Back to Journals » Cancer Management and Research » Volume 14
Use of Honey in the Management of Chemotherapy-Associated Oral Mucositis in Paediatric Patients
Authors Zhang L, Yin Y , Simons A, Francisco NM , Wen F, Patil S
Received 28 March 2022
Accepted for publication 6 July 2022
Published 19 September 2022 Volume 2022:14 Pages 2773—2783
DOI https://doi.org/10.2147/CMAR.S367472
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Video abstract of "Honey used in the management of Chemotherapy-induced oral mucositis" [ID 367472].
Views: 725
Luyang Zhang,1 Yan Yin,1 Alison Simons,2 Ngiambudulu M Francisco,3 Feiqiu Wen,1 Sandip Patil1,4
1Division of Hematology and Oncology, Shenzhen Children’s Hospital, Shenzhen, People’s Republic of China; 2Post Qualifying Practice Department, Birmingham City University, Birmingham, UK; 3Grupo de Investigação Microbiana e Imunológica, Instituto Nacional de Investigação em Saúde (National Institute for Health Research), Luanda, 3635, Angola; 4Pediatric Research Institute, Shenzhen Children’s Hospital, Shenzhen, People’s Republic of China
Correspondence: Sandip Patil, Division of Haematology and Oncology, Shenzhen Children’s Hospital, 7019 Yitian Road, Futian District, Shenzhen, People’s Republic of China, Tel +86-18813934471, Fax +86-755-83008283, Email [email protected]
Background: Oral mucositis (OM) is a critical condition during chemotherapy in both adult and child cancer patients. Paediatric cancer patients have a higher prevalence of OM than adult cancer patients. Honey is a natural product that has been reported to have the best tissue healing properties. The present mini-review focused on the evaluation of the effectiveness of oral care with honey products in the treatment and prophylaxis of chemotherapy-induced OM in child patients.
Methods: A network of electronic English databases including CINAHL, CENTRAL, EMBASE, MEDLINE and PubMed, were used for primary search from April 2010 to April 2020. We have also considered data collected from ClinicalTrials.gov, Web of Science and Google Scholar. PRISMA software was used to build collective data. Controlled trials were included in this review and were critically appraised by Down and Black. The narrative synthesis was performed.
Results: A total number of 346 data of children and adolescents with cancer were considered in this short review. All patients were from three randomized controlled trial articles and two were non-randomised controlled trial articles. Based on the evidence so far revealed, honey may show an effect in the treatment and prophylaxis of OM. The analysis of collected data revealed that the probability value P< 0.05. The honey enhanced recovery time and severity of OM were significantly compared with those without honey treatment receiving group of pediatric patients.
Conclusion: Honey not only has been shown to have the capability for healing injured tissues but it is also a more economical treatment, and it has fewer side effects compared to synthetic drugs. Honey or honey products can prevent chemotherapy-induced OM (CIOM) and be the best treatment to grade I, II and III CIOM. However, it is disappointing that studies involving children as patients were few, and limited data available so far.
