Back to Journals » Cancer Management and Research » Volume 10
Usefulness of plasma D-dimer level for monitoring development of distant organ metastasis in colorectal cancer patients after curative resection
Received 14 June 2018
Accepted for publication 14 August 2018
Published 4 October 2018 Volume 2018:10 Pages 4203—4216
DOI https://doi.org/10.2147/CMAR.S177274
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Video abstract presented by Yi Guo.
Views: 638
Yi Guo, Feng Chen, Wei Cui
State Key Laboratory of Molecular Oncology, Department of Clinical Laboratory, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
Purpose: To investigate the usefulness of plasma D-dimer level for monitoring the development of distant organ metastasis in colorectal cancer (CRC) patients after curative resection.
Patients and methods: One hundred and seventy-eight CRC patients after curative resection were enrolled in the study. Ninety-two patients developed distant organ metastasis during follow-up (metachronous metastasis), and blood was collected on the day metastasis was confirmed. Eighty-six patients had no evidence of metastasis yet, and their blood samples were evaluated at last return visit. The levels of D-dimer, carcinoembryonic antigen (CEA), and lactate dehydrogenase (LDH) between two patient groups were compared. The agreement between D-dimer and CEA (or LDH) was examined. The receiver operator characteristic (ROC) curve was used to evaluate the performance of D-dimer, CEA, LDH, and their combination in detection of distant organ metastasis.
Results: The level of D-dimer in CRC patients with metachronous metastasis was higher than that in non-metastasis patients (P<0.0001). Agreement between D-dimer and CEA was fair (κ=0.416, P<0.0001). D-dimer had a larger area under ROC (AUC) (0.85) compared to CEA (0.72) or LDH (0.68). The specificity of D-dimer (73.3%) was lower than that of CEA (74.4%), but the sensitivity (88.0%) of D-dimer assay was superior to that of CEA assay (65.2%). LDH showed the lowest sensitivity (42.4%) and highest specificity (95.3%) among the three biomarkers. The sensitivity and negative predictive value (NPV) of a combination assay (either D-dimer elevation or CEA elevation) were 94.6% and 91.1%, respectively, and the specificity and positive predictive value of another combination assay (both D-dimer elevation and LDH elevation) were 97.7% and 94.9%, respectively. Parallel test of the three markers improved the sensitivity and NPV to 95.7% and 92.7%, respectively.
Conclusion: Combining with CEA and/or LDH, D-dimer could be a useful surveillance marker for distant organ metastasis in CRC patients after curative resection.
Keywords: D-dimer, colorectal cancer, curative resection, distant organ metastasis, CEA
Corrigendum for this article has been published
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide, and its mortality rate continues to increase yearly in less developed regions.1 According to a report concerning CRC mortality in China,2 the adjusted death rate increased gradually after 2009, with a mortality of 6.15 per 100,000 people in 2009 which further increased to 7.77 per 100,000 in 2011. Distant organ metastasis from CRC is mainly responsible for the high rate of death.3 Approximately 40%–50% of CRC patients will eventually develop distant organ metastasis after resection of the primary tumor (metachronous metastasis).3,4 Therefore, it is necessary for these CRC patients to monitor distant organ metastasis during postoperative visit.
Currently, pathological evidence of malignant cells in distant organs is regarded as the gold standard in CRC metachronous metastasis diagnosis. Imaging techniques, like CT and ultrasound, are commonly used methods for diagnosing distant organ metastasis.5 By comparison, blood tests are relatively non-invasive, and patients do not suffer from ionizing radiation and high cost.6 Therefore, using serum/plasma biomarkers for detection is more suitable for long-term follow-up. Carcinoembryonic antigen (CEA) is the routine clinical biomarker for predicting metachronous metastasis in CRC, and elevated lactate dehydrogenase (LDH) is also reported as a risk factor for development of distant organ metastasis in patients with CRC.7–9 According to different cutoff values, the sensitivity of CEA or LDH used as postoperative surveillance ranged from 51.7% to 70.4%.9–11 Thus, more valuable biomarkers are needed to monitor the development of distant organ metastasis in CRC patients after curative resection.
