Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 11 » Issue 1
Usefulness of the 6-minute walk test as a screening test for pulmonary arterial enlargement in COPD
Authors Oki Y , Kaneko M, Fujimoto Y, Sakai H, Misu S, Mitani Y, Yamaguchi T , Yasuda H, Ishikawa A
Received 11 June 2016
Accepted for publication 18 August 2016
Published 22 November 2016 Volume 2016:11(1) Pages 2869—2875
DOI https://doi.org/10.2147/COPD.S114497
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Yutaro Oki,1,2 Masahiro Kaneko,3 Yukari Fujimoto,1 Hideki Sakai,2 Shogo Misu,1,2 Yuji Mitani,1,4 Takumi Yamaguchi,1,2 Hisafumi Yasuda,1 Akira Ishikawa1
1Department of Community Health Sciences, Kobe University Graduate School of Health Sciences, 2Department of Rehabilitation, 3Department of Respiratory Medicine, Kobe City Medical Center West Hospital, Kobe, 4Department of Rehabilitation, Sapporo Nishimaruyama Hospital, Sapporo, Japan
Purpose: Pulmonary hypertension and exercise-induced oxygen desaturation (EID) influence acute exacerbation of COPD. Computed tomography (CT)-detected pulmonary artery (PA) enlargement is independently associated with acute COPD exacerbations. Associations between PA to aorta (PA:A) ratio and EID in patients with COPD have not been reported. We hypothesized that the PA:A ratio correlated with EID and that results of the 6-minute walk test (6MWT) would be useful for predicting the risk associated with PA:A >1.
Patients and methods: We retrospectively measured lung function, 6MWT, emphysema area, and PA enlargement on CT in 64 patients with COPD. The patients were classified into groups with PA:A ≤1 and >1. Receiver-operating characteristic curves were used to determine the threshold values with the best cutoff points to predict patients with PA:A >1.
Results: The PA:A >1 group had lower forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1:FVC ratio, diffusion capacity of lung carbon monoxide, 6MW distance, and baseline peripheral oxygen saturation (SpO2), lowest SpO2, highest modified Borg scale results, percentage low-attenuation area, and history of acute COPD exacerbations ≤1 year, and worse BODE (Body mass index, airflow Obstruction, Dyspnea, and Exercise) index results (P<0.05). Predicted PA:A >1 was determined for SpO2 during 6MWT (best cutoff point 89%, area under the curve 0.94, 95% confidence interval 0.88–1). SpO2 <90% during 6MWT showed a sensitivity of 93.1, specificity of 94.3, positive predictive value of 93.1, negative predictive value of 94.3, positive likelihood ratio of 16.2, and negative likelihood ratio of 0.07.
Conclusion: Lowest SpO2 during 6MWT may predict CT-measured PA:A, and lowest SpO2 <89% during 6MWT is excellent for detecting pulmonary hypertension in COPD.
Keywords: 6-minute walk test, chronic obstructive pulmonary disease, exercise-induced oxygen desaturation, pulmonary artery
Introduction
Exacerbations of COPD are associated with accelerated loss of lung function, poor quality of life, and mortality.1,2 These events can be predicted by numerous clinical factors, including prior exacerbations, airflow obstruction, symptom burden, gastroesophageal reflux, and leukocytosis.3 It is important to detect COPD exacerbations early and minimize their severity.
Patients with COPD frequently experience significant decreases in oxygen saturation during exercise, attributed to the imbalance between oxygen delivery and exercise-induced demand.4 Exercise-induced oxygen desaturation (EID) is reported to be associated with hospitalization and mortality in patients with COPD.5 The 6-minute walking test (6MWT) has been suggested as the preferred measure to identify patients with COPD and EID.6 EID occurs frequently during the 6MWT in patients with COPD.7 EID has been related to forced expiratory volume in 1 second (FEV1), diffusion capacity of lung carbon monoxide (DLCO), amount of emphysema, and baseline oxygen saturation.8–10
Pulmonary hypertension (PH) is an important factor contributing to acute exacerbation of COPD.11 PH appears when airflow limitation is severe, and is associated with chronic hypoxemia. Pulmonary vascular remodeling in COPD is the main cause of increased pulmonary artery (PA) pressure, and is thought to result from the combined effects of hypoxia, inflammation, and capillary loss in severe emphysema.12 The presence of PH has been shown to increase the hospitalization rate and mortality of patients with COPD.13,14 Computed tomography (CT)-detected PA enlargement is independently associated with acute exacerbations of COPD.15 The PA-to-aorta (PA:A) ratio measured by CT scan outperforms echocardiography for diagnosing resting PH in patients with severe COPD.16 A PA:A >1 indicates lower oxygen saturation at rest than a PA:A <1.15 However, there are no reports on the association between PA:A and EID in patients with COPD.
