Back to Journals » Clinical Interventions in Aging » Volume 14
Usefulness of patellar cartilage cross-sectional area for knee tibiofemoral osteoarthritis in elderly
Authors Bang YS, Park J , Kim J, Choi YS, Lim YS, Cho HR , Kim YU
Received 12 February 2019
Accepted for publication 22 May 2019
Published 5 June 2019 Volume 2019:14 Pages 1021—1026
DOI https://doi.org/10.2147/CIA.S205027
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Yun-Sic Bang,1 Junbeom Park,1 Jihee Kim,1 Young-Soon Choi,2 Young Su Lim,2 Hyung Rae Cho,3 Young Uk Kim2
1Department of Anesthesiology and Pain Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Republic of Korea; 2Department of Anesthesiology and Pain Medicine, Catholic Kwandong University, College of Medicine, International ST. Mary’s Hospital, Incheon, Republic of Korea; 3Department of Anesthesiology and Pain Medine, Myongji Hospital, Hanyang University Medical Center, Seoul, Republic of Korea
Purpose: Knee tibiofemoral osteoarthritis (KOA) is a major health problem, affecting approximately 30% of elderly. Several studies have reported that the loss of patellar cartilage is associated with an increased risk of KOA. However, no study has reported the optimal cut off value of patellar cartilage cross-sectional area (PCA) in KOA. We hypothesize that PCA is a new sensitive morphologic parameter in the diagnosis of KOA. The purpose of this study was to determine whether PCA could be used as an important adjuvant morphological parameter in the diagnosis of KOA.
Patients and methods: Data regarding PCA were collected from 88 subjects with KOA. A total of 77 subjects in the control group underwent knee MRI as part of nonsymptomatic medical examination. T2-weighted axial images were acquired from both groups. Using a picture archiving communications system, we analyzed the cross-sectional area of the patellar cartilage on MRI.
Results: The average PCA was 98.66±22.18 mm2, in the control group, which was significantly (p<0.001) higher than that (59.43±16.11 mm2,) in the KOA group. Receiver operator haracteristic curve analysis was computed to determine the validity of PCA as a predictor of KOA. In the KOA group, the optimal cut offpoint was 76.06 mm,2 with sensitivity of 83.0%, specificity of 83.1%, and AUC of 0.94 (95% CI: 0.90–0.97).
Conclusions: Lower PCA values were associated with a higher possibility of KOA. The optimal cutoff score of PCA might be used to facilitate the evaluation of patients with KOA.
Keywords: knee tibiofemoral osteoarthritis, patellar cartilage, cross-sectional area, cartilage loss
Introduction
Knee tibiofemoral osteoarthritis (KOA) is a chronic joint disease that imposes significant health care burden.1–3 The major feature of KOA is joint space narrowing. Together with meniscal pathology, it contributes to articular cartilage loss on knee radiographs.4 Painful knee and knee deformity due to KOA decrease the strength of the thigh muscle.5 Thus, it is important to detect risk factors in earlier stages of joint disorder development. Previous studies have investigated morphological parameters of KOA, including joint space narrowing,4 the vastus medialis cross-sectional area,6 the strength of quadriceps muscle,7,8 and articular cartilage loss. Patellar cartilage loss is also associated with a higher possibility of KOA.9 Patella plays a major role in knee joint function. Abnormality in patella can lead to changes in the knee joint mechanism.10 Cross-talk between cartilage and subchondral bone could induce catabolism of the cartilage of patella.11,12 It may lead to pathological changes in cartilage. The presence of patellar bone marrow lesions in the elderly is associated with changes of patellar cartilage,10 including patellar cartilage defect progression and patellar cartilage volume loss. Patellar cartilage volume loss tends to increase over time. It might be a major risk factor for secondary KOA.13 However, no studies have reported the clinical optimal cutoff value of patellar cartilage loss on KOA. To evaluate the connection between KOA and patellar cartilage loss, we analyzed the patellar cartilage cross-sectional area (PCA). MRI is superior to radiography for detecting patellar cartilage loss [4]. Thus, we used knee MRI to compare PCA between KOA patients and normal controls. The objective of this study was to determine whether PCA could be used as an important adjuvant morphological parameter in the diagnosis of KOA. We hypothesize that the cross-sectional area of patellar cartilage is an important morphological parameter in the identification of patellar cartilage loss.