It is widely accepted that certain CRC patients present coagulation and fibrinolysis abnormalities.12 D-dimer can reflect activation of coagulation and fibrinolysis system, as it is a degradation product of cross-linked fibrin. Recently, plasma D-dimer level could be determined routinely in clinical laboratories on the basis of several detection kits. Thus, more and more studies find that higher D-dimer levels before operations are correlated with a more advanced CRC tumor stage10,13–18 and a poorer prognosis.14,16,19–23 This is more distinct in the cases of distant organ metastasis, which may result from the increased angiogenesis in the primary tumor as well as the formation of tumor emboli in the circulation.18 Therefore, we speculate that D-dimer levels might be elevated in CRC patients who develop distant organ metastasis. However, the usefulness of D-dimer for monitoring the development of distant organ metastasis in CRC patients after curative resection has not been reported yet.
In the present study, we measured the plasma D-dimer levels of CRC patients with or without metachronous metastasis after operation. The aim was to evaluate the usefulness of D-dimer combined with CEA and/or LDH as a surveillance marker in detection of distant organ metastasis in CRC after curative resection.
Patients and methods
Ethics statement
Oral and written informed consent was provided by all enrolled patients. This study methodology was approved by the Institution Ethics Review Board for Human Studies at the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College. We also have complied with the World Medical Association Declaration of Helsinki regarding ethical conduct of research involving human subjects.
Patients
The medical information of patients diagnosed with CRC in the Cancer Hospital Chinese Academy of Medical Sciences was reviewed from January 2015 to August 2017. The following inclusion criteria were applied: 1) patients who had undergone primary tumor resection with systematic lymph node dissection for a curative intent, 2) who were histologically confirmed by endoscopic or surgical specimen, and 3) who had complete follow-up data. Cases with following conditions were excluded: 1) patients with concomitant malignant diseases, 2) with infections or other inflammatory diseases, 3) with coagulation dysfunction, 4) being treated with anticoagulants or thrombolytic medications, and 5) with distant organ metastasis initially (synchronous metastasis). Distant organ metastasis was defined as metastatic lesion(s) in organs beyond the colorectum. The identification of metastatic lesion(s) was based on the results of imaging examination, image-guided biopsy, or exploratory laparotomy. Finally, the enrolled cohort comprised 178 patients.
Blood collection and biochemical assays
Blood samples from peripheral vein were collected at each return visit after surgical operation, and the values of D-dimer, CEA, and LDH were measured. For patients with distant organ metastasis, blood samples collected on the day when metastasis was confirmed were used; for patients without distant organ metastasis, blood samples collected at last follow-up were used.
After an overnight fast, 3 mL of peripheral blood was drawn into collection tubes with sodium citrate for the measurement of D-dimer, and 5 mL of blood was collected into serum-separating tubes for CEA and LDH detection. D-dimer values were determined with a Microparticle Enzyme Immunoassay Analyzer (SYSMEX CS-5100) using the commercially available D-dimer assay kit. CEA was detected with an electrochemiluminescence-based immunoassay analyzer (Roche Cobas e441). Automatic biochemistry analyzer (Roche Cobas 8000) was used to measure LDH level by spectrophotometry. The reference ranges of D-dimer, CEA, and LDH in the study were (0–0.55 mg/L FEU), (0–5 ng/mL), and (115–220 U/L), respectively.