Keywords: oral mucositis, honey, pediatric patients, chemotherapy
Introduction
Cancer is among the leading causes of death globally, accounting for nearly 10 million deaths in the year 2020, or nearly one in six deaths (Ref. WHO, 2022). Globally, nearly 300,000 children aged 0–17 are diagnosed with cancer every year.1 Chemotherapy has been reported to be efficient for such conditions.2 One of the potential limitations for chemotherapy drugs is that they do not only act on malignant cells but also on normal cells,3 which may lead to oral mucositis (OM), especially in paediatric patients. In severe cases, OM development can increase mortality by almost 40%.4 The OM is an acute inflammation of the oral mucosa with haemorrhage, erythema and edema.5 The development of such inflammatory conditions is higher (up to 45–80%) in paediatric cancer patients compared to adult cancer patients.4 Moreover, children have differed from adults in relation to compliance, acceptance, and reaction to various preventive agents6 due to their compromised immune system. These conditions are dose-limiting, and high-cost effectiveness, which may result in undermining the ideal cancer treatment plan for patients, and ultimately decreasing the chances of survival.7 Keeping in mind, the facts management of OM in children is particularly critical. OM conditions are treated with some mucosal coating agents, analgesia, and cryotherapy in adult patients; nevertheless, paediatric patients have a limited choice.8 Nevertheless, cryotherapy was previously indicated to be performed only on older and cooperative pediatric patients.9 Honey has attracted the attention of medical care in the past decades because of its tissue healing, antibacterial and antioxidant properties,10 and has been shown to be an acceptable traditional medicine (Natural Product) globally.11 Based on the present knowledge, honey is efficiently used to treat chemotherapy-induced OM.12 Honey comprises ~200 substances, including water and carbohydrates, along with other vitamins and enzymes.13,14 Previous studies have indicated that honey enhances tissue healing (conditions: wound, burn, surgical site and ulcer) by stimulating monocytes, which helps to release cytokines.15,16 The inclusion of honey or its products could be optional for chemotherapy-induced OM in cancer patients.17 Several studies reported that conventional honey, including natural honey and commercially available marketed honey, could be effective only for chemotherapy-induced OM but also on radiation- and chemoradiation-induced OM.13,17–19 In contrast, although evidences have demonstrated that Manuka honey has healing properties, four published studies reported on Manuka honey have shown negative results on prophylaxis and treatment of OM.18–21 The reasons for this negative effect remain uncertain, and it may be because that Manuka honey contains unusually high content of methylglyoxal, which is considered a cytotoxic agent.22 Moreover, the intervention for OM requires high compliance; however, the peculiar taste of Manuka honey, which is bitter taste and lower water content, could be likely the reason for a high dropout rate (57.4%) in the trial conducted by Hawley et al.20 Given the lack of intervention for prophylaxis and treatment of OM in children, this report highlights proven evidences of honey in paediatric care to prevent and treat chemotherapy-induced OM. Summarizing pieces of evidence for paediatric clinical practice and the use of honey may open avenues for further studies in this field.
Methodology
In this study, we selected published studies based on oral care with honey or honey products in the treatment and prophylaxis of chemotherapy-induced OM in child patients. We included randomized controlled trials (RCTs) and non-randomized controlled studies (NRSs) of honey or honey products that treat or prevent chemotherapy-induced OM in paediatric patients.
Databases
For searching English literature, we used the following keywords: honey, stomatitis, oral mucositis, oral ulcer, child, paediatric, pediatric, adolescent, and chemotherapy. We used the CINAHL, CENTRAL, EMBASE, and MEDLINE PubMed as our healthcare search databases from April 2010 to April 2020. Data were analyzed using GraphPad PRISMA-8 California, USA. Thus, the present review included a 27-item checklist, which was performed to assess the quality of included studies, which were described earlier.23 The checklist was divided into five sections: reporting, external validity, internal validity-bias, internal validity-confounding (selection bias), and power. The score ranges of Downs and Black were given the following corresponding quality levels: excellent (26–28); good (20–25); fair (15–19); and poor (14).
Study Characteristics
Population
The population was defined as children and adolescents with cancer aged 1–17 years with chemotherapy-induced OM or during the phase of chemotherapy treatment. Interventions: Honey-made products as therapy interventions include natural honey, commercially available marketed honey, and honey ice cube, all these product types were included in this review, whereby, honey was applied between the 7th day and the 14th day after the initiation of chemotherapy treatment when OM peaked or developed. For prophylaxis therapy, honey was applied before the start of chemotherapy and the development of OM.
Outcome Measures
The primary outcomes were the recovery time and the severity of OM. Various types of outcome measurement can cause clinical heterogeneity. Thus, only studies using National Cancer Institute – Common Toxicity Criteria (NCI-CTC) Table S1 and scales developed by the World Health Organization (WHO) were included and the details of the scales were presented in Table S2.24
Inclusion Criteria
The present report includes studies comparing the effect of honey, routine mouth care, no treatment, or any other treatment for the prophylaxis and treatment of OM. Data (published scientific evidences) of all children and adolescents with cancer aged 1–17 years with chemotherapy-induced OM or during the phase of chemotherapy treatment, were included in this review. Studies were selected by whether they meet the inclusion criteria for the studies outlined as follows: RCTs and NRSs that investigated the effectiveness of honey products for patients with chemotherapy-induced OM in preventative or curative groups compared with control groups were included. All studies were in English language, full-text and released before April 2020.