Patients and methods
Patients
This retrospective research was registered at Catholic Kwandong University (CKU), College of Medicine, Incheon, South Korea. The Institutional Review Board of CKU inspected and approved the study protocol (CKU IRB number: IS18RISI0015). Written informed consent was obtained from each patient involved in this research. This original research followed the Declaration of Helsinki in confidentiality of patient data. We reviewed patients who had visited the Catholic Kwandong Pain Clinic and Orthopedic Center between May 2015 and December 2017 and were diagnosed with KOA. Patients aged above 50 years were included if they had clinical manifestations (stiffness, swelling, pain, a grinding, or grating sensation when moving the joint) compatible with KOA and had knee MRI within 1 year of the diagnosis that was available for chart review. Radiographic KOA was defined by a Kellgren–Lawrence (KL) grade of 2 or 3. Patients were excluded if they had a history of inflammatory arthritis, end-stage of KOA (KL grade 4), complex regional pain syndrome, rheumatoid arthritis, or history of total knee replacement.
A total of 88 patients were enrolled after KOA diagnosis was confirmed by 2 board-certified, experienced musculoskeletal radiologists. The KOA group included 20 (KL grade 2=11, KL grade 3=9) (22.7%) males and 68 (KL grade 2=33, KL grade 3=35) (77.3%) women with a mean age of 58.28±6.67 ears (range, 50–83 years) (Table 1). The PCA of patients with KOA was compared to that of a group of control subjects who had taken knee MRI as part of nonsymptomatic medical examination. Patients in the control group had no KOA-related disease. Control subjects were excluded if they had a history of inflammatory arthritis, complex regional pain syndrome, rheumatoid arthritis, or history of total knee surgery. The control group consisted of 77 patients (23 men and 54 women) with a mean age of 57.13±8.19 years (range, 50–79 years) (Table 1).
 | Table 1 Comparison of the characteristics of control and KOA group |
Imaging parameters
Knee MRI was performed with 3T Avanto (Siemens Healthcare, Erlangen, Germany) and 3 T scanners (Achieva; Philips Healthcare, The Netherlands). Knee MRI examination was conducted using transverse turbo spin echo (TSE) to obtain fat-suppressed T2-weighted images with a slice thickness <3.0 mm, repetition time/echo time of 2860-ms/75-ms, field of view of 160×160, and matrix of 448×314. All MRI data were transferred from the MRI unit to an INFINITT system (INFINITT Healthcare Co., Seoul, Korea).
Image analysis
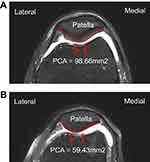
Transverse T2-weighted MR images in individual patients were acquired. An INFINITT picture archiving communications system (INFINITT PACS) was used to measure the PCA by outlining the patellar cartilage contour on the T2-weighted MR image with INFINITT system. INFINITT PACS is an image management system offering streamlined reading workflow task and many value-added features. INFINITT PACS also offers an enterprise imaging solution with the diagnostic viewer (Figure 1).
Statistical analysis
Statistical analysis was carried out using the IBM SPSS version 22 for Windows (IBM Corp, New York). Statistical algorithms were used to calculate the SD. P-values below 0.05 were considered statistically significant. Using the unpaired t-test, we compared the PCA between the KOA and control groups. We use a one-way ANOVA to analyze the correlation between the age-related changes and PCA. The validity of the PCA for diagnosis was calculated using receiver operator characteristic (ROC) curves, sensitivity, and specificity with 95% CIs, optimal cutoff values, area under the curve (AUC).
