Back to Journals » Cancer Management and Research » Volume 13
USP5 Promotes Uterine Corpus Endometrial Carcinoma Cell Growth and Migration via mTOR/4EBP1 Activation
Received 9 November 2020
Accepted for publication 15 April 2021
Published 13 May 2021 Volume 2021:13 Pages 3913—3924
DOI https://doi.org/10.2147/CMAR.S290467
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Yinghua Li, Jian Zhou
The Third Departments of Gynecological Oncology, Hunan Cancer Hospital, Changsha, 410013, Hunan, People’s Republic of China
Correspondence: Jian Zhou
Three Departments of Gynecological Oncology, Hunan Cancer Hospital, No. 283 Tongzipo Road, Yuelu District, Changsha, 410013, Hunan, People’s Republic of China
Tel +86-731-89762061
Fax +86-731-89762063
Email [email protected]
Background: Uterine corpus endometrial carcinoma (UCEC) is a common malignancy worldwide developed in the female reproductive system, which can be life-threatening due to metastasis and poor prognosis. Deubiquitinating enzymes (DUBs) play key roles in ubiquitin–proteasome system. As a member of DUBs, the ubiquitin-specific protease 5 (USP5) has been found to be an oncogene in several cancers. This study aims to explore the function of USP5 in UCEC.
Materials and Methods: Clinical significance of USP5 was assessed from The Cancer Genome Atlas (TCGA) UCEC dataset. Knockdown and overexpression were performed by transfecting the cells with siRNAs and pCDNA3.1 vectors, respectively. CCK8, colony formation, wound healing, transwell, PI, and PI/annexin V staining were conducted to check the effect of USP5 on cellular biology function. Western blot assay was used to detect protein expression.
Results: USP5 was upregulated in UCEC patients. Its downregulation led to decreased migration and proliferation of UCEC cells, and meanwhile, cell cycle arrest and apoptosis were induced. By contrast, USP5 overexpression significantly promoted cell migration and cell mitosis. Further study revealed that USP5 could cause hyperactivation of mTOR/4EBP1 pathway and rapamycin treatment could totally reverse the effects of UPS5 overexpression.
Conclusion: Our data demonstrated that USP5 functioned as an oncogene in UCEC, which provided new insights into the pathogenesis of UCEC and a promising molecular target for UCEC diagnosis and therapy.
Keywords: USP5, UCEC, cell growth, mTOR/4EBP1
Introduction
Uterine corpus endometrial carcinoma (UCEC) is a common gynecologic malignancy in women which is defined as an epithelial neoplasm originating from the endometrium. It is the third most life-threatening cancer in females, after ovarian cancer and cervical cancer. Regardless of staging or grading, cancer metastasis and recurrence are major risks for poor prognosis.1 For these patients, limited treatments can be applied due to the resistance to chemotherapy and hormone therapy.2 Therefore, for the purpose of improving the survival rate of patients with endometrial cancer, it is of urgent need to identify novel therapeutic targets and prognostic biomarkers.
Protein homeostasis is crucial for maintaining normal cellular functions. Accumulation of unfolded, misfolded or damaged proteins is extremely toxic to cells and has been found to be associated with many human diseases including cancers.3,4 Ubiquitination is an important post-translational modification labeling these target proteins for degradation through proteasome. Deubiquitination is a reverse process to remove ubiquitin from proteins, thus keeping a dynamic balance of free ubiquitin pool. This process is mediated by deubiquitinating enzymes (DUBs).5
Ubiquitin-specific proteinase 5 (USP5), also known as ubiquitin isopeptidase T (ISOT), is a cysteine deubiquitinating enzyme belonging to the USP family. It is highly conserved in humans and mice with an amino acid identity of approximately 98%. Unlike other DUBs, USP5 specifically recognizes unanchored polyubiquitin chains and removes proximal ubiquitin.6,7 Increasing evidence has demonstrated that USP5 plays an oncogenic role in various human cancers. In hepatocellular carcinoma, USP5 is highly expressed and positively correlated to malignancy degree. It promotes HCC progression via stabilizing SLUG and alleviating p14ARF-p53 signaling.8,9 Besides, USP5 could activate the Wnt/β-catenin pathway by deubiquitinating β-catenin in non-small cell lung cancer.10 Moreover, USP5 deletion leads to cell cycle arrest and apoptosis in pancreatic ductal adenocarcinoma cells.11
In this study, we aimed to reveal the relationship between USP5 level and UCEC progression, and provided new knowledge for developing targeted therapy for UCEC patients.
