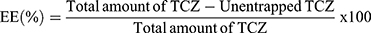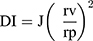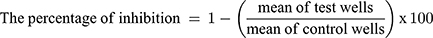Back to Journals » International Journal of Nanomedicine » Volume 16
Use of Novasomes as a Vesicular Carrier for Improving the Topical Delivery of Terconazole: In Vitro Characterization, In Vivo Assessment and Exploratory Clinical Experimentation
Authors Mosallam S, Ragaie MH, Moftah NH, Elshafeey AH , Abdelbary AA
Received 18 October 2020
Accepted for publication 1 December 2020
Published 8 January 2021 Volume 2021:16 Pages 119—132
DOI https://doi.org/10.2147/IJN.S287383
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Shaimaa Mosallam,1 Maha H Ragaie,2 Noha H Moftah,2 Ahmed Hassen Elshafeey,3 Aly Ahmed Abdelbary3,4
1Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, October 6 University, Giza, Egypt; 2Department of Dermatology, STD’s and Andrology, Faculty of Medicine, Minia University, Al-Minya, Egypt; 3Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Cairo University, Cairo, Egypt; 4School of Life and Medical Sciences, University of Hertfordshire Hosted by Global Academic Foundation, Cairo, Egypt
Correspondence: Aly Ahmed Abdelbary
Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Cairo University, Kasr El-Aini, Cairo 11562, Egypt
Tel +201149005526
Email [email protected]
Purpose: This manuscript aimed at encapsulating an antifungal terconazole (TCZ) into innovative novasomes for improving its penetration into the skin and clinically modulating its therapeutic efficacy.
Methods: Novasomes containing free fatty acid (FFA) as a penetration enhancer were formulated using ethanol injection technique based on 24 full factorial design to explore the impact of various formulation variables on novasomes characteristics regarding entrapment efficiency percent (EE%), particle size (PS), polydispersity index (PDI), and zeta potential (ZP). The optimum formulation was chosen using Design-Expert® software and utilized for further explorations.
Results: The chosen formulation (N15; including 100 mg lipid components and Span 80 to oleic acid in a ratio of 2:1 (w/w)) exhibited an EE% = 99.45 ± 0.78%, PS = 623.00 ± 2.97 nm, PDI = 0.40 ± 0.04, and ZP = − 73.85 ± 0.64 mV. N15 showed spherical vesicles with a higher deformability index (DI) (9.62 ± 0.15 g) compared to traditional niosomal formulation (0.92 ± 0.12 g). Further, N15 showed superior inhibition of Candida albicans growth relative to TCZ suspension using XTT (2,3-bis-(2-methyloxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) reduction assay. Moreover, in vivo skin deposition tests revealed a superior TCZ deposition inside the skin from N15 in comparison to traditional niosomal formulation and TCZ suspension. Furthermore, histopathological examination for rats assured the safety of N15 for topical use. A clinical study conducted on infants suffering from napkin candidiasis proved the superiority of N15 to placebo in providing a complete cure of such fungal infections.
Conclusion: Concisely, the obtained outcomes confirmed the pronounced efficacy of N15 to successfully treat skin fungal infections.
Keywords: terconazole, novasomes, free fatty acid, XTT reduction assay, skin deposition, clinical study
Introduction
Fungal infections are among the most often occurring pathogens that damage the skin. Treatment strategies include antifungal agents of both topical and systemic type. Fungal agents used topically are usually favored because of the risks of traditional systemic therapy; including specific organic toxicity, drug–drug interactions, and higher medical cost.1 As the therapeutic efficacy of a drug used locally depends primarily on its capacity to penetrate and permeate the skin, there is an urgent need to create new drug delivery systems that can pass the skin barrier (brick and mortar-like structure). Vesicular systems are one of the most frequently used systems for this purpose.2
Several vesicles such as transfersomes and ethosomes have been established owing to the lack of the capacity of traditional vesicular systems such as liposomes and niosomes to penetrate deeply into the skin and distribute drugs effectively via it.3,4 Besides the preparation of effective delivery forms of the extremely potential antifungal agents available; the recent rapid rise in the usage of antimycotic medications contributes to the development of resistant strains. Thus, there has arisen an immediate therapeutic need for innovative antifungals.
Terconazole (TCZ) is a new broad-spectrum topical antifungal agent that belongs to the drug class triazole and is used primarily in the treatment of vulvovaginal candidiasis. It demonstrates improved mycological and therapeutic cure levels in the management of fungal infections as compared with other imidazole antifungal agents. TCZ works by inhibiting the fungal cytochrome P-450 dependent 14a-demethylase. Thereby, the composition of sterol is changed.5,6 TCZ has a restricted therapeutic application because of its low permeability characteristics. To overcome this problem, TCZ was encapsulated in bilosomes,7 proniosomal gel,8 and polymeric mixed micelles.9
Novasome technology is the proprietary and creative method of encapsulation developed by the IGI laboratories NOVAVAX to solve effectiveness and efficiency-related problems with existing drug delivery systems. Novasomes can be assumed to have enhanced liposomal or niosomal structure, which are typically formulated from a mixture of cholesterol, free fatty acid (FFA), and monoester of polyoxyethylene fatty acid.10 Novasomes have many properties; being a multi-bilayered vesicle with a high capacity central core in a limited size range, it can deliver a wide amount of active ingredients. Different vaccines formulated into novasomes have been approved.11,12
In this manuscript, TCZ was intended to be incorporated into novasomes, as a novel potential nanosystem. In addition, the superiority of novasomes over niosomes in the topical delivery has not been investigated yet. Hence, our aim in this study was to ascertain the high flexibility of novasomes in improving the skin penetration compared to niosomes using TCZ, as a model drug. To accomplish this objective, a full 24 factorial design using Design Expert® software was utilized for formulating novasomes to explore the impact of different formulation variables on the characteristics of the prepared novasomes regarding entrapment efficiency percent (EE%), particle size (PS), polydispersity index (PDI), and zeta potential (ZP) and to choose the optimum formulation. Further, the deformability index (DI) of the optimum novasomes was determined and compared with traditional niosomal formulation to measure the elasticity of the vesicles. Moreover, microbiological efficacy using XTT (2,3-bis-(2-methyloxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) reduction assay for the optimum novasomes compared to TCZ suspension was accomplished to determine the inhibition efficacy of TCZ against Candida albicans. Furthermore, in vivo skin deposition tests of the optimum novasomes were performed compared to traditional niosomal formulation and TCZ suspension. To ensure the safety of the optimum novasomes, histopathological modifications in rat skin were examined. Finally, the optimum formulation was clinically tested for its antifungal efficacy on infants suffering from napkin candidiasis and compared to placebo.