We hypothesized that PA:A correlates with the presence of EID and that 6MWT results are useful for predicting the risk of having a PA:A >1. The present study aimed to examine the relationship between PA:A and EID and develop a simple screening tool by determining the appropriate cutoff score on the 6MWT to predict a PA:A >1 in patients with COPD.
Patients and methods
Study design and patient selection
This study analyzed regularly treated outpatients with COPD between 2014 and 2015 at the Kobe City Medical Center West Hospital. A total of 64 patients with COPD were included after applying the exclusion criteria in this study (Figure 1). The criteria for diagnosing COPD were a smoking history (≥20 pack-years) and postbronchodilator FEV1/forced vital capacity (FVC) <70%. Furthermore, we used the following inclusion criteria to define COPD clinically, all of which had to be fulfilled: symptoms, including cough, sputum production, wheezing, dyspnea, smoking history (≥20 pack-years), existence of emphysema on chest CT, and a physician diagnosis of COPD.17–21 Study-exclusion criteria were as follows: history of lung surgical procedures, exacerbation-related hospitalization 3 months before 6MWT, and patients on long-term oxygen therapy. This study was approved by the ethics committee of Kobe University (N287). All the participants provided written or oral informed consent.
  | Figure 1 Patient flow diagram. |
Clinical characterization
Assessments
A chest physician performed the physical examination for all outpatients. This examination included an assessment of body weight, height, and medical history (eg, pulmonary embolism and sleep apnea syndrome), GOLD (Global initiative for chronic Obstructive Lung Disease) grade 0–4, history of acute exacerbations of COPD within the previous year, COPD Assessment Test, level of dyspnea (using the modified Medical Research Council dyspnea scale), postbronchodilator spirometry, DLCO, 6MWT (according to international recommendations), emphysema area, and PA enlargement on CT. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. GOLD 0 was defined as current and former smokers with a normal postbronchodilator ratio of FEV1:FVC exceeding 0.7 and an FEV1 of at least 80%, symptoms, including cough, sputum production, wheezing, and dyspnea, smoking history (≥20 pack-years), existence of emphysema on chest CT, and a physician diagnosis of COPD.17–21
Six-minute walking test
The 6MWT was performed according to the 2002 American Thoracic Society guidelines.22 Participants were asked to walk indoors on a flat, round, 25 m walking course supervised by a physician and physical therapist. A practice 6MWT was not undertaken. Subjects were encouraged using standard methodology every minute of the 6MWT. A pulse oximeter (WristOx 3150; Nonin Medical, Plymouth, MN, USA) with a finger probe measured peripheral oxygen saturation (SpO2) during 6MWT, and 6MWT-analysis software (WristOx 2; Star Product, Tokyo, Japan) was used. In addition, a modified Borg scale was used to quantify the levels of dyspnea perceived by subjects at each minute during the 6MWT. EID was defined as a nadir SpO2 <90%, SpO2 ≤88%, and ΔSpO2 ≥4%.23–25
Measuring the PA:A ratio
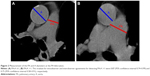
One reviewer, blinded to hemodynamic information, analyzed CT scans (Optima CT 660 Discovery; GE Healthcare, Little Chalfont, UK). Measurements of the diameter of the main PA and the diameter of the aorta at the level of the bifurcation were used to calculate the PA:A ratio, as previously reported.14–16 In cases where the aorta was not uniform in diameter, two measurements were taken 90° apart and the larger diameter used. PA was measured on the line that joins the origin of the left PA and the center of the adjacent ascending aorta on the axial section at the level of PA bifurcation.26 CT-measured relative PA enlargement was defined as PA:A >1 (Figure 2).14–16
Statistical analysis
Results are expressed as counts or median (interquartile range). Data are presented as means and standard deviation or as proportion, as appropriate. Cohen’s κ-coefficient measured intraobserver and interobserver agreements for CT measurements of the PA:A ratio. Bivariate analyses were performed with the use of Pearson’s χ2 test for categorical data and the Mann–Whitney U-test for continuous data when appropriate. Spearman’s rank-correlation coefficient was determined for relationships between the PA:A ratio, lung-function parameters, 6MWT parameters, and CT parameters. Receiver operating characteristic (ROC) curves were used to determine the threshold values with the best sensitivity and specificity to predict PA:A >1, with the best being defined as the point on the ROC curve with the shortest distance from the upper-left corner. Sensitivity, specificity, positive/negative predictive value, and positive/negative likelihood were calculated for lung-function parameters and 6MWT parameters of exacerbation-risk factors on the basis of a previous study.6,27,28
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for the R project (R Foundation for Statistical Computing, Vienna, Austria).29 More precisely, it is a modified version of R Commander designed to add statistical functions frequently used in biostatistics, and P-values <0.05 were considered statistically significant.