Materials and Methods
Cell Culture and Treatment
Human endometriotic stromal cells (ESCs) were isolated from endometriotic tissues as described previously.12 Human UCEC cells Ishikawa, Hec-1-B and KLE were purchased from ATCC. Hec-1-B cells were cultured in DMEM, and KLE cells were cultured in F12. Ishikawa cells were grown in RPMI-1640. To keep cell status, 10% of FBS was added into the medium. To avoid contamination, 1% antibiotics were added into the medium. The cells were grown in a 37°C incubator which contained 5% CO2.
Cell Transfection
siRNA transfection in Hec-1-B and KLE cells was carried out using RNAiMax, following the protocols provided by Invitrogen. Targeted sequences were as follows: siCtrl, UUCUCCGAACGUGUCACGU; siUSP5#1, GCCUCAAGCAGUUGGACAA; siUSP5#2, GGAGUUCUUCCUUCACCUU.
USP5 Overexpression by Vectors
pcDNA3.1 vectors were used to overexpress USP5 in UCEC cells. When efficiently overexpressing USP5, Hec-1-B and KLE cells were subjected to Western blot, MTT, colony formation, transwell, wound healing, cell cycle and apoptosis assay.
Cell Proliferation Assay
Cells were plated in 96-well plate at a density of 2000 cells/well and cultured in a complete medium for 4 days. MTT (JT343, Geneview) was added and incubated for 30 min. The absorbance of the formazan solution was then read spectrophotometrically at 490 nm. All experiments were performed in triplicate.
Colony Formation Assay
Cells were seeded in 6-well plate at a density of 500/well for Hec-1-B and 1000/well for KLE cells and then incubated for 8 days and stained with 0.1% crystal violet for 20 min at room temperature. Colonies containing ≥50 cells were manually counted under a microscope. Each assay was performed in triplicate.
Wound Healing Assay
Cells were plated in 96-well plate and cultured overnight. Wounds were made in confluent monolayer cells using 96 Wounding Replicator (VP Scientific) and cells were cultured in a medium supplemented with 0.5% FBS. Wound healing was detected at 0, 24, 48 hours within the scraped lines, and representative fields at different time points were photographed (at ×100 magnification). Migration area was analyzed by Celigo (Nexcelom).
Transwell Migration Assay
Cells were plated in the upper chamber with serum-free medium in the transwell with 8-um pore filters (3422, Millipore, Billerica, USA), while the lower chambers were filled with complete culture medium. After incubation for 24 hours, cells on the upper membrane surface were removed by cotton tips. Then membranes were washed with PBS, fixed with 4% PFA, washed twice with PBS, and stained with 0.2% crystal violet. The invaded cells from nine fields were counted under a light microscope (at ×200 magnification). All experiments were performed in triplicate.
Cell Cycle Distribution Analysis
Cells were treated as indicated. About 48 h later, the cells were harvested, washed with ice-cold PBS and fixed in 70% ethanol overnight at −20°C. Subsequently, the fixed cells were washed three times with PBS and stained with propidium iodide (PI) solution (50 µg/mL PI and 100 µg/mL RNase A in PBS) in the dark for 30 min. DNA content was measured on a FACScan flow cytometer (BD Biosciences, San Jose, CA, USA) with FlowJo software (version 7.6.1, Tree Star, Ashland, OR, USA). Experiments were repeated three times.
Cell Apoptosis Analysis
To analyze the apoptosis in UCEC cells, USP5 knockdown or overexpressed Hec-1-B and KLE cells were harvested by trypsin without EDTA. The cells were then stained with PI and annexin V and apoptosis was analyzed by flow cytometry, according to the manufacturer's protocols.