Materials
TCZ was provided by Marcyrl Pharmaceutical Industries (Cairo, Egypt). Span 60, Span 80, acetonitrile (HPLC grade), ethanol (95%), and methanol (HPLC grade) were purchased from Merck-Schuchardt, Germany. Stearic acid, oleic acid, cholesterol, disodium hydrogen phosphate, potassium dihydrogen phosphate, sodium chloride, triethanolamine, dimethyl sulfoxide (DMSO), and glacial acetic acid were obtained from El-Nasr Chemicals Co., Cairo, Egypt.
Methods
Formulation of TCZ Loaded Novasomes
TCZ loaded novasomes were formulated by the technique of ethyl alcohol injection adopted via Kakkar and Kaur.13 Novasomes were formulated by varying the surface active agent (SAA) type, FFA type, SAA to FFA ratio (w/w), and total lipid concentration. Briefly, ethyl alcohol was used for dissolving TCZ (10 mg), SAA, FFA, and cholesterol (30 mg) in a 60°C water bath. This was then injected slowly into a five-fold larger vehicle of phosphate-buffered saline (PBS, pH 7.4) that was magnetically stirred at the same temperature. Novasomes dispersions were formed when a sudden turbidity was observed. The outcome was continuously stirred until the full evaporation of ethyl alcohol. For PS reduction, the resulted novasomes dispersions were then sonicated in a sonicator water bath (Type USR3, Julabo Labortechnik, Seelbach, West Germany) for 10 min at 25°C. All the prepared formulations were kept until use at 4°C.14
In Vitro Characterization and Optimization of TCZ Loaded Novasomes
TCZ Loaded Novasomes EE%
The determination of TCZ EE% in novasomes was assessed by indirect measurement of the free amount of TCZ (unentrapped).15 One milliliter of each novasomes formulation was centrifuged by cooling centrifuge (Sigma 3–30 KS, Germany) at 20000 rpm for an hour at 4°C. The clear supernatant was then diluted and TCZ concentration was determined via UV/VIS spectrophotometer (Shimadzu, model UV-1601 PC, Kyoto, Japan) at λmax = 255.8 nm. TCZ EE% was calculated using the following equation:
TCZ Loaded Novasomes PS, PDI, and ZP
The average PS, PDI, and ZP of the various formulated TCZ loaded novasomes were measured by Zetasizer 2000 (Malvern Instrument Ltd., UK). Novasomes formulations were diluted before measurements. Measuring the ZP was carried out with the same equipment. Each sample was measured in triplicate and the mean value was stated.
Experimental Design Construction
A full factorial design (24) was applied through Design-Expert® software (Version 7, Stat-Ease Inc., USA) to assess the influence of different variables in preparing novasomes. Four factors (independent variables) were evaluated, each factor with two levels: SAA type (X1), FFA type (X2), SAA: FFA ratio (w/w) (X3), and total lipid concentration (X4). The EE% (Y1), PS (Y2), PDI (Y3), and ZP (Y4) were designated as dependent variables (Table 1).
 |
Table 1 24 Full Factorial Design Used for Optimization of the Novasomes Formulations |
Optimization of TCZ Loaded Novasomes
The criteria for the optimum formulation selection were set based on the smallest (PS and PDI) and the highest (EE% and ZP (as absolute value)). The solution with desirability outcome close to one was chosen. To confirm the performance of this model, the chosen formulation was formulated, evaluated, and compared to the predicted outcomes. For the purpose of investigating the impact of the incorporated FFA on the vesicular physicochemical characteristics, traditional niosomal formulation was prepared in the previously mentioned manner using the same components of the optimum novasomes without incorporating FFA. The traditional niosomal formulation was characterized with respect to EE%, PS, PDI, and ZP (Table 2).
 |
Table 2 Experimental Runs, Independent Variables, and Measured Responses of 24 Full Factorial Design of Novasomes Formulations Compared to Traditional Niosomal Formulation |
Characterization of the Optimum TCZ Loaded Novasomes
Elasticity Measurement
The elasticity of both the optimum novasomes and traditional niosomal formulation was determined using the extrusion method.16 The formulations were properly diluted (5 folds) before extrusion through a nylon filter of a 220 nm (Jinteng Experiment Equipment Co., Ltd, China)17,18 at a constant pressure of 2.5 bar (Haug Kompressoren AG; Büchi Labortechnik AG, Flawil, Switzerland). The outcomes were expressed as average value ± SD, n = 3. DI was calculated using the equation below:19
where J = weight of sample extruded in 10 min, rv = size of the vesicle after extrusion in nm, and rp = the barrier pore size in nm. Statistical analysis was computed by Student’s t-test utilizing SPSS® program 22.0 (USA). The difference at P ≤ 0.05 was considered significant.
Transmission Electron Microscopy (TEM)
The morphological appearance of the optimum novasomes was visualized by TEM (Joel JEM 1230, Tokyo, Japan). One drop of undiluted formulation was deposited on a film-coated 200-mesh copper grid, negatively stained with one drop of 2% aqueous solution of phosphotungstic acid (PTA), and allowed to dry before TEM observation.20
The Effect of Storage on the Optimum Novasomes
For 90 days, the optimum novasomes formulation was stored at 4°C and 25°C. On the day (0, 45, and 90), samples were drawn from each formulation. The effect of storage was assessed by comparing the initial outcomes with the post storage findings regarding EE%, PS, PDI, and ZP.21 Statistical analysis was computed by Student’s t-test utilizing SPSS® program 22.0. The difference at P ≤ 0.05 was considered significant.