Exclusion Criteria
Patients who were aged less than 1 year and over 17 years, had no capacity for oral feeding, were allergic to honey, with evidence of confirmed co-infection, and were diagnosed with diabetes were excluded. Details of exclusion criteria are shown in Table 1. In addition, studies that used Manuka honey as an intervention were excluded due to its taste and high content of methylglyoxal.22
 |
Table 1 Detailed Exclusion Criteria for the Present Study |
Data Extraction and Processing
Data were extracted from a database that met the objectives of the study and presented in a Microsoft Excel format Table S3. The key extracted data is based on basic information, study characteristics, study design, participants, intervention, and outcomes. The mini-review was performed to integrate each sample to review the effects of treatment compared to the other, especially in RCTs; a large number of patients allow to be included, and thus the smaller, but clinically significant differences may be identified.22 In addition, compare to the short review, we have performed the narrative synthesis is essentially more subjective. Therefore, to avoid potential bias, the Centre for Reviews and Dissemination (CRD) (2009) suggested that the method applied should be transparent and rigorous.25 However, there was heterogeneity within and between studies, including not only the methodology but also the cancer type, OM cause, control arm, assessment scales, and study design. Therefore, data synthesis as part of this study adopted narrative synthesis that would be more appropriate to describe the results. A framework for narrative synthesis is the developing a preliminary synthesis of the findings of included studies; the researcher brought extracted data together and organised and described the findings. Exploring relationships within and between studies – the relationship between characteristics and findings of individual studies, and the findings of different studies were explored, and assessing the robustness of the synthesis – it was the end of the data synthesis process, and the related analysis resulted in a comprehensive assessment of the quality of the evidence.
Interventions and Comparison
All experimental groups in the studies used honey as an intervention, two studies used natural honey,26,27 two studies used commercial honey,12,28 and one study used honey and tulsi in ice cubes as an intervention.28 Tulsi is a herb that has robust evidences supporting its anti-cancer, anti-inflammatory, and anti-stress effects and can protect the body’s DNA against radiation. Except for one study that divided patients into three groups, all studies divided patients into experiment groups and control groups.27 The ice cubes study did not mention the application of routine mouth care,29 all experimental groups in the other three studies received routine mouth care along with honey,26–28 and instead of performing routine mouth care, one study performed the routine practice of analgesic and antiseptic gel application along with honey.12 The control groups in three studies performed the same protocols as the experiment groups but did not use honey.12,26,28 The other two studies performed Benzocaine 7.5% gel27 and plain ice cubes29 as comparisons. Except for the ice cube study that used honey 5 minutes before receiving the MTX treatment,29 all studies performed the honey more than three times a day.12,26–28 The experiment group in two studies received 0.5–1g honey/kg,26,27 one study received 1–2mL each time,12 and the rest of the two studies did not mention the dose of honey.28,29
Outcomes
All studies used OM scales to assess the grade of OM and evaluated the effectiveness of honey in treatment and prophylaxis from different angles. It is difficult to conclude due to numerous variables being involved. In terms of recovery time, four studies reported it, which is defined as the number of days from the beginning of treatment until all ulcers have healed completely.12,26–28 Among them, instead of reporting the recovery time, one study reported the duration of hospitalization.28 In terms of the severity of OM, Bulut and Tüfekci assessed the severity of OM before each session of chemotherapy and on the 1st, 4th, 8th, 12th, 16th, and 21st days after chemotherapy.26 Mishra and Nayak focused on the occurrence of OM from the initiation of MTX administration.29 Thus, the severity of OM was assessed on the 5th and 15th days of the administration of ice cubes. In the study of Al Jaouni et al, OM was assessed before and after chemotherapy, as well as a week after the initiation of chemotherapy.28 Singh et al evaluated OM every other day from the first day of enrollment until OM had healed completely.12
Quality of Included Studies
All included studies were in the fair range based on the Down and black (1998)23; the scores for Singh et al and Abdulrhman et al were 16 and 18,12,27 and another 3 studies were 17.26,28,29 Table S4, presents the detail of the quality assessment of each included study. All studies failed to describe how patients were selected, it is not possible to determine whether the chosen participants were representative of their population12,27,29 Bulut and Tüfekci and Abdulrhman et al included almost all source populations that met the inclusion criteria26,27; Mishra and Nayak recruited almost all participants who were receiving MTX chemotherapy due to few numbers of children with MTX chemotherapy in the Hematology and oncology department.29 Therefore, it cannot be determined whether the participants of the above three studies are representative or not because this appears to be a convenience sampling. Only two studies12,26 blinded the people who assessed the outcomes of the intervention to avoid personal bias.30 Moreover, all studies failed to blind participants to the intervention they received which may have an impact on the reliability of their results.12,26–29 In addition, only three studies in this review were RCTs, and the participants were randomized into groups.27–29 However, none of the studies mentioned whether randomized intervention assignments were concealed from health-care staff and patients.