Western Blot
Cells were washed once with cold PBS. Proteins were extracted with lysis buffer supplemented with proteinase inhibitor. Cell lysates were centrifuged at 12000g for 10 min. The supernatants were collected and protein concentrations were quantified by BCA assay (P0010S, Beyotime). 35ug proteins with loading buffer were then resolved by SDS-PAGE. Western blot was developed using anti-mTOR (2983, CST), anti-p-mTOR (2974, CST), anti-4EBP1 (9644, CST), anti-p-4EBP1 (2855, CST), anti-β-actin (47,778, SantaCruz), mouse anti-rabbit IgG (7074, CST), rabbit anti-mouse IgG (7076, CST).
Bioinformatics Analysis
The Cancer Genome Atlas (TCGA) UCEC dataset was obtained from the TCGA website. Copy number variation rates were analyzed via cBioportal online tools. Gene expression levels were analyzed via GEPIA.
Statistical Analysis
Each experiment was performed at least three times independently. Data were presented as mean ± s.d. Comparisons between two groups were carried out using a 2-tailed unpaired t-test. The level of significance was set at P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***).
Results
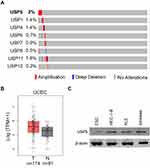
USP5 is Highly Amplified in UCEC Patients
First, we analyzed genetic alteration rates of eight USP family members using 539 UCEC patient samples with copy number data extracted from TCGA database. We found USP5 had the highest amplification rate (Figure 1A). And USP5 mRNA level was relatively higher in tumor tissues than that in nontumor tissues (Figure 1B). We then examined the expression of USP5 in human normal endometrial cells, ESC and endometrial cancer cells, including Ishikawa, HEC-1-B and KLE. We showed that USP5 was highly expressed in cancer cells compared with normal cells (Figure 1C). These indicated an aberrant upregulation of USP5 in UCEC.
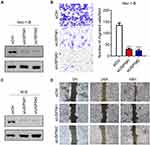
USP5 Knockdown Impedes UCEC Cell Migration
In order to figure out the biological function of USP5 in UCEC, we transiently knocked down USP5 in two UCEC cell lines with two different siRNA sequences. As the Western blot results indicated, all of them could efficiently decrease the protein level of USP5 (Figure 2A and C). In Hec-1-B cells, USP5 downregulation inhibited cell transmigration significantly accessed by transwell assay (Figure 2B). Similar results were obtained for KLE cells by wound healing assay (Figure 2D). These results suggested that USP5 deficiency could impair migration ability of UCEC cells.
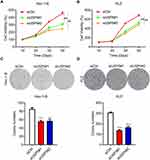
USP5 Downregulation Impairs UCEC Cell Proliferation
USP5 was found to promote cancer cell proliferation.10,11,13 So next we tried to find out the impact of USP5 on UCEC cell proliferation. As shown in Figure 3A, knockdown of USP5 with two siRNAs strongly impaired the viability of Hec-1-B cells. It was also the case in KLE cells (Figure 3B). Besides, the formation of colonies of Hec-1-B and KLE cells was also repressed when USP5 was inadequate (Figure 3C and D). To confirm the role of USP5, we also knocked down USP5 in Ishikawa cells and found that USP5 downregulation suppressed the proliferation of Ishikawa cells (Supplementary Figure 1). These results indicated that USP5 was required for UCEC cell survival.
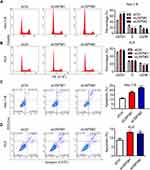
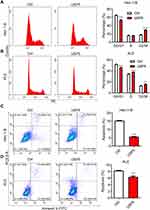
The Decease of USP5 Causes Cell Cycle Arrest and Apoptosis of UCEC Cells
USP5 was shown to modulate cell cycle progression in pancreatic cancer and ovarian cancer.14,15 To reveal the mechanisms underlying the repression of cell proliferation, we analyzed cell cycle distribution of UCEC cells after USP5 knockdown. We observed significant accumulations of Hec-1-B cells in G0/G1-phase transfected with USP5 siRNAs compared to control cells, and meanwhile obvious reductions in S-phase and G2/M-phase (Figure 4A). In KLE cells, USP5 knockdown also led to elevated proportions of cells in G0/G1-phase and decreased proportions in G2/M-phase (Figure 4B). In addition, the apoptotic cells increased apparently after USP5 knockdown in both Hec-1-B and KLE cells (Figure 4C and D), suggesting the cell cycle arrest and apoptosis contributed a lot to cell proliferation inhibition.