Microbiological Efficacy of TCZ Loaded Novasomes for the Treatment of Candida Albicans
Minimum Inhibitory Concentration (MIC) Assay via XTT Reduction Technique
The MIC value represents the lowest concentrations that completely inhibit the growth of Candida albicans (ATCC 90028). This was determined by a micro-well dilution method. The inoculums were primed and their concentrations were maintained to 106 CFU/mL. The TCZ suspension, unmedicated novasomes prepared without the drug, and optimum novasomes were diluted twofold by DMSO in a ninety six-well plate.22 Every well of the microplate was filled with 40 µL of brain heart infusion (the growth medium), 10 µL inoculum, and 50 µL of diluted formulations. DMSO was used as a negative control. The plates were then incubated at 37°C for 24 h followed by 40 µL of tetrazolium salt addition and then incubated in the dark for an hour at 37°C. Any change in color as a result of XTT reduction was calculated at 492 nm by the use of the microtiter plate reader (Tecan Sunrise absorbance reader, UK). The percentage of inhibition was obtained as follows:
In Vivo Studies
Experimental Animals
Eighty-four male Wistar rats were included for the study design under the approval of the ethical committee of the Faculty of Pharmacy, Cairo University ((PT) 1922) with a weight ranging from 150 to 200 g. The use and handling of animals in all studies complied with the EU directive 2010/63/EU for animal experiment. The animals were kept in cages at a temperature of 22°C and relative humidity of 55%. The animals were put in a dark: light cycle of 12 h each and were provided with standard diet and had free access to water. Rats were left for 7 days for adaptation before experiments. In the rat skin deposition study, 72 rats were included, while 12 rats were used for the histopathology study.
Skin Deposition in Rats
Before conducting the study, the rats were divided randomly into group I (control) while group II, group III, and group IV were subjected to topical application of TCZ suspension, traditional niosomal formulation, and the optimum novasomes, respectively. Each group contained 18 rats. The formulations were applied by the aid of bottle caps which acted as a pool of a surface area equal to 4.15 cm2. The bottle caps were fixed to the dorsal skin of each rat that was previously shaved 24 h before the application of the sample.23 A total of 0.5 mL of each formulation was added non-occlusively into the drug pool. At different intervals (1, 2, 4, 6, 8, and 10 h), 3 rats from each group were subjected to anesthesia with excess inhalation of ether then humanely killed using cervical dislocation procedure. The dorsal rat skin in contact with the formulation was removed and washed for 2 successive times with normal saline (5 mL). The removed skin segments were cut into smaller segments and sonicated for 30 min in 5 mL methyl alcohol. The skin homogenate was then filtered using a filter membrane (0.22 μm pore size) and TCZ concentrations were estimated by HPLC. The amount of TCZ deposited was expressed by AUC0-10 (µg.h). At the end of the experiment, incineration was performed for the destruction of animal carcasses.
TCZ HPLC Assay
The assay of TCZ was carried out using HPLC (LC-20AD, Shimadzu, Japan). Chromatographic separations were done by the Pronto SIL® RP-C18 column (SC-150, Germany). The detector was set at 254 nm, the retention time was 3.6 min, and the temperature was maintained at 25 ± 2.0°C. The mobile phase was composed of (acetonitrile: aqueous solution of disodium hydrogen phosphate (20 mM): triethanolamine) in the ratio of 60:39.8:0.2 (v/v/v), respectively, and the pH was adjusted to 4.0 by the aid of glacial acetic acid and delivered at a flow rate of 1 mL/min.7
Histopathological Examination for Rats
The potential for skin irritation and the alteration in the skin structure as a result of the topical application of optimum novasomes were evaluated using in vivo histopathological study. Twelve male Wistar rats were distributed randomly into three groups each group containing four rats. Group I (control), group II (TCZ suspension), and group III (the optimum novasomes). The formulations in groups II and III were applied topically onto the rat skin three times per day for one week,15 then the animals were killed and the skin was removed and examined based on the steps described by Aziz et al.24
Clinical Study
The clinical study was done in compliance with the Declaration of Helsinki. Following the approval of the research ethics committee for experimental and clinical studies at the Faculty of Pharmacy, Cairo University (reference number = (PT) 1922), the clinical study was conducted on 20 infants suffering from diaper dermatitis seen at the dermatology outpatient clinic of Minia University Hospital. The dermatitis was suggestive of napkin candidiasis which is characterized by intense erythema involving the groin creases and perianal skin in association with satellite papules and pustules. All the infants were examined by one member of the study team. The study protocol was explained to the parents and an informed consent was obtained from those willing to participate.
Initial Assessment
All the infants were subjected to medical history taking, including previous candidal infections, past or present use of antibiotics or steroids, recent diarrhea, and diapering practices. To score the severity of the diaper rash three criteria were used: extent, redness, and pustules. The extent was measured in centimeters with the baby in the frog-leg position (hips maximally flexed and abducted). The length is the maximal diameter in the axis parallel to the spinal cord and the width is the maximal diameter in the axis perpendicular to the length. Redness was graded as: (0) none, (1) mild, or (2) marked. Pustules were graded as: (1) one to five pustules, (2) five to 20 pustules, (3) innumerable pustules, (4) confluent pustules, and (5) bullae or erosions.25
To confirm the diagnosis of candidal napkin dermatitis, superficial scrapings from the skin of all infants were obtained, prepared on a glass slide, and covered with a cover slip. Few drops of 20% KOH were then applied until the area under the lip of the cover is filled, followed by gentle heating of the slide to accelerate the destruction of the cutaneous squamous cells. A light microscope (Accu-Scope # 3025 five-headed (A3025-5), Olympus, Tokyo, Japan) was used to examine the sections. Candida appeared as clusters of oval buds with pseudo and true septate mycelium.
