Back to Journals » Clinical Ophthalmology » Volume 8
Use of preservative-free hyaluronic acid (Hylabak®) for a range of patients with dry eye syndrome: experience in Russia
Authors Brjesky V, Maychuk Y, Petrayevsky A, Nagorsky P, Stolz J
Received 6 May 2013
Accepted for publication 17 August 2013
Published 18 June 2014 Volume 2014:8 Pages 1169—1177
DOI https://doi.org/10.2147/OPTH.S47713
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Vladimir Vsevolodovich Brjesky,1 Yury Fedorovich Maychuk,2 Alexey Vladimirovich Petrayevsky,3 Peter Gerrievich Nagorsky4
1Department of Ophthalmology, Pediatric State Medical Academy, Saint Petersburg, 2Moscow Research Institute of Eye Diseases, Moscow, 3Department of Ophthalmology, Volgograd State Medical University, Volgograd, 4Novosibirsk Branch of the Federal State Institute MNTK Eye Microsurgery, Novosibirsk, Russian Federation, Russia
Abstract: Artificial tear preparations are important in the management of dry eye syndrome. We present the findings from four recently published studies conducted in Russia assessing Hylabak® (marketed as Hyabak® in Europe), a preservative-free hyaluronic acid preparation, for the treatment of dry eye syndrome. All studies had an open, noncomparative design, but one compared the findings with those from 25 patients treated with Tear Naturale® in previous studies. A total of 134 children and adults were enrolled, and the etiologies of dry eye syndrome included contact lens use, intensive office work, adenovirus eye infection, postmenopausal status, persistent meibomian blepharitis, Sjögren's syndrome, phacoemulsification with intraocular lens implantation, and refractive surgery. The patients were treated with Hylabak for 2 weeks to 2 months. All studies showed that Hylabak resulted in marked improvement as assessed by subjective sensations/complaints, Schirmer's test, Norn’s test, impression cytology and biomicroscopy, staining, and tear osmolarity. Greater benefits were also reported compared with Tear Naturale, including a faster onset of action. Hylabak was well tolerated. In conclusion, Hylabak provided rapid and safe relief from the signs and symptoms of dry eye syndrome, as well as improvement in objective measures, in a wide range of patients.
Keywords: dry eye, eye drops, artificial tears, hyaluronic acid, Hylabak®, preservative-free
Introduction
Dry eye is a multifactorial disorder of the tear film and ocular surface. Symptoms include discomfort or burning sensation, photophobia, blurred or abnormal vision, and eye watering, and the longer-term sequelae can be serious.1 Factors contributing to dry eye are numerous, and include aging, autoimmune disorders, infection, abnormalities of the lipid tear layer, menopause, wearing contact lenses, exposure to air conditioning, and use of computers. A modern understanding of dry eye includes etiologic factors such as inflammatory mediators, instability of the tear film, meibomian gland dysfunction, and hyperosmolarity of the tear film.2
The incidence of dry eye has risen considerably in recent years,3,4 partly due to increased computer use in air-conditioned offices. Often known as office eye syndrome,5 it results from exposure of the precorneal tear film, corneal epithelium, and conjunctiva to artificial heating and dehumidified air provided by air conditioning and is likely amplified by the reduced eye blink rate and increased tear film evaporation consequent to extended viewing of computer screens. The everyday use of computers may be specifically related to the increased reporting of dry eye syndrome in younger populations,6 as may routine contact lens use, which frequently results in changes in the corneal epithelium and lacrimal film.