Statistical analysis
Statistical analysis was carried out using SPSS software (version 13.0; SPSS Inc., Chicago, IL, USA). Student’s t-test was used to compare age and tumor sizes of the two patient groups, and the results were presented as mean ± SD. Wilcoxon rank-sum test was used compare the values of D-dimer, CEA, and LDH between the two patient groups, and results were reported as median and interquartile ranges. Association among clinicopathological variables was determined by chi-squared tests. Kappa identity test was used to examine the agreement between D-dimer and CEA levels, or between D-dimer and LDH levels. The receiver operator characteristic (ROC) curve was used to evaluate the performance of D-dimer, CEA, and combination of D-dimer and CEA in detection of distant organ metastasis. All tests were two-sided, and a P-value <0.05 was considered statistically significant.
Results
Patients’ characteristics
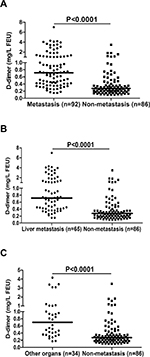
Of the 178 patients enrolled, 92 patients with CRC developed distant organ metastasis during follow-up, and 86 patients had no evidence of distant organ metastasis yet at last return visit. No significant difference was found in age, sex, primary tumor site, tumor size, tumor differentiation, and clinical stage between vtwo patient groups, indicating that the two cohorts were comparable. In the metastasis group, 65 patients had hepatic metastasis, 23 had lung metastasis, and four had metastatic lesions in both the liver and lung. Moreover, in eleven metastatic patients, other distant organs were involved, including brain, bone, and spleen. A detailed compilation of these data are presented in Table 1.
In the metastasis group, 51 patients were ≥60 years, 28 had a history of smoking, and 35 received chemotherapy after operation. In contrast, the non-metastasis group comprised 41 patients ≥60 years, 23 patients with a history of smoking, and 31 patients received chemotherapy. The detailed results are summarized in Table 2.
Comparison of D-dimer values between patients who developed distant organ metastasis and those without metachronous metastasis
Figure 1A shows the distribution of plasma D-dimer levels in CRC patients who developed distant organ metastasis (“metastasis” in the figures) and those without metachronous metastasis (“non-metastasis” in the figures). The results revealed statistically significant differences between the two groups. As shown in Table 1, the median D-dimer values of patients in the metastasis group and those in the non-metastasis group were 0.89 mg/L FEU and 0.28 mg/L FEU, respectively (P<0.0001). With respect to distant organ involvement, plasma D-dimer level was still higher in CRC patients who developed liver metastasis, or other organ metastasis, than in those not demonstrating metastasis (Figure 1B and C). Based on the reference range (0–0.55 mg/L FEU), 76% patients (70/92) showed an elevated plasma D-dimer value in the metastasis group, and only 19% patients (16/86) presented elevated D-dimer values in the non-metastasis group.
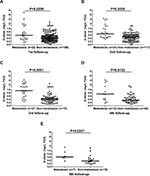
The blood samples obtained from patients during follow-up were checked to determine whether there were still differences in D-dimer levels in every visit of the patients after curative resection. Among 178 CRC patients enrolled, 22 developed distant organ metastasis in their first visit after surgery. In the next four follow-ups, 24, 27, 12, and 7 patients developed distant organ metastasis, respectively. Totally, 92 patients developed distant organ metastasis during five follow-ups. The median interval for occurrence of distant organ metastasis was 8 months (interquartile range 4–11 months). Results showed that the median D-dimer values were elevated in patients who developed distant organ metastasis in each visit (Figure 2). Other details are given in Table 3.
It has been reported that D-dimer concentration increases with age.14,24 Additionally, exposure to cigarette smoke and chemotherapy can also cause elevated D-dimer values.25,26 To mitigate these variables, patients were divided into two groups based on age, smoking history, and chemotherapy. The differences in D-dimer values between the two groups were then analyzed. We found that the median D-dimer values were still higher in patients who developed distant organ metastasis than in those without metachronous metastasis regardless of age over or under 60 years. Similar results were also observed in the smoker vs non-smoker group and chemotherapy vs non- chemotherapy group. More details are presented in Figure 3 and Table 2.