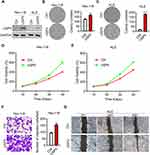
USP5 Overexpression Promotes Migration and Proliferation of UCEC Cells
The above results showed that USP5 was required for UCEC cell function, but whether USP5 expression was sufficient to promote UCEC proliferation and migration should be determined. We therefore overexpressed USP5 in Hec-1-B and KLE cells to observe cell migration and growth. Western blot assay showed that the protein level of USP5 was markedly overexpressed in Hec-1-B and KLE cells (Figure 5A). To our expectation, both Hec-1-B and KLE cells transfected with exogenous USP5 exhibited formed more colonies (Figure 5B and C) and enhanced cell viabilities (Figure 5D and E). Besides, Hec-1-B cells with USP5 overexpression transmigrated more than cells with control vector (Figure 5F). And KLE cells with increased USP5 moved much more rapidly than control cells (Figure 5G).
USP5 Overexpression Promotes Cell Mitosis and Suppresses Cell Apoptosis
Next, we moved further to check whether high USP5 expression level could promote cell division. The proportion of cells in G0/G1-phase showed an obvious decline while a rise in G2/M-phase after USP5 overexpression in Hec-1-B cells (Figure 6A). In USP5-overexpressed KLE cells, the S-phase cells also showed an accumulation (Figure 6B). These results indicated that the division activity of UCEC cells was greatly motivated by an elevated expression level of USP5. What is more, high USP5 level could hinder cell apoptosis, thus protecting cancer cells from death (Figure 6C and D).
USP5 Promotes Cell Proliferation by Activating mTOR/4EBP1 Pathway
mTOR is a protein kinase regulating cell growth and survival and its deregulation has been observed in many cancer types.16,17 For the purpose of unraveling the molecular mechanisms, we carried on detecting the status of mTOR pathway. Interestingly, when USP5 was silenced, the phosphorylated mTOR was slightly declined, as well as the phosphorylation of its downstream target 4EBP1 (Figure 7A). In contrast, overexpressed USP5 resulted in upregulation of p-mTOR and p-4EBP1 (Figure 7B). When Hec-1-B cells were treated with rapamycin, an mTOR inhibitor, the hyperproliferation caused by USP5 overexpression would be completely reversed (Figure 7C and D). The colony formations were nearly blocked as well (Figure 7E). The above results illustrated that USP5 modulated mTOR/4EPB1 activation promote UCEC cell survival.
Discussion
Nowadays, with the development of technology and expansion of knowledge, novel diagnostic markers and efficient targeted therapies have been developed for various cancer types. For the uterine corpus endometrial carcinoma, the common treatment is surgery combined with chemotherapy, which can bring great pain and pity to women. By analyzing the TCGA database, we found an amplification of USP5 in UCEC patients, which is a special DUB in that it only removes the proximal ubiquitin. However, other USPs have either lower amplification rates or deletions in tumor samples, suggesting a unique correlation of USP5 in UCEC progression.
Ubiquitination is a fundamental modification to label proteins for degradation. Deregulation of the balance formed between the two reversed processes could contribute to tumorigenesis.4,18 Several members of the USP family have been shown to facilitate tumor growth or metastasis.19–22 For example, USP7 activation of PI3K/AKT signaling cascade promotes hepatocellular carcinoma growth.23 USP7 contributes to the progression of esophageal carcinoma by regulating JMJD3/CLU axis.24 Silencing of USP8 inhibits the proliferation and migration of lung cancer cells.25 Oncogenic function of USP8 is also observed in gastric cancer, cholangiocarcinoma, and breast cancer.26–28 Apart from them, a growing body of evidence has demonstrated the tumor-promoting role of USP5 in different cancer types.8,10,11,13–15 And in this study, we found that it was also the case in UCEC. When USP5 was knocked down, cell viability was significantly impeded accompanied with blockade of cell cycle transition and induction of apoptosis. However, upregulation of USP5 could greatly drive cell mitosis and inhibit cell death. On the other hand, insufficiency of USP5 weakened cell migration ability which was fully rescued by USP5 overexpression.