Infants Management and Follow Up
All parents received general advice about diaper rash care; including more frequent diaper changes, gentle cleansing of the buttocks at each change. The infants were divided into two groups, each group included 10 infants. In group A, each infant received optimum novasomes and the parents were instructed to use one formulation (10 mg TCZ) which was divided into two equal portions. Application of this formulation was done onto the affected areas after two diaper changes daily for 10 days. In group B, placebo cream was applied to each infant and the parents were instructed to apply it onto the affected areas after diaper changes in the same pattern as group A (twice daily for 10 days). Parents were asked to bring their children for the clinical reassessment and skin scraping tests 10 days after the treatment has begun.
Results and Discussion
Factorial Design Outcomes
A full factorial design is a good tool to recognize the new drug delivery system variables that could affect its characteristics. The design is advantageous in this concern owing to its ability to analyze the effect of various variables on the properties of a new system.26 In this study, the factors were carefully chosen and the levels were selected depending on initial trials (data not shown) to conclude the possible independent variables arrays. 24 full factorial plan was applied and analyzed statistically. The chosen model was the two-factor interaction (2FI). Adequate precision calculated the signal-to-noise ratio in order to confirm that the model could navigate the design space.27 In all responses, a ratio higher than four was obtained as revealed in Table 3. Furthermore, predicted R2 was measured to afford a better vision into the quality of the model.28,29 Hence, the adjusted and predicted (R2) are favored to be close to each other in order to be in rational agreement30,31 and that was the outcome obtained in all the studied responses.
 |
Table 3 24 Factorial Analysis Outcome of Novasomes Formulations and the Predicted and Observed Responses of the Optimum Formulation (N15) |
The Impact of Formulation Variables on the EE% of TCZ Loaded Novasomes
The capability of fabricated novasomes to entrap high percent of TCZ is a crucial constraint for maximum topical drug delivery. The percentage of TCZ encapsulated within novasomes extended from 77.35 ± 0.35 to 100 ± 0.00% (Table 2). The impact of SAA type, FFA type, SAA: FFA ratio (w/w), and total lipid concentration on the EE% of the novasomes is illustrated as response 3D plots in Figure 1A–C. The resulting equation in terms of coded factors was as follows:
EE% = + 95.42 – 0.36X1 + 0.15X2 + 2.01X3 – 0.68X4 – 2.26X1X2 + 1.91X1X3 − 0.88X1X4 + 1.91X2X3 − 1.13X2X4 + 1.58X3X4
Among all of the studied independent variables, only SAA to FFA ratio (w/w) (X3) had a significant impact on EE% (P = 0.0024). It was found that the EE% value increases upon increasing SAA to FFA ratio from 1:1 to 2:1 which could be attributed to the added emulsification and stabilization impact of the lipid material in the presence of high SAA concentration. This result was in agreement with the results obtained by Abdelbary and Fahmy.32 On the contrary, SAA type (X1), FFA type (X2), and total lipid concentration (X4) did not significantly affect EE% (P = 0.548, 0.799, and 0.256, respectively).
The Impact of Formulation Variables on the PS of TCZ Loaded Novasomes
PS is known as one of the main issues affecting the penetration of drugs in the skin.33 Formulating a system of nanoscale range allows greater penetration through the skin strata.34 PS of the prepared novasomes fluctuated between 334.85 ± 0.78 and 1360.00 ± 28.28 nm as revealed in Table 2. The impact of the type of SAA, type of FFA, SAA: FFA ratio (w/w), and total lipid concentration on the PS of TCZ loaded novasomes is demonstrated as response 3D plots in Figure 1D–F. The resulting equation in terms of coded factors was as follows:
PS = + 711.33 – 8.85X1 – 62.99X2 + 126.84X3 + 205.26X4 + 94.70X1X2 – 12.89X1X3 − 6.19X1X4 − 33.36X2X3 − 27.39X2X4 + 47.69X3X4
Factorial analysis of variance showed that SAA type (X1) showed no significant impact on PS (P = 0.588). The FFA type (X2) had a significant influence on PS (P = 0.0008). Novasomes formulated using stearic acid were larger than those prepared using oleic acid. This could be explained by the variation in the FFA melting point, where stearic acid has a melting point of 69°C while oleic acid has a melting point of 13°C. High FFA melting point resulted in higher melting viscosity and consequently decreased sonication step efficiency in PS reduction.35
On the other hand, the SAA to FFA ratio (w/w) (X3) was found to impact the PS (P < 0.0001) significantly. When the weight ratio of SAA to FFA increased from 1:1 to 2:1, the PS increased significantly. This might be due to the direct association between the PS of the vesicles and the previously mentioned increase in the EE%. The higher amount of TCZ encapsulated within the hydrophobic area of the vesicles might be the reason behind increasing the gap between the bilayers and hence PS increased.36 Furthermore, the increase in PS with increasing Span 60 concentration was also reported by Zaki et al37 working on diacerein-loaded niosomes.
Also, the ANOVA results revealed the synergistic effect of the total lipid concentration (X4) on the PS (P < 0.0001). This could be attributed to the existence of a large amount of film-forming materials relative to the hydration medium with resultant accumulation of multiple layers over each other and thus PS increased. Moreover, the existence of a large amount of lipid components resulted in an increase in the amount of lipid particles in each vesicle providing a linear association with the vesicle scale.38
The Impact of Formulation Variables on the PDI of TCZ Loaded Novasomes
With regard to PDI, a value of 0 indicates a fully mono-dispersed population of particles whereas a value of 1 represents an extremely poly-dispersed population of particles.39 The PDI of all the formulated novasomes formulations varied from 0.26 ± 0.01 to 0.88 ± 0.07 (Table 2). The impact of SAA type, FFA type, SAA: FFA ratio (w/w), and total lipid concentration on the PDI of TCZ loaded novasomes is illustrated graphically as response 3D plots in Figure 2A–C. The resulting equation in terms of coded factors was as follows:
PDI = + 0.56 + 0.01X1 – 0.05X2 + 0.03X3 + 0.10X4 – 0.08X1X2 + 0.01X1X3 + 0.01X1X4 + 0.03X2X3 – 0.02X2X4 – 0.01X3X4
According to the outcomes of the study plan, only the type of FFA (X2) (P = 0.0231) and total lipid concentration (X4) (P < 0.0001) had a significant impact on the PDI. On the contrary, SAA type (X1) and SAA: FFA ratio (w/w) (X3) did not significantly affect PDI (P = 0.562 and 0.0896, respectively). Regarding the FFA type (X2), it was found that oleic acid-forming vesicles displayed significantly lower PDI and good homogeneity relative to stearic acid. This could be explained by the lower PS obtained by oleic acid-forming vesicles. These findings were in agreement with the previous findings of Abd-Elal et al40 working on zolmitriptan-loaded novasomes. With respect to total lipid concentration (X4), it was clear that there was a direct association between lipid concentration and PDI. The increase in lipid concentration led to an increase in PS and thus might have increased heterogeneity of the vesicles.