Effects of Interventions
All included studies confirmed the effectiveness of honey in the prophylaxis and treatment of OM among child patients with chemotherapy.12,26–29 The actual results will be presented by narrative synthesis due to the existing clinical and methodological heterogeneity that was explained in the Methodology section. P-value is a statistical approach that was used for measuring the effect of honey application in this mini-review. The characteristics of the experimental and control groups of each study are presented in Table 2. The overall data on recovery time and the OM status can be seen in Table 3.
 |
Table 2 Detailed Characteristics of Participants Included in the Present Studies (Experimental and Control Groups) |
 |
Table 3 Characteristics of Recovery Time and the Severity of OM |
Results
A total of 51 original studies were recorded from the electronic databases by performing PRISM-8 (Figure 1). Among which 8 results were obtained from CENTRAL, 9 from MEDLINE, 12 from CINAHL, 12 results from EMBASE and 10 from Web of Science. After the analysis of each article, 16 studies that included adults or non-cancer patients, and used propolis as intervention, were excluded due to the inconsistency with the predefined inclusion criteria for this short review. Furthermore, 7 studies were excluded due to the following reasons: three studies investigated the effectiveness of radiation-reduced OM, one piece of grey literature did not report results, two studies were published in the symposium without sufficient information and one study has no full-text access S5.
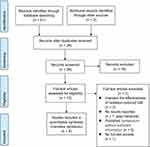 |
Figure 1 The study selection process is presented as PRISMA flow diagram. Notes: Adapted from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71. Creative Commons Attribution (CC BY 4.0) license (https://creativecommons.org/licenses/by/4.0/legalcode).33 |
Characteristics of the Participants
A total of 51 studies were included, which was published so far. The detailed data are present in Table 2. All participants did not suffer from OM in the phase of prophylaxis; participants in the treatment phase had varying grades of chemotherapy-induced OM that are present in Table 2. Instead of only reporting chemotherapy, two studies reported the specific chemotherapy drugs that patients were undergoing, which was methotrexate (MTX). Moreover, one study enrolled participants who were treated with chemo/radiotherapy; the limitation of this study was that they failed to report the ratio of chemotherapy and radiotherapy.
The Recovery Time
Three studies assessed the effectiveness of honey by investigating the recovery time. One study focused on the duration of hospitalisation for OM patients.28 In the study of Abdulrahman et al, in grade II OM, the recovery time was 3.6±0.8 days in the honey group, and 4.6±0.9 days in the control group, which is a statistical significance between the honey group and control group (P=0.0017).27 In grade III OM, the recovery time between the honey and control groups, which were 5.4±1.11 days in the honey group, and 8.6±1.0 days in the control group, differ significantly (P=0.0001). It shows significantly faster healing in the honey group compared to the control group. Combining grades II and III, the recovery time was 4.25±1.25 days in the honey group and 6.20±2.47 days in the control group, which shows honey can produce faster healing compared with the control group (P=0.0005; with a statistical power of 96.2%). Bulut and Tüfekci reported that the recovery duration in the honey group was 4.869±4.341 days before OM developed and 14.857±2.905 days after OM developed, which was shorter than the control group was 19.282±1.805 days.26 The recovery duration differs significantly between the two groups (p=0.000). Al Jaouni et al compared the duration of hospitalization between the honey group and the control group.28 The duration of hospitalization for OM children was significantly reduced in the honey group (mean 7±3 days) as compared to the control group (mean 13±5 days) (p<0.001). The study by Singh et al (2019) compared the duration of OM between the honey group and the control group. The median duration of OM in the honey group was 4 days (IQR: 4–6 days) and was 6d (IQR: 6–8 days) in the control group. All the above findings show significantly faster healing in the honey group (p<0.01).