It has been reported that USP5 could protect SLUG and β-catenin from being degraded to promote tumor progression.8,10 Here we found that USP5 could mediate mTOR/4EBP1 activation. The usage of mTOR inhibitor rapamycin could absolutely block the hyperproliferation caused by high USP5 level. However, based on the immunoblotting results, USP5 did not regulate the protein abundance of mTOR and 4EBP1, suggesting that USP5 activation of mTOR/4EBP1 is not depending on direct interaction and ubiquitination manner. Through what mechanisms USP5 modulated mTOR activity remained to be explored.
Taken together, we uncovered a close relationship between USP5 expression level and UCEC progression, providing strong evidence for USP5 to become a potential therapy target and prognostic molecule.
Abbreviations
UCEC, uterine corpus endometrial carcinoma; DUBs, deubiquitinating enzymes; USP5, ubiquitin-specific protease 5; ISOT, isopeptidase T; qRT-PCR, real-time polymerase chain reaction; TCGA, The Cancer Genome Atlas.
Data Sharing Statement
The data generated during this study are included in the manuscript.
Funding
This project was supported by Scientific research project of Hunan Provincial Health Commission (20201401).
Disclosure
These authors declare no competing interests.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Chaudhry P, Asselin E. Resistance to chemotherapy and hormone therapy in endometrial cancer. Endocr Relat Cancer. 2009;16(2):363–380. doi:10.1677/ERC-08-0266
3. Cook C, Stetler C, Petrucelli L. Disruption of protein quality control in parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2(5):a009423. doi:10.1101/cshperspect.a009423
4. Tu Y, Chen C, Pan J, Xu J, Zhou ZG, Wang CY. The Ubiquitin Proteasome Pathway (UPP) in the regulation of cell cycle control and DNA damage repair and its implication in tumorigenesis. Int J Clin Exp Pathol. 2012;5(8):726–738.
5. Wilkinson KD, Tashayev VL, O’Connor LB, Larsen CN, Kasperek E, Pickart CM. Metabolism of the polyubiquitin degradation signal: structure, mechanism, and role of isopeptidase T. Biochemistry. 1995;34(44):14535–14546. doi:10.1021/bi00044a032
6. Ning F, Xin H, Liu J, et al. Structure and function of USP5: insight into physiological and pathophysiological roles. Pharmacol Res. 2020;157:104557. doi:10.1016/j.phrs.2019.104557
7. Dayal S, Sparks A, Jacob J, Allende-Vega N, Lane DP, Saville MK. Suppression of the deubiquitinating enzyme USP5 causes the accumulation of unanchored polyubiquitin and the activation of p53. J Biol Chem. 2009;284(8):5030–5041. doi:10.1074/jbc.M805871200
8. Meng J, Ai X, Lei Y, et al. USP5 promotes epithelial-mesenchymal transition by stabilizing SLUG in hepatocellular carcinoma. Theranostics. 2019;9(2):573–587. doi:10.7150/thno.27654
9. Liu Y
10. Ma X, Qi W, Pan H, Yang F, Deng J. Overexpression of USP5 contributes to tumorigenesis in non-small cell lung cancer via the stabilization of beta-catenin protein. Am J Cancer Res. 2018;8(11):2284–2295.
