Back to Journals » Drug Design, Development and Therapy » Volume 13
Use of lidocaine to prevent postoperative coughing after partial laryngectomy: comparison of three delivery methods
Authors Wang Y , Lu WS, Qiao H , Zhao J, Fan Q
Received 13 January 2019
Accepted for publication 3 April 2019
Published 27 May 2019 Volume 2019:13 Pages 1835—1841
DOI https://doi.org/10.2147/DDDT.S201416
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Yuezhi Wang,1,* Wei S Lu,2,* Hui Qiao,2 Jian Zhao,3 Qing Fan2
1Department of Gerontology, Huashan Hospital of Fudan University, Shanghai 200040, People’s Republic of China; 2Department of Anesthesiology, The Eye, Ear, Nose and Throat Hospital of Fudan University, Shanghai 200031, People’s Republic of China; 3Department of Gerontology, Shanghai Sixth People’s Hospital Jinshan Branch, Shanghai, 201500, People’s Republic of China
*These authors contributed equally to this work
Objective: To compare the effectiveness of lidocaine administration (intravenous injection, dripping via the tracheostomy tube, and spraying into the tracheostomy incision) on postoperative coughing after partial laryngectomy.
Patients and methods: A total of 115 male patients with laryngeal carcinoma scheduled for partial laryngectomy under general anesthesia were randomized into three groups. In group I (n=35), 2% lidocaine hydrochloride (1.5 mg/kg) was slowly infused intravenously. In group II (n=40), 2% lidocaine hydrochloride (1.5 mg/kg) was dripped into the tracheostomy tube upon completion of surgery. In group III (n=40), 7% lidocaine aerosol (5 sprays, 22.5mg) was sprayed into the tracheostomy incision before tracheostomy tube placement. We recorded incidences of coughing, incisional bleeding, and hemodynamic changes when leaving the postanesthesia care unit (T1), and 6 hrs (T2) and 24 hrs (T3) after surgery.
Results: The coughing scores and incisional bleeding scores were significantly lower in group II and III than that in group I at T1, T2, and T3. Group II and III had significantly lower heart rate than group I at T1 and T2. Compared with group I, mean arterial pressure decreased significantly in group II (T1 and T2) and group III (T1 and T3).
Conclusion: In patients undergoing partial laryngectomy, spraying 7% lidocaine aerosol into the tracheostomy incision before placing the tracheostomy tube or instilling 2% lidocaine hydrochloride into the tracheostomy tube upon completion of surgery effectively prevented postoperative coughing, which reduced the risk of bleeding from the incision and thus facilitates postoperative rehabilitation.
Keywords: lidocaine, laryngeal carcinoma, partial laryngectomy, postoperative bucking
Introduction
Laryngeal cancer is the most common malignant tumors of the head and neck in the ENT hospital. Over 13,000 new laryngeal cancer cases occur in the United States with prevalence of 3.1 per 100,000, 40% of which is locally advanced and 95% is squamous cell cancer. The partial laryngectomy (PLE) is an essential technique in modern laryngeal organ preservation surgery. There are various types of PLE depending on the anatomical structures that have to be removed, including cordectomy, frontolateral laryngectomy, hemilaryngectomy, supracricoid partial laryngectomy. A balloon-type silica gel tracheostomy tube (TOT) must be placed in the tracheostomy incision following PLE to maintain ventilation. Because the airway is controlled by rich innervation from the vagus nerve, TOT placement can cause severe airway responsiveness, which is seen as severe coughing and increased airway secretions.1–3 Bucking causes not only increased blood pressure and heart rate but also leads to sudden increases in jugular arterial/venous pressure, which can increase incisional bleeding. Incisional bleeding and increased airway secretions further aggravate bucking. No consensus and recommended therapeutic option exist for postoperative coughing and bucking after PLE.
Intravenous injection of lidocaine,4–6 dripping of lidocaine into the endotracheal tube,7 or spraying lidocaine into the airway can effectively prevent airway responsiveness caused by the insertion and withdrawal of the tracheal tube;8,9 however, it is unclear which method is more effective in alleviating bucking and incisional bleeding following PLE. Therefore, we evaluated one of three methods (intravenously injecting 2% lidocaine, instilling 2% lidocaine into the TOT, and spraying 7% lidocaine into the tracheostomy incision) in patients after PLE and observed the incidences of bucking and incisional bleeding to determine the safest, most effective, and simplest method to prevent bucking after PLE to alleviate patient discomfort and to reduce postoperative complications.
