-7827-12-12
185. Testuri M, Daghero H, Rey G, Acosta G, Bernachin J, Marco M. Expression of membrane type- matrix metalloproteinases in common epithelial malignant tumors of the ovary. JSM Clin Pathol. 2019;4:7.
186. Afzal S, Lalani E-N, Poulsom R, et al. MT1-MMP and MMP-2 mRNA expression in human ovarian tumors: possible implications for the role of desmoplastic fibroblasts. Hum Pathol. 1998;29(2):155–165. doi:10.1016/s0046-8177(98)90226-x
187. Adley BP, Gleason KJ, Yang XJ, Stack MS. Expression of membrane type 1 matrix metalloproteinase (MMP-14) in epithelial ovarian cancer: high level expression in clear cell carcinoma. Gynecol Oncol. 2009;112(2):319–324. doi:10.1016/j.ygyno.2008.09.025
188. Wang H, Qi C, Wan D. MicroRNA-377-3p targeting MMP-16 inhibits ovarian cancer cell growth, invasion, and interstitial transition. Ann Transl Med. 2021;9(2):124. doi:10.21037/atm-20-8027
189. Bruney L, Conley KC, Moss NM, Liu Y, Stack MS. Membrane-type I matrix metalloproteinase-dependent ectodomain shedding of mucin16/ CA-125 on ovarian cancer cells modulates adhesion and invasion of peritoneal mesothelium. Biol Chem. 2014;395(10):1221–1231. doi:10.1515/hsz-2014-0155
190. Kaimal R, Aljumaily R, Tressel SL, et al. Selective blockade of matrix metalloprotease-14 with a monoclonal antibody abrogates invasion, angiogenesis, and tumor growth in ovarian cancer. Cancer Res. 2013;73(8):2457–2467. doi:10.1158/0008-5472.CAN-12-1426
191. Klupp F, Neumann L, Kahlert C, et al. Serum MMP7, MMP10 and MMP12 level as negative prognostic markers in colon cancer patients. BMC Cancer. 2016;16(1):494. doi:10.1186/s12885-016-2515-7
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.