A variety of treatments are available for dry eye, including surgery, moisture-retaining spectacles, and anti-inflammatory drugs, but artificial tear preparations are the cornerstone of dry eye management across the severity spectrum and almost 20 of these products are currently available in Russia.7 They are widely used for the prolonged treatment of patients with early-stage dry eye syndrome and for improving ocular comfort in patients with transient secondary tear film instability (eg, office workers). Some such preparations have been developed based on the naturally occurring polysaccharide, hyaluronic acid. This has excellent water-retaining and lubricant properties, as well as viscoelastic effects that aid vision during blinks but maintain hydration and lubrication between blinks.8,9 Studies have shown good efficacy in patients with dry eye syndrome.10–14
Hyperosmolarity has recently been identified as an important factor in the etiology of dry eye; tear instability and hyperosmolarity are now considered to be interacting and key mechanisms in dry eye and precursors to inflammatory processes and corneal damage.15 Indeed, the most recent definition of dry eye by the Dry Eye Workshop (2007) makes specific mention of tear hyperosmolarity. Clinical studies also suggest that hypotonic eye drops have an advantage over isotonic eye drops in the treatment of dry eye.16–19
Whilst the efficacy of hyaluronate-containing tear substitutes has been well demonstrated, there remain issues over the use of preservatives in such therapies, particularly when the treatment is likely to be long-term. It is now generally recognized that elimination of preservatives such as benzalkonium is important in the long-term safety and tolerability of ocular preparations,20,21 to the extent that some authorities consider the elimination of preservatives from tear substitutes as one of the most critical advances in the treatment of dry eye.22
The preservative-free hyaluronic acid preparation, Hylabak® (Laboratoires Thea, Clermont Ferrand, France), has recently been developed to address this issue. Hylabak comprises hyaluronic acid 0.15% and actinoquinol in a hypo-osmolar, preservative-free ABAK® bottle (Laboratoires Thea). Sterility of the open container is assured by the ABAK system, that consists of a multidose eye drop dispenser closed by an adapted, small-pore sterilizing filter.23
We present here the findings from four recently published studies with broadly comparable methodologies conducted in Russia that have assessed Hylabak for the treatment of dry eye.24–27 Patients enrolled in these studies had a range of symptoms and etiologies ranging from contact lens use and visually intensive occupations to persistent meibomian blepharitis, Sjögren’s syndrome, and the sequelae of ocular surgery.
Materials and methods
Patient characteristics and a methodologic summary of the four individual studies are shown in Table 1. All four studies were conducted in Russia and had an open, noncomparative design. One study,25 however, compared its findings with those from 25 patients treated with Tear Naturale® (0.1% dextran 70, 0.3% hydroxypropyl methylcellulose, preserved with benzylalkonium [0.05%], Alcon laboratories, Fort Worth, TX, USA) in previous studies.28
  | Table 1 Patient characteristics and methodologic summary of four open studies of Hylabak® in patients with dry eye syndrome |
A total of 134 children and adults (aged 7–55 years) were enrolled in the studies. There were various etiologies accounting for the dry eye syndrome, including contact lens use, intensive office work, adenovirus eye infection, postmenopausal status, persistent meibomian blepharitis, Sjögren’s syndrome, phacoemulsification with intraocular lens implantation, and refractive surgery (Table 2).
  | Table 2 Etiology of dry eye syndrome in each study |
The patients were treated with Hylabak for 2 weeks to 2 months, depending on the study, and assessments were performed at various times during treatment. All studies assessed subjective sensations/complaints using a 0–4 scale (0, absence of symptoms; 1, slight feeling of discomfort; 2, evident feeling of discomfort; 3, the worst feeling of discomfort), tear production (Schirmer’s test, without anesthesia) and tear stability/break-up time (Norn’s test);29 other assessments (impression cytology and biomicroscopy, staining, tear osmolarity) performed in the individual studies are shown in Table 1.
Results
All four studies showed an improvement in dry eye syndrome of varying etiologies with Hylabak treatment (Table 2). A brief summary of the results from each of the individual studies is given below.
Study 1
Maychuck and Yani24 conducted an open study in 40 patients with dry eye syndrome (adenovirus infection, soft contact lens use, phacoemulsification with intraocular lens implantation, and refractive surgery) who were treated for 28 days. Assessments were performed on days 1, 7, 14, 21, and 28, and included subjective complaints, Schirmer’s test, Norn’s test, meniscometry, osmometry, and conjunctival xerosis.