Patients and methods
This study was registered with the China Clinical Research Information Service (Registration number, ChiCTR-IOR-14005407) and conformed to the tenets of the Declaration of Helsinki. After obtaining approval from Ethics Committee of Eye, Ear, Nose, and Throat Hospital affiliated with Fudan University (No. KY 2014–046) and written informed consent from patients, we enrolled 120 adult male patients who were 35–73 years of age, American Society of Anesthesiologists (ASA) physical status I and II, and undergoing elective PLE (Oncology classification: cT1NM–cT3NM) between Nov 2014 and July 2017 (prospective and randomized controlled trial). The exclusion criteria included uncontrolled diseases related to cardiovascular and respiratory system, uncontrolled diabetes, abnormalities in coagulation function and allergy history of local anesthetics. Patients’ body weights ranged from 48 to 89 kg, and no patients had heart, lung, or coagulation function abnormalities. There was also no history of tracheostomy, chronic tracheal inflammation, asthma, throat radiation, intraoperative laryngeal topical anesthesia or hypersensitivity to lidocaine in any patient.
After the patients entered the operating room, electrocardiograms, non-invasive blood pressure, and pulse oxygen saturation were routinely monitored, and intravenous access in the upper extremity was established.
Sufentanil (3 μg/kg), propofol (2–3 mg/kg), and rocuronium bromide (0.6 mg/kg) were given intravenously for anesthesia induction, followed by orotracheal intubation. Maintenance of anesthesia: The concentration of end-expiratory sevoflurane was maintained at 1–1.2 minimum alveolar concentration and the infusion rate of remifentanil was 0.05–0.15 μg/kg/min, dose adjusted according to blood pressure and heart rate. During intraoperative mechanical ventilation, end-tidal carbon dioxide pressure was maintained at 35–45 mmHg. The surgical procedures were performed by three attending surgeons who had been subspecially trained in head and neck surgery for more than five years, performed PLE for more than 20 cases and used a similar stepwise technique. Tracheostomy was the first surgical procedure, and after the oral endotracheal tube was removed, another endotracheal tube was inserted into the tracheostomy incision. The total intraoperative dose of sufentanil was 5–6 μg/kg. Upon completion of surgery, oxycodone 0.1 mg/kg was immediately injected intravenously, followed by intravenous patient-controlled analgesia. Background infusion of sufentanil at a dose of 0.03–0.04 μg/kg/h and dexmedetomidine at a dose of 0.04 μg/kg/h were also administered, along with self-controlled analgesia with sufentanil (2 μg) plus dexmedetomidine (2 μg). The patients were randomized into three groups postoperatively with a random number table: group I, 2% lidocaine hydrochloride was slowly infused intravenously at a dose of 1.5 mg/kg immediately after surgery; group II, 2% lidocaine hydrochloride was dripped into the TOT at a dose of 1.5 mg/kg upon completion of surgery; and group III, 7% lidocaine aerosol was sprayed into the tracheostomy incision before TOT placement (5 sprays, 4.5 mg/spray, total dosage: 22.5 mg). The balloon pressure of endotracheal TOT was 60 cmH2O and the balloon would be evacuated for 30 min each 4 h after 12 h. The nasogastric feeding tube was used in all patients.
Nurse anesthetists who were blinded to the grouping conditions recorded the severities of bucking and incisional bleeding when patients left the postanesthesia care unit (PACU) (T1), 6 hrs after surgery (T2), and 24 hrs after surgery (T3). Bucking was scored after 5 mins of observation as follows: 0, no bucking; 1, mild bucking (1–2 times); 2, moderate bucking (3–4 times); and 3, severe bucking (≥5 times).10 The degree of incisional bleeding was recorded as follows: a grid was drawn on the gauze pad under the TOT to divide the pad into 9 squares: 0, no blood or almost no blood on the pad; 1, only 1 square contained blood; and 9, all 9 squares contained blood. Based on the blood volume in the drainage ball of the negative pressure drain, we also recorded the number of patients who required a change in the gauze pad because of excessive bleeding (score =9). Hemodynamic changes at T1, T2, and T3, as well as the use of vasoactive drugs (nicardipine, atropine, ephedrine, and esmolol), were also recorded. When blood pressure increased by 30% compared with baseline, nicardipine (0.5 mg/administration) was injected intravenously; if blood pressure decreased by 30% compared with baseline, ephedrine (6 mg/administration) was injected intravenously. If heart rate was <50 beats/min, atropine (0.3 mg/administration) was injected intravenously; if heart rate was >100 beats/min, esmolol (20–40 mg/administration) was injected intravenously. Recovery time (duration from the end of surgery to eye-opening upon calling), Ramsay sedation score when leaving the PACU and visual analog scale (VAS) pain scores were also recorded.