The Impact of Formulation Variables on the ZP of TCZ Loaded Novasomes
The formulated novasomes showed negative ZP values ranging from −54.10 ± 0.42 to −81.50 ± 0.14 mV as revealed in Table 2, which is considered a sufficient charge to protect them from accumulation and fusion upon storage.41 Because all formulations had (-ve) ZP, the difference in ZP will be discussed in terms of its absolute value in order to prevent misperception.42 The impact of SAA type, FFA type, SAA: FFA ratio (w/w), and total lipid concentration on the ZP of novasomes is illustrated graphically as response 3D plots in Figure 2D–F. The resulting equation in terms of coded factors was as follows:
ZP = - 68.48 – 2.32X1 + 0.78X2 + 1.10X3 – 1.16X4 – 5.83X1X2 – 2.51X1X3 – 1.44X1X4 + 0.43X2X3 + 0.53X2X4 + 1.07X3X4
Based on the outcomes of the study plan, only the type of SAA (X1) had a significant impact on the ZP (P = 0.0046). Oppositely, FFA type (X2), SAA: FFA ratio (w/w) (X3), and total lipid concentration (X4) did not significantly affect ZP of novasomes (P = 0.297, 0.1477, and 0.127, respectively). Concerning the SAA type (X1), Span 80 was found to gain a higher vesicular bilayer charge compared to that obtained by Span 60. This might be attributed to the more hydrophilic nature of Span 60 (HLB 4.7) over Span 80 (HLB 4.3) which might have led to shielding of the (-ve) charge by residing on the surface of the vesicular bilayer contributing to the masking of its charge. Consequently, this has resulted in a significantly lower ZP value.24,43
Optimum Novasomes Formulation Selection
The Design-Expert® software was adopted to analyze the prepared 16 formulations aiming to select the optimum novasomes formulation. The main objective of desirability is to predict the optimum levels for the factors under investigation and to assist in selecting the formulation of choice. The optimum novasomes formulation (accomplishing maximum EE%, the maximum absolute value of ZP, and minimum PS and PDI) was N15 formulation where it possessed an EE% of 99.45 ± 0.78%, PS of 623.00 ± 2.97 nm, PDI of 0.40 ± 0.04, and ZP of −73.85 ± 0.64 mV. To assure the rationality of this process, the predicted and observed responses of N15 were compared and the results are revealed in Table 3. A high match was noted between the observed and predicted outcomes. Thereby, further characterization was carried out on the optimum novasomes (N15).
Characterization of the Optimum TCZ Loaded Novasomes
Elasticity Measurement
The importance of elasticity measurement lies in its ability to explain the high vesicular membrane deformability of novasomes which permits them to squeeze through the skin smaller than their own diameter. This property reduces the risk of vesicle rupture while squeezing.13 DI value of the optimum novasomes (N15) (9.62 ± 0.15 g) was significantly (P < 0.05) higher than that of the traditional niosomal formulation (0.92 ± 0.12 g) which could be explained by the presence of penetration enhancer (FFA) in novasomes bilayers. A penetration enhancer shows different affinity for the curved surface based on its hydrophilic/hydrophobic properties. Hence, it displays different distribution properties within the vesicles. The simultaneous presence of destabilizing penetration enhancer and its tendency to redistribute in the bilayers lead to more elastic vesicles.44 The obtained results confirmed the superiority of novasomes over traditional niosomal formulation owing to their inherent flexibility through the pores of skin.13
Transmission Electron Microscopy (TEM)
TEM was used to describe the morphology of the prepared system.45 As displayed in Figure 3, the TEM micrograph N15 showed spherical non-aggregated vesicles with a smooth surface and narrow size.
 |
Figure 3 Morphological visualization of the optimum novasomes (N15). Abbreviation: N, novasome. |
The Effect of Storage on the Optimum Novasomes
At the end of the experiment, the stored vesicles did not show any clumps or changes in their appearance. Stability analysis showed no obvious alteration (P > 0.05) regarding EE%, PS, PDI, and ZP of the vesicles upon storage compared to the fresh ones as revealed in Table 4. These results indicated good stability of N15 upon storage.
 |
Table 4 Effect of Storage on N15 Physical Properties |
Microbiological Efficacy of TCZ Loaded Novasomes for the Treatment of Candida Albicans
Minimum Inhibitory Concentration (MIC) Assay via XTT Reduction Technique
Candida albicans was selected for the in vitro antifungal test, as it is the prime reason for the superficial and disseminated fungal infections in humans.46 XTT reduction assay offers an advantage over the agar diffusion technique due to its ability to quantify the activity of Candida. It measures the cell activity through the quantified colorimetric estimation of the intracellular formazan compound which is released upon the reduction of XTT.47,48 The in vitro antifungal activity of TCZ suspension, unmedicated N15, and N15 is shown in Figure 4.