Results
Demographic characteristics (gender, body mass index, age) were not significantly different between the two groups (Table 1). The average PCA was 98.66±22.18 mm2 in the control group and 59.43±16.11 mm2 in the KOA group. KOA patients had significantly (p<0.001) lower PCA than control subjects (Table 1). The mean PCA of the control group was 97.92±22.39 mm2 in subjects aged 50–59 years, 101.22±18.91 mm2 in subjects aged 60–69 years, and 98.92±28.48 mm2 in subjects aged 70–79 years (Table 2). There were no statistically significant difference between PCA and age-related morphological changes in one-way ANOVA (F=0.128; df=2; p=0.880) in the control group. The mean PCA of KOA group was measured at 57.79±14.98 mm2 for patients aged 50–59 years, 61.96±18.28 mm2 for patients aged 60–69 years, and 60.38±12.07 mm2 for patients aged 70–83 years (Table 3). There were no statistically significant relationships between PCA and age-related changes in the KOA group either (F=0.666; df=2; p=0.516). Regarding the validity of PCA as a predictor of KOA, ROC curve analysis showed that the optimal cutoff point of the PCA was 76.06 mm2, with sensitivity of 83.0%, specificity of 83.1% (Table 4), and AUC of 0.94 (95% CI: 0.90–0.97) (Figure 2).
 | Table 2 Age distribution of patients with mean PCA of control group |
 | Table 3 Age distribution of patients with mean PCA of KOA group |
 | Table 4 Sensitivity and specificity of each cutoff point of the PCA |
Discussion
Patellar cartilage defects of the knee are a frequent pathologic condition associated with reduced quality of life and knee pain. Because of their limited healing potential, patellar cartilage defects tend to increase over time and may be considered a major risk factor for KOA.9 KOA characterized by biochemical and structural cartilage abnormalities may ultimately become functionally and symptomatically debilitating.14–16 Painful knee and knee deformity due to KOA result in reduced muscle strength of the thigh.5 With expectations and hope for early intervention, there is a major interest in finding a reliable and sensitive morphological parameter to identify these biochemical and structural changes at various stages of the KOA.14,17 Previous studies have investigated the association of signs and symptoms of KOA with knee cartilage volume loss,18,19 vastus medialis cross-sectional area,6 mean cartilage thickness over the knee, total subchondral bone area,20 and thigh muscle volumes5 on knee MRI. Yamauchi et al have reported that individual muscle volumes of knee flexors and extensors of patients with KOA can be assessed from a single-slice cross-sectional area using MRI.5 Pan et al have insisted that the number of painful sites can independently predict volume loss of knee cartilage, suggesting that widespread knee pain may be a rapid marker of more early cartilage loss of knee in those without radiographic KOA.18 Buck et al have reported that the subchondral bone and mean cartilage thickness can define an effective subset for detecting structural change in cartilage in KOA.20 However, there are no previous reports correlating KOA and PCA as a morphologic parameter on MRI. In the human body, the patellar cartilage is the thickest cartilage.21 Recurrent patellar instability problems or dislocations can lead to degenerative cartilage changes that can eventually lead to KOA.21 Our results demonstrate that PCA is associated with KOA. The correlation between PCA and KOA is explained by the decrease in PCA associated with an increase in KOA. One explanation for this association is infiltration of inflammatory factors known to be critical contributors to KOA pathogenesis.22 In the pathogenesis of inflammation processes, inflammatory factors such as chemokines, cytokines, proteolytic, prostanoids, vascular growth factors, and enzymes are released. These factors can activate peripheral nociceptors.23 These inflammatory cytokines are associated with matrix degradation and increased cartilage turnover.18 Mostrom et al have insisted that degenerative changes of cartilage are due to altered biomechanics in the femoropatellar joint.21 Repetitive mechanical load is an important for regulating the chondrocytes metabolic activity while deformation of the orientation of the collagen network and cells can stimulate the synthesis of matrix components.24 The instability enhanced by malalignment and loss of congruity can potentially lead to an increased shear stress of the load-bearing area. In the end, this may lead to proteoglycans loss.21 Based on this evidence, a higher level of systemic inflammation can lead to more loss of PCA.
In our study, the best cutoff point for PCA was 76.06 mm2, with sensitivity of 83.0%, specificity of 83.1%, and AUC of 0.94 (95% CI: 0.90–0.97). Our findings suggest that PCA is an accurate, objective, and adjuvant morphological parameter for KOA prediction.