Results
The current analysis comprised 64 patients who were separated into groups on the basis of PA:A >1 (n=29) and ≤1 (n=35). Participants had a mean age of 73 (68–79) years. Fifty were male (78.1%) and 14 were female (21.9%). The κ-values for intraobserver and interobserver agreements for detecting PA:A >1 were 0.87 (95% confidence interval [CI] 0.74–0.99) and 0.75 (95% CI 0.58–0.91), respectively.
Differences in the PA:A ratio between both groups were driven by the diameter of PA (2.9 [2.7–3.3] cm in PA:A ≤1 vs 3.7 [3.5–3.9] cm in PA:A >1, P=0.002), because no differences were detected in the diameter of aortae (3.7 [3.4–3.9] cm vs 3.5 [3.3–3.7] cm, P=0.20). There were no significant differences between the two groups with regard to age, sex, BMI, pack-years, modified Medical Research Council dyspnea scale, GOLD, COPD Assessment Test, baseline pulse rate, or baseline modified Borg Scale (P>0.05). On the other hand, there were significant differences between the two groups with regard to FEV1 (71.6% [60.5%–80.8%] vs 52.6% [39.6%–72.1%], P=0.013), FVC (82.3% [50.3%–93.6%] vs 75.8% [42.7%–86%], P=0.04), FEV1:FVC ratio (68% [61%–73.3%] vs 53.8% [48.8%–69.4%], P=0.023), DLCO (72.5% [55.5%–82.9%] vs 44.6% [37.7%–49.6%], P=0.005), BODE (BMI, obstruction [airflow], dyspnea, and exercise performance) index (2 [1–3] vs 4 [2–5], P<0.001), 6MW distance (6MWD; 450 m [400–510.5] vs 325 m [238–466], P<0.001), baseline SpO2 (97% [95%–97.5%] vs 95% [93%–96%], P=0.001), lowest SpO2 (92% [91%–94%] vs 86% [84%–88%], P<0.001), highest modified Borg scale result (2 [0–5] vs 5 [2–5], P=0.04), low-attenuation area (LAA; 6.8% [2.8%–14.7%] vs 25.4% [11.3%–33.4%], P<0.001), and history of acute exacerbations of COPD within the previous year (1 [2.9%] vs 7 [24.1%], P=0.019) (Table 1).
The PA:A ratio demonstrated a significant linear correlation with lowest SpO2 (r=−0.68, r2=0.46; P<0.001), DLCO (r=−0.61, r2=0.37; P<0.001), 6MWD (r=−0.43, r2=0.18; P<0.001), BODE index (r=0.41, r2=0.17; P<0.001), baseline SpO2 (r=−0.36, r2=0.13; P=0.003), LAA (r=0.36, r2=0.13; P=0.004), FVC (r=−0.34, r2=0.12; P=0.006), FEV1 (r=−0.29, r2=0.08; P=0.019), and highest pulse rate (r=0.26, r2=0.07; P=0.035) (Table 2).
Using ROC curves, the threshold values with the best cutoff point, sensitivity, and specificity to predict PA:A >1 were determined for SpO2 during the 6MWT (best cutoff point 89%, area under curve [AUC] 0.94, 95% CI 0.88–1), DLCO (best cutoff point 51%, AUC 0.87, 95% CI 0.78–0.96), 6MWD (best cutoff point 388 m, AUC 0.75, 95% CI 0.62–0.87), and BODE index (best cutoff points 4, AUC 0.74, 95% CI 0.61–0.87) (Table 3, Figure 3). The performance data on the 6MWT and lung function for predicting PA enlargement are depicted in Table 4. SpO2 <90%, SpO2 ≤88%, and ΔSpO2 ≥4% during 6MWT were 94.3 (80.8–99.3), 97.1 (85.1–99.9), and 45.7 (28.8–63.4), respectively, for specificity, 93.1 (77.2–99.2), 95.8 (78.9–99.9), and 59.6 (44.3–73.6), respectively, for positive predictive value, and 16.2 (4.2–62.8), 27.7 (4–193.3), and 1.8 (1.3–2.4), respectively, for positive likelihood ratios.