11. Li XY, Wu HY, Mao XF, Jiang LX, Wang YX. USP5 promotes tumorigenesis and progression of pancreatic cancer by stabilizing FoxM1 protein. Biochem Biophys Res Commun. 2017;492(1):48–54. doi:10.1016/j.bbrc.2017.08.040
12. Yang H, Hu T, Hu P, Qi C, Qian L. miR‑143‑3p inhibits endometriotic stromal cell proliferation and invasion by inactivating autophagy in endometriosis. Mol Med Rep. 2021;23(5). doi:10.3892/mmr.2021.11995
13. Potu H, Peterson LF, Pal A, et al. Usp5 links suppression of p53 and FAS levels in melanoma to the BRAF pathway. Oncotarget. 2014;5(14):5559–5569. doi:10.18632/oncotarget.2140
14. Du Y, Lin J, Zhang R, et al. Ubiquitin specific peptidase 5 promotes ovarian cancer cell proliferation through deubiquitinating HDAC2. Aging. 2019;11(21):9778–9793. doi:10.18632/aging.102425
15. Kaistha BP, Krattenmacher A, Fredebohm J
16. Alabdullah ML, Ahmad DA, Moseley P, Madhusudan S, Chan S, Rakha E. The mTOR downstream regulator (p-4EBP1) is a novel independent prognostic marker in ovarian cancer. J Obstet Gynaecol. 2019;39(4):522–528. doi:10.1080/01443615.2018.1534091
17. Hua H, Kong Q, Zhang H, Wang J, Luo T, Jiang Y. Targeting mTOR for cancer therapy. J Hematol Oncol. 2019;12(1):71.
18. Fraile JM, Quesada V, Rodriguez D, Freije JM, Lopez-Otin C. Deubiquitinases in cancer: new functions and therapeutic options. Oncogene. 2012;31(19):2373–2388. doi:10.1038/onc.2011.443
19. Ning J, Zhang J, Liu W, Lang Y, Xue Y, Xu S. Overexpression of ubiquitin-specific protease 22 predicts poor survival in patients with early-stage non-small cell lung cancer. Eur J Histochem. 2012;56(4):e46. doi:10.4081/ejh.2012.e46
20. Yun SI, Kim HH, Yoon JH, et al. Ubiquitin specific protease 4 positively regulates the WNT/beta-catenin signaling in colorectal cancer. Mol Oncol. 2015;9(9):1834–1851. doi:10.1016/j.molonc.2015.06.006
21. Yang B, Zhang S, Wang Z, et al. Deubiquitinase USP9X deubiquitinates beta-catenin and promotes high grade glioma cell growth. Oncotarget. 2016;7(48):79515–79525. doi:10.18632/oncotarget.12819
22. Wu X, Liu M, Zhu H, et al. Ubiquitin-specific protease 3 promotes cell migration and invasion by interacting with and deubiquitinating SUZ12 in gastric cancer. J Exp Clin Cancer Res. 2019;38(1):277. doi:10.1186/s13046-019-1270-4
23. Ye M, He J, Zhang J, et al. USP7 promotes hepatoblastoma progression through activation PI3K/AKT signaling pathway. Cancer Biomark. 2021:1–11. doi:10.3233/CBM-200052
24. Li N, Zhao Z, Liu P, et al. Upregulation of deubiquitinase USP7 by transcription factor FOXO6 promotes EC progression via targeting the JMJD3/CLU axis. Mol Ther Oncolytics. 2021;20:583–595. doi:10.1016/j.omto.2020.12.008
25. Rong Z, Zhu Z, Cai S, Zhang B. Knockdown of USP8 inhibits the growth of lung cancer cells. Cancer Manag Res. 2020;12:12415–12422. doi:10.2147/CMAR.S259191
26. Sun J, Shen D, Zheng Y, et al. USP8 inhibitor suppresses HER-2 positive gastric cancer cell proliferation and metastasis via the PI3K/AKT signaling pathway. Onco Targets Ther. 2020;13:9941–9952. doi:10.2147/OTT.S271496
27. Jing X, Chen Y, Chen Y, et al. Down-regulation of USP8 inhibits cholangiocarcinoma cell proliferation and invasion. Cancer Manag Res. 2020;12:2185–2194. doi:10.2147/CMAR.S234586
28. Shin S, Kim K, Kim H, et al. Deubiquitylation and stabilization of Notch1 intracellular domain by ubiquitin-specific protease 8 enhance tumorigenesis in breast cancer. Cell Death Differ. 2020;27(4):1341–1354. doi:10.1038/s41418-019-0419-1
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.