In this analysis, we used knee MRI. This is because MRI is a noninvasive, useful, and sensitive method to detect composition changes of cartilage degeneration.25 Knee MRI also has been instrumental in exploring functional adaptation, maintenance, development, and degeneration of articular cartilage, making it possible to extract geometric dimensions of the tissue.20,26 In the analysis of KOA, morphologic measures of cartilage are frequently acquired to assess disease progression or status.20 Metrics of cartilage morphology (thickness, volume) based on knee MRI have been well investigated.27
Our study only included individuals aged above 50 years. This is because Mosher et al have reported that patella cartilage changes in early KOA may occur mainly and primarily in the superficial layer where collagen matrix first degenerates.28
The present study had some limitations. First, we could not compare PCA with patients’ clinical outcomes such as visual analog scale or knee injury and osteoarthritis outcome score subscales. Previous studies have indicated that clinical outcome is not significantly associated with all morphologic parameters.29 The pathophysiology of KOA is complex. Although the loss of the patellar cartilage is a major factor, inflammatory effects and some other causes such as depression and subjective quality of feeling may also affect pain intensity and unpleasantness.30–36 Second, there might be measurement errors associated with analyzing the PCA on knee MRI. We measured PCA in axial T2 images. However, these axial images might be inhomogeneous due to differences in cutting angle of knee MR images resulting from technical insufficiency and individual anatomic variations. Third, knee joint cartilage can be assessed anatomically in eight regions (Central lateral femoral condyle, Posterior lateral femoral condyle, Lateral tibial plateau, Trochlea, Central medial femoral condyle, Posterior medial femoral condyle, Medial tibial plateau, and Patella).14 However, this study only used PCA measurement. Therefore, our results might be limited regarding the measurement of other morphologic changes. Fourth, one of the findings of the study is that “there were no statistically significant difference between PCA and age-related morphological changes.” However, the distributions of subjects among the three age groups were highly skewed in both KOA and control groups. The majorities of the subjects fall in the age group of 50–59 (N>50), while only 8 and 4 subjects were in the age group of 70–79 in control and KOA groups, respectively. Due to the extremely small number of subjects in the older age group, the statistical power can be limited to conclude age-related morphological differences among these 3 groups. Fifth, the knee joint is consisted of tibiofemoral joint and patellofemoral joint. However, we did not analyze the relationship between tibiofemoral and patellofemoral joints osteoarthritis. In the future study, we have a plan to investigate the relationship between PCA and both tibiofemoral and patellofemoral joints osteoarthritis. Sixth, our study only included individuals aged above 50 years. Therefore, these results may not be generalized to individuals <50 years old and early-stage KOA (KL grade 4). Another weakness of this study was its retrospective nature. Despite these limitations, this is the first paper that describes the association of PCA with KOA. These results may facilitate the diagnosis of KOA.
Conclusion
PCA is a new sensitive parameter for the diagnosis of KOA. With a best cut offpoint of 76.06 mm2, it had sensitivity of 83.0%, specificity of 83.1%, and AUC of 0.94. We expect that this measurement tool can be used to facilitate the evaluation of patients with KOA.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All authors report no conflicts of interest in this work.
References
1. Farber JM. The knee, osteoarthritis and biomarkers. Osteoarthritis Cartilage. 2018;26:845–846. doi:10.1016/j.joca.2018.01.023
2. Khan M, Khanna V, Adili A, Ayeni OR, Bedi A, Bhandari M. Knee osteoarthritis: when arthroscopy can help. Pol Arch Intern Med. 2018;128:121–125. doi:10.20452/pamw.4186
3. Veronese N, Stubbs B, Solmi M, et al. Knee osteoarthritis and risk of hypertension: a longitudinal cohort study. Rejuvenation Res. 2018;21:15–21. doi:10.1089/rej.2017.1917
4. Saunders J, Ding C, Cicuttini F, Jones G. Radiographic osteoarthritis and pain are independent predictors of knee cartilage loss: a prospective study. Intern Med J. 2012;42:274–280. doi:10.1111/j.1445-5994.2011.02438.x
5. Yamauchi K, Yoshiko A, Suzuki S, et al. Estimation of individual thigh muscle volumes from a single-slice muscle cross-sectional area and muscle thickness using magnetic resonance imaging in patients with knee osteoarthritis. J Orthop Surg (Hong Kong). 2017;25:2309499017743101. doi:10.1177/2309499017743101
6. Wang Y, Wluka AE, Berry PA, et al. Increase in vastus medialis cross-sectional area is associated with reduced pain, cartilage loss, and joint replacement risk in knee osteoarthritis. Arthritis Rheum. 2012;64:3917–3925. doi:10.1002/art.34681
7. Lloyd DG, Buchanan TS. Strategies of muscular support of varus and valgus isometric loads at the human knee. J Biomech. 2001;34:1257–1267.