Discussion
We were able to reveal three main findings in the present study. First, we found a strong association between the PA:A ratio and lowest SpO2 during the 6MWT. For this reason, a consistent finding in patients with COPD is the close relationship between severity of hypoxemia and PA pressure or pulmonary vascular resistance, supporting a major role for alveolar hypoxia.30 Alveolar hypoxia causes constriction of the resistance PAs, and sustained alveolar hypoxia induces pulmonary vascular remodeling.28 Pathological studies of lung specimens from patients with COPD have shown extensive pulmonary vascular remodeling, with prominent intimal thickening and medial hypertrophy. For this reason, chronic alveolar hypoxia plays an important role in pulmonary vascular remodeling.28 In a previous study, patients with PA:A >1 showed lower resting SpO2, higher usage rates of supplemental oxygen, and an indirect association with EID.15 In the present study, the lowest SpO2 during the 6MWT to predict PA:A >1 was considered to be a beneficial result based on the ROC curves. Lowest SpO2 <89% during the 6MWT is excellent in the detection of PH. These results suggest that the lowest SpO2 during the 6MWT is a very helpful measure and screening test for the PA:A ratio. For example, it might be possible to easily screen for pulmonary artery expansion by means of the six-minute walking test in a home-care situation, where it would be difficult to perform CT imaging.
Second, with regard to the relationship between PA:A ratio and lung function, correlations were observed among FEV1, DLCO, and LAA. One of the factors that may play a role in causing PH to advance in patients with COPD is the destruction of lung parenchyma, leading to loss of part of the pulmonary vascular bed,30,31 and the burden of airway remodeling influencing PA-pressure increase.28 A previous study included patients with airflow-obstruction severity greater than moderate, and our study included mild airflow obstruction and smokers with normal spirometry.15 Therefore, regardless of the severity of airflow obstruction, PA enlargement may be progressing. Undiagnosed COPD is a problem worldwide.18 GOLD 0 has been reported to be an exacerbation risk; therefore,18 early detection and not just spirometry evaluation is important from multiple perspectives.18,32 From the viewpoint of early detection of PA enlargement, a definition of EID as SpO2 <90% may be a good start.
Third, there are many causes for acute COPD exacerbations. However, these findings may imply that PA:A >1 is one of the multiple risk factors for acute COPD exacerbations. One reason for this is that PA:A is associated with PH16 and PH is also a risk factor for acute COPD exacerbations.33 Furthermore, a previous study reported an association between the PA:A ratio and acute COPD exacerbation.15 These results suggest that screening for the PA:A ratio without CT using the 6MWT may indicate the risk of acute COPD exacerbations at an early stage.
Limitations
This study had some limitations, including small size, single-center experience, and retrospective design. In addition, this study also included COPD subjects who did not fit the GOLD criteria. Furthermore, because healthy controls do not have respiratory symptoms and there are no control data for the measurement items pertaining to such individuals, healthy controls were not included in the present study. However, it has been reported that the presence of clinical symptoms and low DLCO in smokers, even with normal spirometry, increases the risk of progression to airflow obstruction in COPD.17–20 Therefore, the present study’s results during the 6MWT could be useful to screen for PH at an early COPD stage, even in GOLD 0 patients. Finally, according to a previous study, left ventricular dysfunction causes PA enlargement. However, echocardiography was not performed in all subjects, and this information could not be included because it was unavailable from the medical history, although we observed clinically relevant associations between CT-measured PA:A ratios and 6MWT results.
Conclusion
The current study’s findings suggest that there is a strong association between PA:A ratio and lowest SpO2 during the 6MWT. The 6MWT is a simple, noninvasive, and reproducible measurement tool. Lowest SpO2 during the 6MWT is a very helpful measurement to predict PA:A ratios on CT, and lowest SpO2 of <89% during the 6MWT is excellent to screen for PH in COPD.
Acknowledgments
The authors would like to thank Kentaro Iwata, Kazuki Takahashi, Shigefumi Murakami, Yu Watanabe, Yoji Yamada, Yusuke Iwata, Takuya Sawada, Kanji Yamada, Kaoru Hanaie, Ken Umehara, and Kana Michiue of the Department of Community Health Sciences, Kobe University Graduate School of Health Sciences for constructive comments on the manuscript. We also thank Enago (Tokyo, Japan) for the English-language review.