The MIC for N15 was lower than that of the unmedicated N15 and TCZ suspension (0.98, 125.00, and 3.90 µg/mL, respectively). The lower the MIC value, the higher the efficacy of the formulation is. Remarkably, the unmedicated formulation revealed a therapeutic potential against fungal infections. This could be due to that oleic acid contains fixed bend C=C bonds. Hence, it occupies a wider cross section when enters into the fungal membrane.49 Higher fungicidal behavior results because of oleic acid enhanced freedom of mobility within the membrane. Therefore, it should be noted that increased oxidative stress arising from the inclusion of polyunsaturated lipids in the membrane will lead to the antifungal action of FFA.50 N15 accomplished four-folds decrease in the MIC in comparison to TCZ suspension which could be attributed to the high discharge and eventual diffusion of TCZ from N15, together with the antifungal action of FFA in comparison with the TCZ suspension.51
In Vivo Studies
Skin Deposition in Rats
The skin deposition test of TCZ from N15 relative to TCZ suspension and traditional niosomal formulation is shown in Figure 5. N15 showed a higher TCZ amount accumulated in the skin through most of the experiment duration in comparison to TCZ suspension and traditional niosomal formulation. The AUC0-10 for N15 was 151.06 µg.h which was approximately 2 folds greater than that of TCZ suspension (75.24 µg.h) and was approximately 1.7 folds higher than that achieved from traditional niosomal formulation (86.40 µg.h). The higher skin deposition of TCZ from N15 might be due to the capability of novasomes, being highly deformable vesicles based on elasticity results, to partition themselves via stratum corneum interstices carrying the entrapped TCZ to deeper skin layers following topical application.52 In addition, since oleic acid is a skin penetration enhancer, the increased permeation of N15 would be directly related to the capacity of oleic acid to create along with stratum corneum lipids a new type of lipid domain which is responsible for the reduced ability of skin barrier function.53 Another speculated theory is that oleic acid reduces the phase transition temperature of the skin lipids resulting in improved freedom of mobility or fluidity of skin lipids and thereby reduces diffusion resistance.54–56
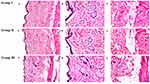
Histopathological Examination for Rats
The investigation of the stained rat skin sections under the light microscope of the three groups; group I: untreated control, group II: treated with TCZ suspension, and group III: treated with N15 is displayed in Figure 6. The untreated group revealed normal skin layers; the epidermis, dermis in addition to the adipose subcutis and muscular layer. With respect to group II and group III, there was neither inflammation nor skin irritation. Normal skin architecture was observed and all the underlying connective tissue was unharmed, indicating the acceptable skin safety profile of N15 for topical use.
Clinical Study
No significant differences were detected between the two groups with respect to the infants’ medical history, personal habits, or their clinical characteristics that could affect the resolution of candidal diaper dermatitis. Skin scrapings revealed Candida albicans in all of the 20 infants. Clinical evaluation of group A (received formulation N15) revealed that 100% of the infants (n = 10) improved in all the three clinical criteria of napkin candidiasis after 10 days of therapy (Figure 7). In addition, skin scrapings became negative for Candida albicans after 10 days of therapy in 100% of infants (n = 10) as demonstrated in Table 5. On the other hand, clinical evaluation of group B (received placebo) revealed that 100% of the infants (n = 10) did not show any improvement in all of the three clinical criteria of napkin candidiasis after 10 days of therapy and the skin scrapings also remained positive for Candida albicans (Figure 7 and Table 5). These results indicate the therapeutic efficacy of formulation N15 for the treatment of Candida albicans infections.
 |
Table 5 Clinical and Mycological Evaluation of Napkin Candidal Dermatitis in 20 Infants Before and After 10 Days of Treatment |
Conclusions
In this study, novasomes were explored as an innovative system for the delivery of TCZ topically. A 24 factorial design was used in selecting the optimum formulation (N15) which possessed small PS, rounded morphology, and high EE%. Microbiological assessment of N15 showed its successful potential against Candida albicans relative to TCZ suspension. Owing to its highly elastic properties, it also displayed superior deposition in the skin as compared to traditional niosomal formulation and TCZ suspension. Additionally, N15 confirmed its dermatological safety when added to the skin. Furthermore, clinical experiments proved the superiority of N15 compared to placebo in providing a complete clinical cure of Candida albicans infections. Concisely, the outcomes concluded that novasomes are promising vesicles for the topical delivery of TCZ for treating skin fungal infections.
Disclosure
No conflicts of interest were reported by the authors.
References
1. Kumar L, Verma S, Bhardwaj A, Vaidya S, Vaidya B. Eradication of superficial fungal infections by conventional and novel approaches: a comprehensive review. Artif Cells Nanomed Biotechnol. 2014;42(1):32–46. doi:10.3109/21691401.2013.769446
2. Sinico C, Fadda AM. Vesicular carriers for dermal drug delivery. Expert Opin Drug Deliv. 2009;6(8):813–825. doi:10.1517/17425240903071029
3. Cevc G, Blume G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim Biophys Acta. 1992;1104(1):226–232. doi:10.1016/0005-2736(92)90154-E
4. Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M. Ethosomes—novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release. 2000;65(3):403–418. doi:10.1016/S0168-3659(99)00222-9
5. Tolman EL, Isaacson DM, Rosenthale ME, et al. Anticandidal activities of terconazole, a broad-spectrum antimycotic. Antimicrob Agents Chemother. 1986;29(6):986–991. doi:10.1128/AAC.29.6.986
6. Cauwenbergh G, Vanden HB. Terconazole. Pharmacology of a new antimycotic agent. J Reprod Med. 1989;34(8 Suppl):588–592.
7. Abdelbary AA, Abd-Elsalam WH, Al-mahallawi AM. Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: in vitro characterization, ex vivo permeation and in vivo safety assessment. Int J Pharm. 2016;513(1–2):688–696. doi:10.1016/j.ijpharm.2016.10.006
8. Abdou EM, Ahmed NM. Terconazole proniosomal gels: effect of different formulation factors, physicochemical and microbiological evaluation. J Pharm Drug Deliv. 2016;5(1):1–6.
9. Abd-Elsalam WH, El-Zahaby SA, Al-Mahallawi AM. Formulation and in vivo assessment of terconazole-loaded polymeric mixed micelles enriched with Cremophor EL as dual functioning mediator for augmenting physical stability and skin delivery. Drug Deliv. 2018;25(1):484–492. doi:10.1080/10717544.2018.1436098
10. Singh A, Malviya R, Sharma PK. Novasome-a breakthrough in pharmaceutical technology a review article. Adv Biol Res. 2011;5(4):184–189.