Results for patients divided into those with mild or moderate disease showed that subjective complaints were reduced or had disappeared from the first days of treatment irrespective of symptom severity. Complaints were typical of dry eye syndrome and included feelings of a foreign body in the eye, eye reddening, eyelid edema, itching, burning sensation, and variation in visual acuity. This was confirmed by objective measures. Tear production (Schirmer’s test) and precorneal tear break-up time (Norn’s test) were increased at each assessment in patients with both mild and moderate disease (Table 2). Similarly, there was an improvement to near normal values in tear osmolarity from 327 ± 0.6 and 336 ± 1.2 mOsm/L in the mild and moderate groups, respectively, on day 1 to 286 ± 1.7 and 302 ± 1.5 mOsm/L, respectively, on day 28. In both mild and moderate groups combined, the height of the tear meniscus increased markedly from 0.38 ± 0.2 mm on day 1 to 0.58 ± 0.2 mm on day 28, and conjunctival xerosis fell from 4.55 ± 0.5 points on day 1 to 0.4 ± 0.4 points on days 28. There was early corneal epithelialization followed by formation of a completely stable epithelial cover. Treatment was well tolerated and no allergic reactions were observed. The authors concluded that Hylabak showed good therapeutic efficacy in the treatment of dry eye syndrome of varying etiologies and severity, and that it was well tolerated during prolonged use.
Study 2
Brjesky et al25 conducted an open study in 32 patients with dry eye syndrome (deficiency of tear film production, persistent meibomian blepharitis, Sjögren’s syndrome) who were treated for 28 days, and the results were compared with those from 25 patients treated with Tear Naturale in previous studies. Assessments were performed on days 3, 7, 14, and 28, and included subjective discomfort, Schirmer’s test, Norn’s test, tear meniscus index, and basal and total tear production.
Patients were assessed according to the etiology of their dry eye syndrome. Subjective discomfort, measured on a four-point scale from 0 (no symptoms) to 3 (worst feelings of discomfort), was significantly (P < 0.05 versus baseline) reduced by Hylabak from day 7 onwards in postmenopausal women (2.0 ± 0.2 at baseline versus 1.2 ± 0.1 on day 28) and from day 3 onwards in patients with persistent meibomian blepharitis (2.1 ± 0.2 at baseline versus 0.6 ± 0.1 on day 28), or Sjögren’s syndrome (2.6 ± 0.2 at baseline versus 1.4 ± 0.2 on day 28) (Figure 1). This compares with findings using Tear Naturale in previous studies in which significant differences were reported from day 7 onwards in postmenopausal women (2.0 ± 0.1 at baseline versus 1.4 ± 0.2 on day 28) and those with meibomian blepharitis, and from day 14 onwards (1.9 ± 0.2 at baseline versus 0.8 ± 0.1 on day 28) in those with Sjögren’s syndrome (2.6 ± 0.2 at baseline versus 1.7 ± 0.1 on day 28).
  | Figure 1 Change in subjective discomfort score during 28 days of treatment with Hylabak® in (A) postmenopausal women, (B) patients with persistent meibomian blepharitis, and (C) Sjögren’s syndrome (n=32)25 compared with a similar group of patients treated with Tear Naturale® (n=25).28 |
Objective signs (four-point scale) were significantly (P < 0.05) reduced by Hylabak from day 14 onwards in postmenopausal women (1.4 ± 0.1 at baseline versus 0.3 ± 0.1 on day 28), in patients with Sjögren’s syndrome (2.1 ± 0.2 at baseline versus 1.3 ± 0.1 on day 28), and from day 7 onwards in those with meibomian blepharitis (1.1 ± 0.1 at baseline versus 0.6 ± 0.1 on day 28, Figure 2). In contrast, no significant changes were seen with Tear Naturale in patients with meibomian blepharitis (1.2 ± 0.1 at baseline versus 0.7 ± 0.2 on day 28) or Sjögren’s syndrome (2.1 ± 0.3 at baseline versus 1.5 ± 0.1 on day 28), and a significant decrease was seen only on day 28 in postmenopausal women (1.3 ± 0.2 at baseline versus 0.5 ± 0.1 on day 28).
  | Figure 2 Change in objective signs score during 28 days of treatment with Hylabak® in (A) postmenopausal women, (B) patients with persistent meibomian blepharitis, and (C) Sjögren’s syndrome (n=32)25 compared with a similar group of patients treated with Tear Naturale® (n=25).28 |
Tear film stability (Norn’s test) was significantly increased from day 3 onwards in all groups of patients given Hylabak, and in postmenopausal women and patients with Sjögren’s syndrome given Tear Naturale; patients with meibomian blepharitis given Tear Naturale had a significant improvement only from day 7 onwards.