Hilgers and colleagues reported that 64% of patients experienced bucking symptoms after total laryngectomy.11 Considering this percentage, power analyses indicated that an estimated sample of at least 34 patients would be required to detect a significant difference between the three groups with α =0.05 and power =0.8 accounting for expected standard deviations. Forty patients per group were enrolled considering potential loss of protocol violation.
Statistical analyses were performed using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). Age, body weight, duration of surgery, recovery time, Ramsay score, and VAS pain score were analyzed using one-way analysis of variance, and inter-group comparisons were evaluated using Dunnett’s multiple comparison tests (compared with group I). The bucking score was analyzed using the chi-square test. Mean arterial pressure (MAP), heart rate, and incisional bleeding score were analyzed using two-way analysis of variance after repeated measures, and results at the same time point were compared using post hoc Bonferroni test. A P-value <0.05 was considered statistically significant.
Results
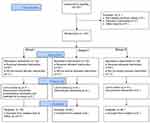
Of the 120 PLE patients enrolled in this study, four patients from group I required further interventions because of severe bucking and asking for quit from the study; one patient in group I underwent a second surgery because of excessive incisional bleeding 3 hrs after surgery. Data from these five patients did not enter the final analysis. A total of 115 patients completed the study (Figure 1).
Patients’ general conditions, smoking rate, cTNM classification and surgical times were not significantly different among the three groups (Table 1).
 | Table 1 Patients’ characteristics |
Coughing scores were significantly lower in groups II and III compared with group I at T1 (P<0.0001), T2 (P=0.002), and T3 (P=0.0011), and lowest in group III (Table 2). Groups II and III had significantly lower incisional bleeding scores than did group I at all three time points: at T1, the mean incisional bleeding score was 2.2 in group I, 1.4 in group II (P<0.05), and 0.9 in group III (P<0.001); at T2, the mean incisional bleeding score was 4.5 in group I, 2.5 in group II (P<0.001), and 2.1 in group III (P<0.001); and at T3, the mean incisional bleeding score was 7.4 in group I, 4.7 in group II (P<0.001), and 4.6 in group III (P<0.001) (Table 2).
 | Table 2 Changes in coughing and bleeding score at different postoperative time points |
Heart rate was significantly lower in group II (74 bpm and 73 bpm) and group III (75 bpm and 75 bpm) than that in group I (82 bpm and 80 bpm) at T1 and T2 respectively (P<0.01). At T3, the mean heart rate was lower in both group II and group III, but the difference was not statistically significant (P>0.05). At T1, MAP was 106 mmHg in group I, 102 mmHg in group II (P<0.01), and 101 mmHg in group III (P<0.001); at T2, MAP was 102 mmHg in group I, 98 mmHg in group II (P<0.01), and 100 mmHg in group III (P>0.001); and at T3, MAP was 97 mmHg in group I, 94 mmHg in group II (P>0.05), and 93 mmHg in group III (P<0.001). The patients in group I had the highest nicardipine use rate (37%) and the lowest ephedrine use rate (0%) in the PACU.
Recovery time, time in PACU and VAS pain score were not significantly different among the three groups. The Ramsay sedation score was 1.8 in group I, which was significantly lower than that in group II (2.1) (P<0.05) and group III (2.3) (P<0.05). The VAS pain score when leaving the PACU was 3.1, 2.9, and 2.9 in group, II, and III, respectively (P>0.05). No obvious lidocaine-associated complications were seen 24h after surgery in any group (Table 3).
 | Table 3 Recovery variables and adverse events |
Discussion
In this study, we compared the impacts of three delivery methods (in group I, 2% lidocaine hydrochloride was infused intravenously at a dose of 1.5 mg/kg; in group II, 2% lidocaine hydrochloride was dripped through the TOT at a dose of 1.5 mg/kg; and in group III, 7% lidocaine aerosol was sprayed into the tracheostomy incision with 5 sprays of 4.5 mg/spray) on bucking, incisional bleeding, heart rate, and blood pressure after PLE. We found that the incidence of bucking significantly decreased in groups II and III, and, in particular, group III received a smaller dose of lidocaine (22.5 mg) with better effectiveness.