11. Gregoriadis G. Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol. 1995;13(12):527–537. doi:10.1016/S0167-7799(00)89017-4
12. Chambers MA, Wright DC, Brisker J, et al. A single dose of killed Mycobacterium bovis BCG in a novel class of adjuvant (Novasome) protects guinea pigs from lethal tuberculosis. Vaccine. 2004;22(8):1063–1071. doi:10.1016/j.vaccine.2003.05.002
13. Kakkar S, Kaur IP. Spanlastics—a novel nanovesicular carrier system for ocular delivery. Int J Pharm. 2011;413(1–2):202–210. doi:10.1016/j.ijpharm.2011.04.027
14. Al-mahallawi AM, Khowessah OM, Shoukri RA. Nano-transfersomal ciprofloxacin loaded vesicles for non-invasive trans-tympanic ototopical delivery: in-vitro optimization, ex-vivo permeation studies, and in-vivo assessment. Int J Pharm. 2014;472(1–2):304–314. doi:10.1016/j.ijpharm.2014.06.041
15. Abdelbary AA, AbouGhaly MH. Design and optimization of topical methotrexate loaded niosomes for enhanced management of psoriasis: application of Box–Behnken design, in-vitro evaluation and in-vivo skin deposition study. Int J Pharm. 2015;485(1–2):235–243. doi:10.1016/j.ijpharm.2015.03.020
16. van den Bergh BA, Wertz PW, Junginger HE, Bouwstra JA. Elasticity of vesicles assessed by electron spin resonance, electron microscopy and extrusion measurements. Int J Pharm. 2001;217(1–2):13–24. doi:10.1016/S0378-5173(01)00576-2
17. El Zaafarany GM, Awad GA, Holayel SM, Mortada ND. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int J Pharm. 2010;397(1–2):164–172. doi:10.1016/j.ijpharm.2010.06.034
18. Lei W, Yu C, Lin H, Zhou X. Development of tacrolimus-loaded transfersomes for deeper skin penetration enhancement and therapeutic effect improvement in vivo. Asian J Pharm Sci. 2013;8(6):336–345. doi:10.1016/j.ajps.2013.09.005
19. Gupta PN, Mishra V, Rawat A, et al. Non-invasive vaccine delivery in transfersomes, niosomes and liposomes: a comparative study. Int J Pharm. 2005;293(1–2):73–82. doi:10.1016/j.ijpharm.2004.12.022
20. Basha M, Abd El-Alim SH, Shamma RN, Awad GE. Design and optimization of surfactant-based nanovesicles for ocular delivery of Clotrimazole. J Liposome Res. 2013;23(3):203–210. doi:10.3109/08982104.2013.788025
21. Zeb A, Qureshi OS, Kim HS, Cha JH, Kim HS, Kim JK. Improved skin permeation of methotrexate via nanosized ultradeformable liposomes. Int J Nanomedicine. 2016;11:3813–3824. doi:10.2147/IJN.S109565
22. Tunney MM, Ramage G, Field TR, Moriarty TF, Storey DG. Rapid colorimetric assay for antimicrobial susceptibility testing of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2004;48(5):1879–1881. doi:10.1128/AAC.48.5.1879-1881.2004
23. Shen LN, Zhang YT, Wang Q, Xu L, Feng NP. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. Int J Pharm. 2014;460(1–2):280–288. doi:10.1016/j.ijpharm.2013.11.017
24. Aziz DE, Abdelbary AA, Elassasy AI. Fabrication of novel elastosomes for boosting the transdermal delivery of diacerein: statistical optimization, ex-vivo permeation, in-vivo skin deposition and pharmacokinetic assessment compared to oral formulation. Drug Deliv. 2018;25(1):815–826. doi:10.1080/10717544.2018.1451572
25. Rebora A, Marples RR, Kligman AM. Experimental infection with Candida albicans. Arch Dermatol. 1973;108(1):69–73. doi:10.1001/archderm.1973.01620220041010
26. Araujo J, Gonzalez-Mira E, Egea M, Garcia M, Souto E. Optimization and physicochemical characterization of a triamcinolone acetonide-loaded NLC for ocular antiangiogenic applications. Int J Pharm. 2010;393(1–2):168–176. doi:10.1016/j.ijpharm.2010.03.034
27. De Lima LS, Araujo MDM, Quináia SP, Migliorine DW, Garcia JR. Adsorption modeling of Cr, Cd and Cu on activated carbon of different origins by using fractional factorial design. Chem Eng J. 2011;166(3):881–889. doi:10.1016/j.cej.2010.11.062
28. Chauhan B, Gupta R. Application of statistical experimental design for optimization of alkaline protease production from Bacillus sp. RGR-14. Process Biochem. 2004;39(12):2115–2122. doi:10.1016/j.procbio.2003.11.002
29. Kaushik R, Saran S, Isar J, Saxena R. Statistical optimization of medium components and growth conditions by response surface methodology to enhance lipase production by Aspergillus carneus. J Mol Catal B: Enzym. 2006;40(3–4):121–126. doi:10.1016/j.molcatb.2006.02.019
30. Annadurai G, Ling LY, Lee JF. Statistical optimization of medium components and growth conditions by response surface methodology to enhance phenol degradation by Pseudomonas putida. J Hazard Mater. 2008;151(1):171–178. doi:10.1016/j.jhazmat.2007.05.061
31. Bendas ER, Abdelbary AA. Instantaneous enteric nano-encapsulation of omeprazole: pharmaceutical and pharmacological evaluation. Int J Pharm. 2014;468(1–2):97–104. doi:10.1016/j.ijpharm.2014.04.030
32. Abdelbary G, Fahmy RH. Diazepam-loaded solid lipid nanoparticles: design and characterization. AAPS PharmSciTech. 2009;10(1):211–219. doi:10.1208/s12249-009-9197-2
33. Verma DD, Verma S, Blume G, Fahr A. Particle size of liposomes influences dermal delivery of substances into skin. Int J Pharm. 2003;258(1–2):141–151. doi:10.1016/S0378-5173(03)00183-2
34. Du Plessis J, Ramachandran C, Weiner N, Müller D. The influence of particle size of liposomes on the deposition of drug into skin. Int J Pharm. 1994;103(3):277–282. doi:10.1016/0378-5173(94)90178-3
35. Wong PCH, Heng PWS, Chan LW. Viscosity–temperature relationship of lipid‐based excipients amenable for spray congealing: derivation of a rheological parameter with good correlation to particle size. Eur J Lipid Sci Technol. 2016;118(7):1062–1073. doi:10.1002/ejlt.201500410
36. Hathout RM, Mansour S, Mortada ND, Guinedi AS. Liposomes as an ocular delivery system for acetazolamide: in vitro and in vivo studies. AAPS PharmSciTech. 2007;8(1):E1–E12. doi:10.1208/pt0801001
37. Zaki RM, Ali AA, El Menshawe SF, Bary AA. Formulation and in vitro evaluation of diacerein loaded niosomes. Int J Pharm Pharm Sci. 2014;6(2):515–521.