Agreement between D-dimer and CEA (or LDH)
Clinical data showed that CRC patients who developed distant organ metastasis had significantly higher CEA values than those without metachronous metastasis (15.73 ng/mL vs 2.92 ng/mL, P<0.0001) (Table 1). Moreover, the serum LDH value was significantly higher in patients with metastasis than in those without metastasis (192 U/L vs 161 U/L, P<0.0001) (Table 1). According to the reference range mentioned earlier, CEA values were elevated in 72% patients (66/92) who had developed distant organ metastasis, and LDH values were elevated in 29% of them (27/92). In the non-metastasis group, 42% (36/86) and 5% (4/86) of patients presented elevated CEA and LDH values, respectively.
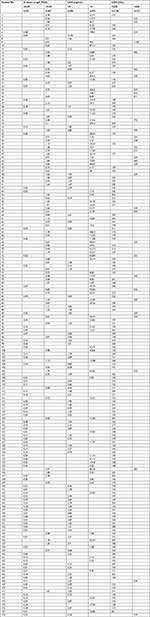
Previous studies also reported that elevated CEA and LDH values indicate increased risk for distant organ metastasis in CRC,8,9,27,28 so we further investigated the agreement between D-dimer and CEA or LDH values. Table 4 shows that agreement between D-dimer and CEA values was fair (κ=0.416, P<0.0001). In contrast, the identity of D-dimer with LDH was inferior (κ=0.293, P<0.0001) (Table 4). Detailed values of D-dimer, CEA, and LDH in CRC patients enrolled in this study are listed in Table S1.
Plasma D-dimer value as an indicator for distant organ metastasis in CRC patients after curative resection
As D-dimer values were markedly increased in CRC patients who developed distant organ metastasis, we inferred that plasma D-dimer level could indicate metastases in distant organs.
An ROC curve was used to evaluate the performance of D-dimer, CEA, and LDH on indicating distant organ metastasis in CRC patients after curative resection. The area under the curve (AUC) for D-dimer performance was 0.85, while the AUC for CEA and LDH was 0.72 and 0.68, respectively (Figure 4). A reasonable cutoff value of 0.415 mg/L FEU for D-dimer was determined using the Youden’s index. Similarly, the cutoff value for CEA assay was set at 6.095 ng/mL, and the cutoff value for LDH was 208 U/L. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy of D-dimer, CEA , LDH, and their combination for indicating distant organ metastasis are summarized in Table 5.
Discussion
Hypercoagulability and hyperfibrinolysis have been demonstrated in CRC.29 Mutant oncogenes, such as K-ras, promote colorectal carcinoma cells to express tissue factor (TF).30,31 Once tumor cells detach from primary location and invade into blood vessels, TF on the cytomembrane could initiate the blood coagulation cascades and participate in fibrin formation. Enhanced activity of urokinase-type plasminogen activator (u-PA) has also been demonstrated in colon cancer cells.32 u-PA could activate plasminogen and participate in fibrinolysis. Consequently, large quantities of D-dimer are produced due to fibrin degradation. According to the above-mentioned studies, D-dimer levels should be elevated when patients develop distant organ metstasis, as more tumor cells have invaded into the circulation. Therefore, we checked the D-dimer levels in the CRC patients who have developed distant organ metstasis after curative resection, and investigated its usefulness for monitoring the development of distant organ metastasis in this study.
Our results revealed that CRC patients who developed distant organ metastasis during follow-up had higher plasma D-dimer levels than those without metachronous metastasis. These results were not affected by patient age, smoking history, or chemotherapy. Moreover, D-dimer level was significantly consistent with CEA level in patients with CRC. Combining with CEA and/or LDH, D-dimer contributed to detect distant organ metastasis in postoperative CRC patients.