Most laryngeal cancer patients are male, and surgery for laryngeal cancer can be classified as partial or total laryngectomy based on the range of tumor involvement. However, in this study, we performed only PLE to avoid bias caused by two different surgical procedures. Upon completion of PLE surgery, a balloon-type silica gel TOT is placed in the tracheostomy incision to maintain ventilation; the tracheostomy incision is closed after a laryngeal cavity forms. Because the airway is richly innervated and controlled by the vagus nerve, TOT placement can cause severe airway responsiveness, seen as severe bucking and increased airway secretions. Severe bucking can lead to circulatory and respiratory adverse events and difficulty in healing the tracheostomy fistula;12 severe bucking can also increase intracranial and intraocular pressures.13 Soltani et al reported that intravenous administration of lidocaine effectively reduced airway responsiveness.4–6 Yamasaki et al found that instilling lidocaine into the tracheal tube effectively reduced airway responsiveness during emergence from anesthesia and tracheal extubation,7 and Dreher et al found that spraying lidocaine aerosol under fiberoptic bronchoscopy was more effective in preventing cough than lidocaine instillation.8 In our current study, the bucking score was significantly better in groups II and III than in group I at all three time points (T1, T2, and T3), possibly because intravenously-injected lidocaine has a shorter duration in suppressing the cough center and therefore is less effective for laryngeal carcinoma patients in whom the TOT has been in place for a long period of time. In group II, after the lidocaine was dripped, the liquid flowed into the respiratory tract at the lower edge of the tube; however, lidocaine could also block the airway mucosa by diffusion where the tube contacted the mucosa. In group III, the 7% lidocaine aerosol sprayed via the tracheostomy incision acted directly on the airway mucosa where the TOT balloon contacted the mucosa, and although the dose was low (22.5 mg), the bucking score was the lowest in this group. Good local anesthesia of airway could reduce the secretions. A fewer cough could prevent high blood pressure which decreased the bleeding of incision so that lesser blood flew to the airway. Both of these could cut down the coughing in the airways. We excluded patients who had previously undergone tracheostomy and who had received a TOT because these patients were more tolerant of the tube, and would have a lower postoperative bucking incidence.
Severe bucking can further aggravate incisional bleeding for the following reasons: first, bucking activates the circulatory system and increases arterial blood pressure; second, severe bucking leads to increased intrathoracic pressure and jugular venous pressure; and finally, incomplete ligation of the branches of the superior laryngeal artery and superior thyroid artery during PLE can easily cause postoperative incisional bleeding.14 In our study, one patient returned to the operating room to undergo a hemostatic procedure for excessive incisional bleeding because of incomplete ligation of the superior laryngeal artery. Blood flowing into the lower respiratory tract increases the incidence of bucking. In our study, incisional bleeding scores were significantly lower in groups II and III than in group I at all three time points (T1, T2, and T3), possibly because of the relatively lower bucking scores and MAP in groups II and III.
Previous studies suggested that coughing on emergence could result in hypertension, tachycardia, and tachyarrhythmias.9 In our study, group I had a higher bucking score than groups II and III, which also explained higher MAP and heart rate in group I; also, group I had the highest postoperative nicardipine use rate in the PACU (37%) and the lowest ephedrine use rate (0%). The decreased airway responsiveness, along with the sedative effect of dexmedetomidine, prolonged the recovery time (14.0 min in group II and 14.3 min in group III, vs 12.8 min in group I) and increased the Ramsay sedation score when leaving the PACU (2.1 in group II and 2.3 in group III, vs 1.8 in group I).
In our study, we also considered that cough suppression might not be conducive to the drainage of airway secretions and might lead to respiratory complications; however, no lidocaine-associated adverse reactions such as accumulation of respiratory secretions, respiratory depression, asthma, or respiratory infections were seen during follow-up.