38. Wang Z, He X. Dynamics of vesicle formation from lipid droplets: mechanism and controllability. J Chem Phys. 2009;130(9):094905. doi:10.1063/1.3079097
39. Zeisig R, Shimada K, Hirota S, Arndt D. Effect of sterical stabilization on macrophage uptake in vitro and on thickness of the fixed aqueous layer of liposomes made from alkylphosphocholines. Biochim Biophys Acta. 1996;1285(2):237–245. doi:10.1016/S0005-2736(96)00167-8
40. Abd-Elal RM, Shamma RN, Rashed HM, Bendas ER. Trans-nasal zolmitriptan novasomes: in-vitro preparation, optimization and in-vivo evaluation of brain targeting efficiency. Drug Deliv. 2016;23(9):3374–3386. doi:10.1080/10717544.2016.1183721
41. Ruckmani K, Sankar V. Formulation and optimization of zidovudine niosomes. AAPS PharmSciTech. 2010;11(3):1119–1127. doi:10.1208/s12249-010-9480-2
42. Abdelbary AA, Li X, El-Nabarawi M, Elassasy A, Jasti B. Effect of fixed aqueous layer thickness of polymeric stabilizers on zeta potential and stability of aripiprazole nanosuspensions. Pharm Dev Technol. 2013;18(3):730–735. doi:10.3109/10837450.2012.727001
43. Wilson B, Samanta MK, Santhi K, Kumar KPS, Paramakrishnan N, Suresh B. Poly (n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer’s disease. Brain Res. 2008;1200:159–168. doi:10.1016/j.brainres.2008.01.039
44. Bsieso EA, Nasr M, Moftah NH, Sammour OA, Abd El Gawad NA. Could nanovesicles containing a penetration enhancer clinically improve the therapeutic outcome in skin fungal diseases? Nanomedicine. 2015;10(13):2017–2031. doi:10.2217/nnm.15.49
45. Al-mahallawi AM, Abdelbary AA, Aburahma MH. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int J Pharm. 2015;485(1–2):329–340. doi:10.1016/j.ijpharm.2015.03.033
46. Bondaryk M, Kurzątkowski W, Staniszewska M. Antifungal agents commonly used in the superficial and mucosal candidiasis treatment: mode of action and resistance development. Postepy Dermatol Alergol. 2013;30(5):293–301. doi:10.5114/pdia.2013.38358
47. Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods. 1991;142(2):257–265. doi:10.1016/0022-1759(91)90114-U
48. Jahn B, Martin E, Stueben A, Bhakdi S. Susceptibility testing of Candida albicans and Aspergillus species by a simple microtiter menadione-augmented 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide assay. J Clin Microbiol. 1995;33(3):661–667. doi:10.1128/JCM.33.3.661-667.1995
49. Avis TJ, Bélanger RR. Specificity and mode of action of the antifungal fatty acid cis-9-heptadecenoic acid produced by pseudozyma flocculosa. Appl Environ Microbiol. 2001;67(2):956–960. doi:10.1128/AEM.67.2.956-960.2001
50. Thibane VS, Kock JL, Ells R, Van Wyk PW, Pohl CH. Effect of marine polyunsaturated fatty acids on biofilm formation of Candida albicans and Candida dubliniensis. Mar Drugs. 2010;8(10):2597–2604. doi:10.3390/md8102597
51. Shahin M, Hady SA, Hammad M, Mortada N. Novel jojoba oil-based emulsion gel formulations for clotrimazole delivery. AAPS PharmSciTech. 2011;12(1):239–247. doi:10.1208/s12249-011-9583-4
52. Peira E, Trotta M, Carlotti ME, Gallarate M, Chirio D. Elastic positively-charged liposomes for topical administration of acyclovir. J Drug Deliv Sci Technol. 2007;17(5):321–324. doi:10.1016/S1773-2247(07)50049-3
53. Tanojo H, Bos-van Geest A, Bouwstra JA, Junginger HE, Boodé HE. In vitro human skin barrier perturbation by oleic acid: thermal analysis and freeze fracture electron microscopy studies. Thermochim Acta. 1997;293(1–2):77–85. doi:10.1016/S0040-6031(97)00063-4
54. Ongpipattanakul B, Burnette RR, Potts RO, Francoeur ML. Evidence that oleic acid exists in a separate phase within stratum corneum lipids. Pharm Res. 1991;8(3):350–354. doi:10.1023/A:1015845632280
55. Naik A, Pechtold LA, Potts RO, Guy RH. Mechanism of oleic acid-induced skin penetration enhancement in vivo in humans. J Control Release. 1995;37(3):299–306. doi:10.1016/0168-3659(95)00088-7
56. Walker RB, Smith EW. The role of percutaneous penetration enhancers. Adv Drug Deliv Rev. 1996;18(3):295–301. doi:10.1016/0169-409X(95)00078-L
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.