Tear meniscus index significantly improved from day 3 onwards in all groups of patients, irrespective of treatment. Increases from 1.1 ± 0.1, 1.9 ± 0.1, and 1.1 ± 0.1 at baseline to 2.1 ± 0.2, 2.6 ± 0.1, and 2.2 ± 0.1 at day 28 were observed with Hylabak in postmenopausal women and those with meibomian blepharitis or Sjögren’s syndrome, respectively. The corresponding increases in patients given Tear Naturale were from 1.1 ± 0.1, 1.8 ± 0.2, and 1.1 ± 0.1 at baseline to 1.9 ± 0.1, 2.4 ± 0.1, and 1.9 ± 0.1 on day 28.
Basal and total tear production was not significantly changed by either treatment in any group of patients. Hylabak had no toxic effects and no allergic reactions were observed. The results showed that both Hylabak and Tear Naturale were effective in treating the various etiologies of dry eye syndrome, but that benefits with regard to subjective discomfort, objective signs, and tear film stability were more marked with Hylabak (although the differences between the groups did not reach statistical significance).
Study 3
In an open study by Petrayevsky et al,26 32 women with dry (office) eye syndrome due to intensive use of personal computers in an air-conditioned office environment were treated for 2 weeks. Assessments performed before and after treatment included recording of subjective complaints, Schirmer’s test, Norn’s test, and cytologic analysis of the bulbar conjunctiva. The patients were divided into those with mild symptoms and those with moderate symptoms.
Subjective signs and symptoms, measured on a four-point scale from 0 (no symptoms) to 3 (severe), were improved after Hylabak treatment in patients with mild or moderate symptoms (Figure 3). Patients with mild symptoms showed nonspecific objective symptoms (eg, local edema of the bulbar conjunctiva involving the free edge of the eyelid and mild hyperemia), whilst those with moderate symptoms showed both specific (eg, reduction of tear meniscus at the lid margin) and nonspecific objective symptoms, all of which were improved or resolved after Hylabak treatment. Functional tests (Schirmer’s test and tear break-up time assessed by Norn’s test) were also significantly (P < 0.01) improved after Hylabak (Table 2), with the exception of Schirmer’s test in patients with mild symptoms.
  | Figure 3 Change in subjective signs and symptoms score during 2 weeks of treatment with Hylabak® in office workers with (A) mild or (B) moderate symptoms (n=32).26 |
Cytologic examination of the conjunctiva showed that 50% of patients with mild symptoms had early loss of goblet cells and the remainder had total loss of goblet cells, all without keratinization (Tseng stage 1 and 2, respectively). There was a marked improvement after Hylabak treatment, with 28% of patients showing normalization and 61% with Tseng stage 1 (mild dry eye population). Among the patients with moderate symptoms, Tseng stage 1 was seen in 36% and Tseng stage 2 in 64% at baseline. Normalization occurred in 29% of patients and 57% had Tseng stage 1 after treatment.
The authors concluded that Hylabak resulted in subjective and objective improvement, as well as normalization of functional tests and the cytologic profile of the conjunctiva, in a population suffering from office eye syndrome.
Study 4
Thirty children and adolescents were enrolled in this open study by Nagorsky et al,27 and were assessed according to whether they wore soft contact lenses during the day or orthokeratologic lenses at night. Treatment was given for 2 months. Assessments performed before treatment and after 1 and 2 months included subjective sensations, Schirmer’s test, Norn’s test, meniscometry and conjunctival xerosis. The main focus of the study was soft contact lens wearers. Amongst these 20 patients, there was a marked improvement in subjective complaints after one month of Hylabak treatment, that was increased further after 2 months (Figure 4). When asked about the duration of the most comfortable period of wearing their contact lenses, the patients reported a more than three-fold increase with Hylabak treatment. This was supported by improvements in objective measures to within the normal range (Table 3). There was also a significant (P < 0.05) increase in the height of the tear meniscus, from 0.62 ± 0.20 mm to 0.95 ± 0.20 mm after 2 months, and a reduction in the intensity of corneal xerotic changes. There was also a reduction in corneal xerotic change intensity with Hylabak treatment in the 10 patients wearing orthokeratologic lenses, and patients reported that it was faster, easier, and more comfortable to manipulate their lenses. Hylabak was well tolerated. There were no cases of unpleasant sensation, discomfort, or prolonged blurred vision, and most patients noted improved comfort compared with previous eye drops. The authors concluded that Hylabak had good therapeutic efficacy and was easy to use, cost-effective, and well tolerated in this group of young patients.