The Severity of OM
Four of the five included studies assessed the effectiveness of honey by investigating the severity of OM.12,26,28,29 One study set two honey groups.26 The OM degree decreased gradually in the honey group (patients with grade III and V OM) after the fourth follow-up day (p<0.001). The severity of OM was higher in the honey group compared to the control group on day 4 (3.58±0.47 vs 1.79±1.08, p=0.000); however, the OM degree was noticeably lower on day 21 (0.14±0.36 vs 1.76±1.03, p= 0.000). And, 92.9% of the children achieved full recovery and 7.1% at a mild level in the honey group. In the control group, only 5.1% of the children obtained full recovery and the rate of mild-level OM was 71.8%; the remaining 23.1% of the children experienced severe OM. However, none of the children developed severe OM in the honey group after the eighth follow-up day. Recovery status differs significantly between the two groups (p<0.01), which indicates honey can reduce the severity of OM. Meanwhile, the severity of OM was significantly lower in the other honey group (patients without OM and before chemotherapy was initiated) compared to the control group (0.43±0.58 vs 1.79±1.08, p=0.000) on day 4 and day 21 (0.17±0.38 vs 1.76±1.03, p= 0.000). The OM grade increased gradually on the 4th, 8th, and 12th days and slightly decreased on the 16th and 21st days in the control group, and a significant difference in OM degree on the different follow-up days (p<0.001). Also, 82.6% of the children achieved full recovery and 17.4% at a mild level in the honey group before OM occurred. In the control group, only 5.1% of the children obtained full recovery and the rate of mild-level OM was 71.8%; the remaining 23.1% of the children experienced severe OM. However, none of the children developed severe OM in the honey group. Recovery status differed significantly between the two groups (p<0.01).
Al Jaouni et al recruited participants before OM was developed.28 The prophylaxis and treatment were not investigated separately, and participants were receiving honey before OM occurred, and when the children experienced OM with grades III and V. Compared with the control group, the incidence of grade III and V OM was significantly reduced in the honey group (20% in honey versus 55% in control; P=0.02). Mishra and Nayak enrolled participants before OM occurred.29 The incidence of OM was considerably lower in the experimental group (honey and tulsi ice cubes) as compared to the control group (plain ice cubes). Forty percent of the children experienced OM (mild-moderate) in the experimental group and 90% (65% mild-moderate OM and 25% severe OM) in the control group on day 5 (p<0.001). As well as the assessment on the 15th day showed that all OM was at mild-moderate grade, and the incidence of OM was 15% in the experimental group and 80% in the control group (p<0.001). The severity of OM was significantly reduced in the experimental group compared to the control group (0.4±0.50 vs 1.75±0.96, p=0.001) on day 5 and day 15 (0.15±0.36 vs 1.1±0.71, p= 0.001). The severity of OM was significantly reduced on follow-up days in the honey group compared to the control group (p<0.01), and 12% of the children achieve a recovery on the third day in the experiment group. The rate of the grade 0 differs significantly between both the groups, which was 50% on the 5th day and 92% on the 7th day in the honey group, and 8% on the 5th day and 54% on the 7th day in the control group (p<0.01). All children recovered from OM on day 9 in the honey group and on day 13 in the control group.