Increased D-dimer level was associated with a shorter survival time, and thus many studies have explored the application of D-dimer as a prognostic marker for CRC.16,22,23 However, the relationship between plasma D-dimer and distant organ metastasis was seldom reported. Kilic et al17 enrolled 54 CRC patients with synchronous metastasis, and found that there was a difference in D-dimer values between the metastatic positive group and the metastatic negative group before chemotherapy (373.7 IU/mL vs 603 IU/mL). Another study demonstrated that preoperative D-dimer level in patients with Dukes D CRC was significantly higher than in those with Dukes A, B, or C cancer.10 In the study mentioned above, 23 patients with Dukes D stage underwent operation (exploratory laparotomy) for diagnosis intent, and all were confirmed as having synchronous metastasis. By comparison, our study revealed the first observation that plasma D-dimer level is higher in CRC patients who developed distant organ metastasis after curative resection of the primary tumor.
Presently, the most common tumor marker applied to assess the risk of metachronous metastasis in CRC is serum CEA.33,34 LDH is another reflector of tumor burden, and linked to distant organ metastasis in CRC.9,17 Similarly, distributions of CEA and LDH were both markedly higher among CRC patients with metachronous metastasis in our study. Moreover, we found that D-dimer rise was fairly consistent with higher CEA, suggesting that elevated D-dimer level might also indicate the occurrence of distant organ metastasis in patients with resected CRC. However, the agreement of D-dimer with LDH was relatively low. The reason might be that most values of LDH still fell within the reference range in the metastasis group, although they were elevated compared to those in non-metastasis patients.
CEA is recommended as a biomarker for predicting tumor recurrence for CRC in the guidelines issued by the National Comprehensive Cancer Network in 2017.33,34 Elevated LDH levels also indicate an increased risk for metachronous metastasis in CRC.9 Therefore, we compared the performance of D-dimer level in detecting metachronous metastasis with that of CEA or LDH. The results of ROC curve showed that AUC of D-dimer assay (0.8, 95%CI: 0.74–0.87) is larger than that of CEA (0.72, 95%CI: 0.64–0.80) or LDH (0.68, 95%CI: 0.60–0.76), indicating that D-dimer had the advantage over CEA or LDH in differentiating patients who developed distant organ metastasis from CRC after undergoing primary tumor resection. The specificity of D-dimer alone (73.3%) was lower than that of CEA alone (74.4%), but the sensitivity (88.0%), PPV (77.9%), NPV (85.1%), and accuracy (80.9% vs 69.7%) in D-dimer assay were superior to those in CEA assay (65.2%, 73.2% and 80.9%, respectively). Other studies also reported that CEA assay had a moderate sensitivity and specificity for distant organ metastasis in patients with CRC,7,10,17 mainly because CEA is relatively insensitive to non-liver metastasis.27,35 By comparison, LDH showed the lowest sensitivity (42.4%) and highest specificity (95.3%) among the three biomarkers. Dong et al36 found that the sensitivity of LDH for predicting brain metastasis in triple-negative breast cancer was 44.0%, which was similar to our result. Different combinations could further improve diagnostic performance. The sensitivity and NPV of a combination assay (either D-dimer elevation or CEA elevation) increased to 94.6% and 91.1%, respectively, and the specificity and PPV of another combination assay (both D-dimer elevation and LDH elevation) were 97.7% and 94.9%, respectively. Parallel test of the three markers increased the sensitivity and NPV to 95.7% and 92.7%, respectively. The results suggested that the combination of serum/plasma biomarkers could be warning indicators for detailed imaging examination. In general, CRC patients are followed up routinely at 2-month intervals for the first 2 years and at 6-month intervals thereafter. At each visit, blood samples are collected. However, chest radiographs and abdominopelvic CT are only performed 6 months postoperatively and then at yearly intervals. Therefore, if levels of D-dimer, CEA, or LDH are elevated in a CRC patient after curative resection during a return visit, timely imaging examination for distant organ metastasis should be recommended.
There surely are some potential limitations in the study. Firstly, patients with other cancers could also show elevated D-dimer level, so this feature is not an exclusive indicator for distant organ metastasis only in patients with CRC. Secondly, relevance between D-dimer values and distant organ metastasis in CRC still requires further validation with prospective trials.