8. Winby CR, Lloyd DG, Besier TF, Kirk TB. Muscle and external load contribution to knee joint contact loads during normal gait. J Biomech. 2009;42:2294–2300. doi:10.1016/j.jbiomech.2009.06.019
9. Mehl J, Feucht MJ, Bode G, Dovi-Akue D, Sudkamp NP, Niemeyer P. Association between patellar cartilage defects and patellofemoral geometry: a matched-pair MRI comparison of patients with and without isolated patellar cartilage defects. Knee Surg Sports Traumatol Arthrosc. 2016;24:838–846. doi:10.1007/s00167-014-3385-7
10. Wang J, Antony B, Zhu Z, et al. Association of patellar bone marrow lesions with knee pain, patellar cartilage defect and patellar cartilage volume loss in older adults: a cohort study. Osteoarthritis Cartilage. 2015;23:1330–1336. doi:10.1016/j.joca.2015.02.018
11. Hunter DJ, Gerstenfeld L, Bishop G, et al. Bone marrow lesions from osteoarthritis knees are characterized by sclerotic bone that is less well mineralized. Arthritis Res Ther. 2009;11:R11. doi:10.1186/ar2684
12. Martig S, Boisclair J, Konar M, Spreng D, Lang J. MRI characteristics and histology of bone marrow lesions in dogs with experimentally induced osteoarthritis. Vet Radiol Ultrasound. 2007;48:105–112.
13. Schinhan M, Gruber M, Vavken P, et al. Critical-size defect induces unicompartmental osteoarthritis in a stable ovine knee. J Orthop Res. 2012;30:214–220. doi:10.1002/jor.21521
14. Bittersohl B, Hosalkar HS, Sondern M, et al. Spectrum of T2* values in knee joint cartilage at 3 T: a cross-sectional analysis in asymptomatic young adult volunteers. Skeletal Radiol. 2014;43:443–452. doi:10.1007/s00256-013-1806-1
15. Le J, Peng Q, Sperling K. Biochemical magnetic resonance imaging of knee articular cartilage: T1rho and T2 mapping as cartilage degeneration biomarkers. Ann N Y Acad Sci. 2016;1383:34–42. doi:10.1111/nyas.13189
16. Sabatini L, Nicolaci G, Atzori F, et al. Biochemical stress evaluation after medial parapatellar and subvastus approach in total knee replacement. Musculoskelet Surg. 2017;102:185–190.