There are several limitations in our study. First, we did not include a control group (injection of normal saline upon completion of surgery) because of severe and persistent bucking, which is morally unacceptable. Second, there is no internationally recognized scoring system for bucking. Third, the same surgery performed by different surgeons might have different effectiveness, which might affect the research outcomes. Fourth, we hadn’t collected aspirated/swallowed/expectorated bleeding quantity, because these data couldn’t be calculated exactly and which was positive correlation with oozing on the gaze. Finally, the sample size was small.
Conclusion
In patients undergoing PLE, spraying 7% lidocaine aerosol into the tracheostomy incision (5 sprays, 4.5 mg/spray) before placing the TOT, or instilling 2% lidocaine hydrochloride into the TOT at a dose of 1.5 mg/kg upon completion of surgery effectively prevents postoperative bucking. It is recommended that using either of these methods alone may reduce the risk of incisional bleeding and thus facilitate postoperative rehabilitation. Notably, the tracheostomy incision method requires a lower dose.
Data Sharing Statement
Data will not be shared because a further study based on the present data will be subsequently carried out.
Author contributions
Qing Fan conceived of the study and participated in its design and coordination. Yuezhi Wang and Hui Qiao conducted the study and helped to revise the study protocol. Weisha Lu and Jian Zhao collected the data and performed the statistical analysis. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Karlsson JA, Sant’ Ambrogio G, Widdicombe J. Afferent neural pathways in cough and reflex bronchoconstriction. J Appl Physiol. 1985;65:1007–1023. doi:10.1152/jappl.1988.65.3.1007
2. Sant’ Ambrogio G. Afferent pathways for the cough reflex. Bull Eur Physiopathol Respir. 1987;23:19–23.
3. Widdicombe JG. Sensory neurophysiology of the cough reflex. J Allergy Clin Immunol. 1996;98:S84–90.
4. Sanikop C, Bhat S. Efficacy of intravenous lidocaine in prevention of post extubation laryngospasm in children undergoing cleft palate surgeries. Indian J Anaesth. 2010;54:132–136. doi:10.4103/0019-5049.63654
5. Soltani HA, Aghadavoudi O. The effect of different lidocaine application methods on postoperative cough and sore throat. J Clin Anesth. 2002;14:15–18.
6. Zamora Lozano J, Cruz Villasenor JA, Rodriguez Reyes J. Comparison of topical, intravenous, and intracuff lidocaine for reducing coughing after extubation during emergence from general anesthesia. Rev Esp Anestesiol Reanim. 2007;54:596–601.
7. Yamasaki H, Takahashi K, Yamamoto S. Efficacy of endotracheal lidocaine administration with continuous infusion of remifentanil for attenuating tube-induced coughing during emergence from total intravenous anesthesia. J Anesth. 2013;27:822–826. doi:10.1007/s00540-013-1627-3
8. Dreher M, Comelissen CG, Reddemann MA. Nebulized versus standard lidocaine during flexible bronchoscopy: A randomized controlled trial. Respiration. 2016;92:266–273. doi:10.1159/000449135
9. Minogue SC, Ralph J, Lampa MJ. Laryngotracheal topicalization with lidocaine before intubation decreases the incidence of coughing on emergence from general anesthesia. Anesth Analg. 2004;99:1253–1257. doi:10.1213/01.ANE.0000132779.27085.52
10. Guler G, Akin A, Tosun Z. Single-dose dexmedetomidine attenuates airway and circulatory reflexes during extubation. Acta Anaesthesiol Scand. 2005;49:1088–1091. doi:10.1111/j.1399-6576.2005.00780.x
11. Hilgers FJ, Ackerstaff AH, Aaronson NK. Physical and psychosocial consequences of total laryngectomy. Clin Otolaryngol Allied Sci. 1990;15:421–425.
12. DE Virgilio A, Simonelli M, Greco A. Tracheocutaneous fistula in patients undergoing supracricoid partial laryngectomy: the role of chronic aspiration. Acta Otorhinolaryngol Ital. 2015;35:9–14.
13. Stone DJ, Gal TJ. Airway management. In: Miller RD, editor. Anesthesia.
14. Fagan C, Frizelle HP, Laffey J. Effects of intracuff lidocaine on endotracheal-tube-induced emergence phenomena after general anesthesia. Anesth Analg. 2000;91:201–205.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.