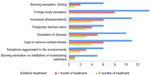  | Figure 4 Change in number of patients with subjective complaints during 2 months of treatment with Hylabak® in children and adolescents wearing soft contact lenses (n=20).27 |
Discussion
All four studies found that Hylabak resulted in a marked improvement in patients with dry eye syndrome of heterogeneous etiology. Subjective sensations and complaints were reduced, and these findings were supported by results from a wide range of objective measures, including Schirmer’s test, Norn’s test, impression cytology and biomicroscopy, staining, and tear osmolarity. Complete resolution of signs and symptoms and normalization of functional tests and cytologic profile was reported in some patients. Hylabak resulted in rapid relief from symptoms. In one study assessing effects during the first week of treatment, there were statistically significant improvements in subjective discomfort, tear film stability, and tear meniscus index from day 3 onwards, with objective signs improving significantly from day 7 in some patients. In contrast, significant improvements with Tear Naturale did not occur until later in treatment. In addition, benefits on subjective discomfort, objective signs, and tear film stability were more marked with Hylabak than with Tear Naturale. Good tolerability was reported throughout the studies, with no toxic or allergic reactions.
Not all artificial tear preparations are suitable for all types of dry eye syndrome patients. Whilst preserved artificial tears may be more convenient for short-term use, for example, a preservative-free formulation would be more appropriate where treatment is likely to continue on a long-term basis. A review of the patients included in the four studies discussed here shows that Hylabak was safe and effective in a wide range of dry eye syndrome etiologies including contact lens use, intensive exposure to air-conditioning and computer use, eye infection, postmenopausal status, meibomian blepharitis, Sjögren’s syndrome, phacoemulsification with intraocular lens implantation, and refractive surgery. The majority of the patients were reported to have mild to moderate disease. There was also a wide age range, with one study enrolling children from 7 years of age. Hylabak proved to be well tolerated, effective and easy to use in these young patients, and patients reported that they found contact lens use faster, easier, and more comfortable compared with their previous eye drops. Treatment was given daily in all four studies, suggesting that Hylabak is suitable for patients with secondary transitional dry eye syndrome, such as office workers who require regularly improved ocular comfort. The findings in this wide-ranging population suggest that Hylabak is an ideal choice for first-line treatment of dry eye syndrome, irrespective of the etiology of the disorder. In conclusion, Hylabak provided rapid and safe relief from the signs and symptoms of dry eye syndrome, as well as improvement in objective measures, in patients with a wide range of dry eye etiologies.
Acknowledgment
Editorial support for this paper was provided by Jane Irons. Editorial assistance was provided by Dr JF Stolz who was remunerated by Laboritoires Thea.
Disclosure
The authors report no conflicts of interest in this work.
References
[No authors listed]. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:75–92. | |
Paiva CS, Pflugfelder SC. Rationale for anti-inflammatory therapy in dry eye syndrome. Arq Bras Oftalmol. 2008;71:89–95. | |
Murube J, Németh J, Höh H, et al. The triple classification of dry eye for practical clinical use. Eur J Ophthalmol. 2005;15:660–667. | |
Brzhevsky VV. Dry eye syndrome. In: Maychuk DY, editor. Red Eye Syndrome. Moscow, Russian Federation: OOO Publications Ophthalmology; 2010. | |
Sommer HJ, Johnen J, Schongen P, Stolze HH. Adaptation of the tear film to work in air-conditioned rooms (office-eye syndrome). Ger J Ophthalmol. 1994;3:406–408. | |
Brzhesky VV. Dry-eye syndrome in young people: the unsolved problem of modern age. Modern Optometry. 2007;2:38–43. | |