17. Burstein D, Gray M, Mosher T, Dardzinski B. Measures of molecular composition and structure in osteoarthritis. Radiol Clin North Am. 2009;47:675–686. doi:10.1016/j.rcl.2009.04.003
18. Pan F, Laslett L, Tian J, et al. Association between pain at sites outside the knee and knee cartilage volume loss in elderly people without knee osteoarthritis: a prospective study. Arthritis Care Res (Hoboken). 2017;69:659–666. doi:10.1002/acr.22964
19. Teichtahl AJ, Davies-Tuck ML, Wluka AE, Jones G, Cicuttini FM. Change in knee angle influences the rate of medial tibial cartilage volume loss in knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:8–11. doi:10.1016/j.joca.2008.05.013
20. Buck RJ, Wyman BT, Le Graverand MP, Wirth W, Eckstein F, Investigators A. An efficient subset of morphological measures for articular cartilage in the healthy and diseased human knee. Magn Reson Med. 2010;63:680–690. doi:10.1002/mrm.22207
21. Bengtsson Mostrom E, Lammentausta E, Finnbogason T, Weidenhielm L, Janarv PM, Tiderius CJ. Pre- and postcontrast T1 and T2 mapping of patellar cartilage in young adults with recurrent patellar dislocation. Magn Reson Med. 2015;74:1363–1369. doi:10.1002/mrm.25511
22. Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–635. doi:10.1038/nrrheum.2010.159
23. Dimitroulas T, Duarte RV, Behura A, Kitas GD, Raphael JH. Neuropathic pain in osteoarthritis: a review of pathophysiological mechanisms and implications for treatment. Semin Arthritis Rheum. 2014;44:145–154. doi:10.1016/j.semarthrit.2014.05.011
24. Madry H, Luyten FP, Facchini A. Biological aspects of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2012;20:407–422. doi:10.1007/s00167-011-1705-8
25. Zuo H, Yao W, Qu N, Yang S, Wang J, Cui X. Quantitative evaluation in combination with nonquantitative evaluation in early patellar cartilage osteoarthritis at 3.0 T. Clin Interv Aging. 2014;9:1133–1143. doi:10.2147/CIA.S65871
26. Eckstein F, Hudelmaier M, Putz R. The effects of exercise on human articular cartilage. J Anat. 2006;208:491–512. doi:10.1111/j.1469-7580.2006.00546.x
27. Eckstein F, Buck RJ, Burstein D, et al. Precision of 3.0 Tesla quantitative magnetic resonance imaging of cartilage morphology in a multicentre clinical trial. Ann Rheum Dis. 2008;67:1683–1688. doi:10.1136/ard.2007.076919
28. Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8:355–368. doi:10.1055/s-2004-861764
29. Kim YU, Kong YG, Lee J, et al. Clinical symptoms of lumbar spinal stenosis associated with morphological parameters on magnetic resonance images. Eur Spine J. 2015;24:2236–2243. doi:10.1007/s00586-015-4197-2
30. Choi JW, Lee JH, Ki M, et al. The comparison of two different intraarticular injections using a sonographic anterolateral approach in patients with osteoarthritic knee. Korean J Pain. 2018;31(4):289–295. doi:10.3344/kjp.2018.31.4.289
31. Ismail EA, Sayed JA, Bakri MH, Mahfouz RZ. Comparison of intrathecal versus intra-articular dexmedetomidine as an adjuvant to bupivacaine on postoperative pain following knee arthroscopy: a randomized clinical trial. Korean J Pain. 2017;30(2):134–141. doi:10.3344/kjp.2017.30.2.134
32. Kim BG, Kim H, Lim HK, Yang C, Oh S, Lee BW. A comparison of palonosetron and dexamethasone for postoperative nausea and vomiting in orthopedic patients receiving patient-controlled epidural analgesia. Korean J Anesthesiol. 2017;70(5):520–526. doi:10.4097/kjae.2017.70.5.520
33. Kim MK, Moon HY, Ryu CG, Kang H, Lee HJ, Shin HY. The analgesic efficacy of the continuous adductor canal block compared to continuous intravenous fentanyl infusion with a single-shot adductor canal block in total knee arthroplasty: a randomized controlled trial. Korean J Pain. 2019;32(1):30–38. doi:10.3344/kjp.2019.32.1.30
34. Kwon D, Kim BG, Yang C, Won J, Kim Y. Inadvertent thermal injury following knee arthroscopic surgery in a pediatric patient. Korean J Anesthesiol. 2018;71(2):157–160. doi:10.4097/kjae.2018.71.2.157
35. Lee JH, Kim D, Seo D, Son JS, Kim DC. Validity and reliability of the Korean version of the Quality of Recovery-40 questionnaire. Korean J Anesthesiol. 2018;71(6):467–475. doi:10.4097/kja.d.18.27188
36. Schwenk ES, Mariano ER. Designing the ideal perioperative pain management plan starts with multimodal analgesia. Korean J Anesthesiol. 2018;71(5):345–352. doi:10.4097/kja.d.18.00217
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.