Conclusion
Our study demonstrated that D-dimer level was elevated in postoperative CRC patients who developed distant organ metastasis. Combining with CEA and/or LDH, D-dimer could be a useful surveillance marker for distant organ metastasis in CRC after curative resection.
Acknowledgments
This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (Grant No 2017-I2M-3-005), the National Natural Science Foundation of China (Grant No 81778272) and Beijing Hope Run Special Fund of Cancer Foundation of China (LC2015B13).
Author contributions
YG contributed to the study design, data collection and analysis, and wrote the main manuscript text; FC conceived the study and contributed to statistical analysis; WC revised the manuscript and funded the study. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. | ||
Fang JY, Dong HL, Sang XJ, et al. Colorectal Cancer Mortality Characteristics and Predictions in China, 1991–2011. Asian Pac J Cancer Prev. 2015;16(17):7991–7995. | ||
Chuang SC, Su YC, Lu CY, et al. Risk factors for the development of metachronous liver metastasis in colorectal cancer patients after curative resection. World J Surg. 2011;35(2):424–429. | ||
Kim CH, Huh JW, Kim HJ, et al. Factors influencing oncological outcomes in patients who develop pulmonary metastases after curative resection of colorectal cancer. Dis Colon Rectum. 2012;55(4):459–464. | ||
Memon AA, Sundquist K, Pirouzifard M, et al. Identification of novel diagnostic biomarkers for deep venous thrombosis. Br J Haematol. 2018;181(3):378–385. | ||
Tsurusaki M, Sofue K, Murakami T. Current evidence for the diagnostic value of gadoxetic acid-enhanced magnetic resonance imaging for liver metastasis. Hepatol Res. 2016;46(9):853–861. | ||
Su BB, Shi H, Wan J. Role of serum carcinoembryonic antigen in the detection of colorectal cancer before and after surgical resection. World J Gastroenterol. 2012;18(17):2121–2126. | ||
Laubert T, Bente V, Freitag-Wolf S, et al. Aneuploidy and elevated CEA indicate an increased risk for metachronous metastasis in colorectal cancer. Int J Colorectal Dis. 2013;28(6):767–775. | ||
Wu XZ, Ma F, Wang XL. Serological diagnostic factors for liver metastasis in patients with colorectal cancer. World J Gastroenterol. 2010;16(32):4084–4088. | ||
Oya M, Akiyama Y, Yanagida T, Akao S, Ishikawa H. Plasma D-dimer level in patients with colorectal cancer: its role as a tumor marker. Surg Today. 1998;28(4):373–378. | ||
Flamini E, Mercatali L, Nanni O, et al. Free DNA and carcinoembryonic antigen serum levels: an important combination for diagnosis of colorectal cancer. Clin Cancer Res. 2006;12(23):6985–6988. | ||
Kawai K, Watanabe T. Colorectal cancer and hypercoagulability. Surg Today. 2014;44(5):797–803. | ||
Edwards CM, Warren J, Armstrong L, Donnelly PK. D-dimer: a useful marker of disease stage in surgery for colorectal cancer. Br J Surg. 1993;80(11):1404–1405. | ||
Oya M, Akiyama Y, Okuyama T, Ishikawa H. High preoperative plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer. Jpn J Clin Oncol. 2001;31(8):388–394. | ||
Xu G, Zhang YL, Huang W. Relationship between plasma D-dimer levels and clinicopathologic parameters in resectable colorectal cancer patients. World J Gastroenterol. 2004;10(6):922–923. | ||
Zhu L, Liu B, Zhao Y, et al. High levels of D-dimer correlated with disease status and poor prognosis of inoperable metastatic colorectal cancer patients treated with bevacizumab. J Cancer Res Ther. 2014;10(8):246–251. | ||
Kilic L, Yildiz I, Sen FK, et al. D-dimer and international normalized ratio (INR) are correlated with tumor markers and disease stage in colorectal cancer patients. Cancer Biomark. 2015;15(4):405–411. | ||