Author contributions
YO was involved in the conception, hypothesis, outline, and design of the study, data acquisition, analysis, and interpretation, drafting the article, and its revision prior to submission. MK, YF, and HY were involved in the conception, hypothesis, outline, and design of the study, data acquisition, and revision of the article prior to submission. AI was involved in the conception, hypothesis, outline, and design of the study, data acquisition and analysis, drafting the article, and its revision prior to submission. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. | ||
Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. | ||
Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. | ||
Stolz D, Boersma W, Blasi F, et al. Exertional hypoxemia in stable COPD is common and predicted by circulating proadrenomedullin. Chest. 2014;146:328–338. | ||
Andrianopoulos V, Wouters EF, Pinto-Plata VM, et al. Prognostic value of variables derived from the six-minute walk test in patients with COPD: results from the ECLIPSE study. Respir Med. 2015;109:1138–1146. | ||
Knower MT, Dunagan DP, Adair NE, Chin R Jr. Baseline oxygen saturation predicts exercise desaturation below prescription threshold in patients with chronic obstructive pulmonary disease. Arch Intern Med. 2001;161:732–736. | ||
Jenkins S, Čečins N. Six-minute walk test: observed adverse events and oxygen desaturation in a large cohort of patients with chronic lung disease. Intern Med J. 2011;41:416–422. | ||
Andrianopoulos V, Franssen FM, Peeters JP, et al. Exercise-induced oxygen desaturation in COPD patients without resting hypoxemia. Respir Physiol Neurobiol. 2014;190:40–46. | ||
van Gestel AJ, Clarenbach CF, Stöwhas AC, et al. Prevalence and prediction of exercise-induced oxygen desaturation in patients with chronic obstructive pulmonary disease. Respiration. 2012;84:353–359. | ||
Kim C, Seo JB, Lee SM, et al. Exertional desaturation as a predictor of rapid lung function decline in COPD. Respiration. 2013;86:109–116. | ||
Kessler R, Faller M, Weitzenblum E, et al. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:219–224. | ||
Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J. 2008;32:1371–1385. | ||
Barberà JA. Mechanisms of development of chronic obstructive pulmonary disease-associated pulmonary hypertension. Pulm Circ. 2013;3:160–164. | ||
Wells JM, Dransfield MT. Pathophysiology and clinical implications of pulmonary arterial enlargement in COPD. Int J Chron Obstruct Pulmon Dis. 2013;8:509–521. | ||
Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367:913–921. | ||
Iyer AS, Wells JM, Vishin S, Bhatt SP, Wille KM, Dransfield MT. CT scan-measured pulmonary artery to aorta ratio and echocardiography for detecting pulmonary hypertension in severe COPD. Chest. 2014;145:824–832. | ||
Paulin LM, Diette GB, Blanc PD, et al. Occupational exposures are associated with worse morbidity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:557–565. | ||
Regan EA, Lynch DA, Curran-Everett D, et al. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175:1539–1549. | ||
Harvey BG, Strulovici-Barel Y, Kaner RJ, et al. Risk of COPD with obstruction in active smokers with normal spirometry and reduced diffusion capacity. Eur Respir J. 2015;46:1589–1597. | ||
Lutchmedial SM, Creed WG, Moore AJ, Walsh RR, Gentchos GE, Kaminsky DA. How common is airflow limitation in patients with emphysema on CT scan of the chest?Chest. 2015;148:176–184. | ||
Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. | ||
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. | ||
Golpe R, Pérez-de-Llano LA, Méndez-Marote L, Veres-Racamonde A. Prognostic value of walk distance, work, oxygen saturation, and dyspnea during 6-minute walk test in COPD patients. Respir Care. 2013;58:1329–1334. | ||
Casanova C, Cote C, Marin JM, et al. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest. 2008;134:746–752. | ||
Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B. Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial. Chest. 2010;138:179–187. | ||
Mahammedi A, Oshmyansky A, Hassoun PM, Thiemann DR, Siegelman SS. Pulmonary artery measurements in pulmonary hypertension: the role of computed tomography. J Thorac Imaging. 2013;28:96–103. | ||
Spruit MA, Watkins ML, Edwards LD, et al. Determinants of poor 6-min walking distance in patients with COPD: the ECLIPSE cohort. Respir Med. 2010;104:849–857. | ||
Dournes G, Laurent F, Coste F, et al. Computed tomographic measurement of airway remodeling and emphysema in advanced chronic obstructive pulmonary disease correlation with pulmonary hypertension. Am J Respir Crit Care Med. 2015;191:63–70. | ||
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. | ||
Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:189–194. | ||
Minai OA, Chaouat A, Adnot S. Pulmonary hypertension in COPD: epidemiology, significance, and management. Chest. 2010;137:39S–51S. | ||
Snider GL. Nosology for our day: its application to chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:678–683. | ||
Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:158–164. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.