Doughty MJ, Glavin S. Efficacy of different dry eye treatments with artificial tears or ocular lubricants: a systematic review. Ophthalmic Physiol Opt. 2009;29:573–583. | |
Snibson GR, Greaves JL, Soper ND, Tiffany JM, Wilson CG, Bron AJ. Ocular surface residence times of artificial tear solutions. Cornea. 1992;11:288–293. | |
Nakamura M, Hikida M, Nakano T, Ito S, Hamano T, Kinoshita S. Characterization of water retentive properties of hyaluronan. Cornea. 1993;12:433–436. | |
Condon PI, McEwen CG, Wright M, Mackintosh G, Prescott RJ, McDonald C. Double blind, randomised, placebo controlled, crossover, multicentre study to determine the efficacy of a 0.1% (w/v) sodium hyaluronate solution (Fermavisc) in the treatment of dry eye syndrome. Br J Ophthalmol. 1999;83:1121–1124. | |
Aragona P, Papa V, Micali A, Santocono M, Milazzo G. Long term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eye. Br J Ophthalmol. 2002;86:181–184. | |
Johnson ME, Murphy PJ, Boulton M. Effectiveness of sodium hyaluronate eyedrops in the treatment of dry eye. Graefes Arch Clin Exp Ophthalmol. 2006;244:109–112. | |
Vogel R, Crockett RS, Oden N, Laliberte TW, Molina L; Sodium Hyaluronate Ophthalmic Solution Study Group. Demonstration of efficacy in the treatment of dry eye disease with 0.18% sodium hyaluronate ophthalmic solution (Vismed, Rejena). Am J Ophthalmol. 2010;149:594–601. | |
Baeyens V, Bron A, Baudouin C; Vismed/Hylovis Study Group. Efficacy of 0.18% hypotonic sodium hyaluronate ophthalmic solution in the treatment of signs and symptoms of dry eye disease. J Fr Ophtalmol. 2012;35:412–419. French. | |
Tomlinson A, Khanal S, Ramaesh K, Diaper C, McFadyen A. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci. 2006;47:4309–4315. | |
Stahl U, Willcox M, Stapleton F. Role of hypo-osmotic saline drops in ocular comfort during contact lens wear. Cont Lens Anterior Eye. 2010;33:68–75. | |
Aragona P, Di Stefano G, Ferreri F, et al. Sodium hyaluronate eye drops of different osmolarity for the treatment of dry eye in Sjogren’s syndrome patients. Br J Ophthalmol. 2002;86:879–884. | |
Troiano P, Monaco G. Effect of hypotonic 0.4% hyaluronic acid drops in dry eye patients: a cross-over study. Cornea. 2008;27:1126–1130. | |
Papa V, Aragona P, Russo S, et al. Comparison of hypotonic and isotonic solutions containing sodium hyaluronate on the symptomatic treatment of dry eye patients. Ophthalmologica. 2001;215:124–127. | |
Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 201;2:312–334. | |
Furrer P, Mayer JM, Gurny R. Ocular tolerance of preservatives and alternatives. Eur J Pharm Biopharm. 2002;53:263–280. | |
Gabisson P, Briat B, Le Foll J, et al. Handiness and acceptability of the new ABAK bottle in chronically treated patients. A cross-sectional, retrospective and multicentre study. Ann Pharm Fr. 2011;69:22–29. French. | |
López-García JS, García-Lozano I. Use of containers with sterilizing filter in autologous serum eyedrops. Ophthalmology. 2012;119:2225–2230. | |
Maychuk YF, Yani EV. New hyaluronic-acid-based artificial tear drug Hylabak for dry eye therapy. J Cataract Refract Surg. 2011;11:48–50. | |
Brjesky VV, Prozornaya LP, Sadovnikova NN, Radhuan MR. New preservative-free hyaluronic acid preparations in treatment of patients with dry eye syndrome. Ocular Pharmacology Issues. 2011;IV(2):99–104. | |
Petrayevsky AV, Trishkin KS, Lyovina OV. The dry eye syndrome in female office workers: a clinical and cytological analysis of results of treatment with Hylabak. Russian Ophthalmological Journal. 2012;2:49–53. | |
Nagorsky PG, Belkina VV, Nesterova LY. Effect of tear-replacement therapy (Hylabac 0.15%) on the dry-eye syndrome intensity in children and adolescents wearing contact lenses. Russian Ophthalmology Journal. 2011;2:32–36. | |
Brjesky VV, Prozornaya LP. New artificial tear preparation Ophtholique in treating patients with dry eye syndrome of different genesis. Ophthalmological Journal. 2009;II:63–68. | |
Norn MS. Desiccation of the precorneal film. I. Corneal wetting time. Acta Ophthalmol. 1969;47:865–880. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