Tekeşin K, Bayrak S, Esatoğlu V, Özdemir E, Özel L, Melih Kara V. D-Dimer and Carcinoembryonic Antigen Levels: Useful Indicators for Predicting the Tumor Stage and Postoperative Survival. Gastroenterol Res Pract. 2016;2016:4295029. | ||
Blackwell K, Hurwitz H, Liebérman G, et al. Circulating D-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma. Cancer. 2004;101(1):77–82. | ||
Kilic M, Yoldas O, Keskek M, et al. Prognostic value of plasma D-dimer levels in patients with colorectal cancer. Colorectal Dis. 2008;10(3):238–241. | ||
Stender MT, Larsen TB, Sørensen HT, Thorlacius-Ussing O. Preoperative plasma D-dimer predicts 1-year survival in colorectal cancer patients with absence of venous thromboembolism (VTE): a prospective clinical cohort study. J Thromb Haemost. 2012;10(10):2027–2031. | ||
Yamamoto M, Yoshinaga K, Matsuyama A, et al. Plasma D-dimer level as a mortality predictor in patients with advanced or recurrent colorectal cancer. Oncology. 2012;83(1):10–15. | ||
Motavaf E, Sunesen KG, Stender MT, Thorlacius-Ussing O. Prognostic value of preoperative D-dimer and carcinoembryonic antigen levels in patients undergoing intended curative resection for colorectal cancer: a prospective cohort study. Int J Colorectal Dis. 2014;29(11):1427–1432. | ||
Harper PL, Theakston E, Ahmed J, Ockelford P. D-dimer concentration increases with age reducing the clinical value of the D-dimer assay in the elderly. Intern Med J. 2007;37(9):607–613. | ||
Caponnetto P, Russo C, di Maria A, et al. Circulating endothelial-coagulative activation markers after smoking cessation: a 12-month observational study. Eur J Clin Invest. 2011;41(6):616–626. | ||
di Nisio M, Ferrante N, de Tursi M, et al. Incidental venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Thromb Haemost. 2010;104(5):1049–1054. | ||
Pakdel A, Malekzadeh M, Naghibalhossaini F. The association between preoperative serum CEA concentrations and synchronous liver metastasis in colorectal cancer patients. Cancer Biomark. 2016;16(2):245–252. | ||
Koukourakis MI, Giatromanolaki A, Simopoulos C, Polychronidis A, Sivridis E. Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clin Exp Metastasis. 2005;22(1):25–30. | ||
Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost. 2013;11(2):223–233. | ||
Shirasawa S, Furuse M, Yokoyama N, Sasazuki T. Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science. 1993;260(5104):85–88. | ||
Yu JL, May L, Lhotak V, et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105(4):1734–1741. | ||
Harvey SR, Sait SN, Xu Y, Bailey JL, Penetrante RM, Markus G. Demonstration of urokinase expression in cancer cells of colon adenocarcinomas by immunohistochemistry and in situ hybridization. Am J Pathol. 1999;155(4):1115–1120. | ||
National Comprehensive Cancer Network (NCCN), Clinical Practice Guidelines in Oncology, Colon Cancer, Version 2; 2017. Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed March 13, 2017. | ||
National Comprehensive Cancer Network (NCCN), Clinical Practice Guidelines in Oncology, Recral Cancer, Version 3; 2017. Available from: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed March 13, 2017. | ||
Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen C. An evaluation of the carcinoembryonic antigen (CEA) test for monitoring patients with resected colon cancer. JAMA. 1993;270(8):943–947. | ||
Dong T, Liu Z, Xuan Q, Wang Z, Ma W, Zhang Q. Tumor LDH-A expression and serum LDH status are two metabolic predictors for triple negative breast cancer brain metastasis. Sci Rep. 2017;7(1):6069. |
Supplementary material
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.