Discussion
We focused on the effect of oral care treatment with honey products in the treatment of chemotherapy-induced OM in paediatric patients. Based on the evidence reported in our study, honey products have a beneficial effect on the treatment and prophylaxis of OM. Honey significantly decreased the OM grade and provided faster healing for OM in included studies. Honey can treat grade I, II, and III chemotherapy-induced OM, and prevent patients from developing severe chemotherapy-induced OM in children. To the best of our knowledge, no data was reported on the disadvantages of conventional honey in OM patients except for Manuka honey, which failed to show positive results. Our report represents the recovery time of OM and the severity of OM was different from lab to lab or hospital to hospital. However, in terms of treatment, the recovery time of OM was reported in four studies and showed statistical significance between the honey group and the control group.12,26–28 Concerning grades of OM, very limited data is available. The present report reflects that honey treatment in grade V OM may not be significant compared to grade I, II, and III OM.12,26–28 However, the severity of such conditions may decrease during chemotherapy.12,26–29 So far co-infection has not been considered by any researcher except Al Jaouni et al and the results of this study showed a statistically significant reduction of bacterial and fungal infections among paediatric cancer patients undergoing chemo/radiotherapy who are receiving honey as an intervention for OM.28 Honey + Tulsi (ratio not reported) showed the best effect as compared to cryotherapy.29
The potential limitation of our present search is the limited number of data, while no data are available on chemotherapy drugs used in recovery. Moreover, yet to explain the effectiveness of honey products in the prevention and treatment of OM, authors accept scientific flora carry out further investigations that are needed. Honey as a more economical treatment enables it to be the intervention of choice for OM.31 Honey shows a significant effect without sex disparity.32 Globally, studies have proven that radiotherapy, chemotherapy, or a combination of both can cause OM.22
Honey reduced the recovery time and the stage of OM, which made it an effective intervention in the prevention and treatment of OM in paediatric oncology patients. Furthermore, not only has been shown to have the capability for healing injured tissues but it is also a more economical treatment, and it has fewer side effects compared to synthetic drugs.
Conclusion
In conclusion, this study suggests that honey must be included as one of the treatments or prevention of choice for the grade I, II, and III chemotherapy-induced OM. However, further studies on the treatment and prevention of chemotherapy-induced OM in children are needed due to the different pathomechanism between radiotherapy and chemotherapy and the characteristics of OM in children healing faster than in adults.
Data Sharing Statement
All data files mentioned in this manuscript are available.
Ethics Approval and Consent to Participate
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee, Shenzhen Children’s Hospital, Reference number: 2018 (013) on dated 2018/09/03.
Informed Consent Statement
Due to the retrospective nature of the study, the Ethics Committee of Shenzhen Children’s Hospital, Shenzhen determined that patients’ consent was not required. Data were kept confidentially and in compliance with the Declaration of Helsinki.
Acknowledgments
We would like to thank Prof. Liu from the Department of Hematology and Oncology, Shenzhen Children’s Hospital and Samantha Toland from Birmingham City University for their constant support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and also agree to be accountable for all aspects of the work.
Funding
This work was supported by Shenzhen Fund for Guangdong Provincial High-Level Clinical Key Specialties (No. SZGSP012) and Shenzhen Key Medical Discipline Construction Fund (No. SZXK034).
Disclosure
The authors declare no conflicts of interest in relation to this work
References
1. Steliarova-Foucher E, Colombet M, Ries L, et al. International incidence of childhood cancer, 2001-10: a population-based registry study. Lancet Oncol. 2017;18(6):719–731. doi:10.1016/S1470-2045(17)30186-9
2. Bartucci M, Dattilo R, Martinetti D, et al. Prevention of chemotherapy-induced anemia and thrombocytopenia by constant administration of stem cell factor. Clin Cancer Res. 2011;17(19):6185–6191. doi:10.1158/1078-0432.CCR-11-1232
3. Hendrawati S, Nurhidayah I, Mediani HS, Mardhiyah A. The incidence of mucositis in children with chemotherapy treatment. J Nursing Care. 2019;2(1):23–31. doi:10.24198/jnc.v2i1.20129
4. Miller MM, Donald DV, Hagemann TM. Prevention and treatment of oral mucositis in children with cancer. J Pediatr Pharmacol Ther. 2012;17(4):340–350. doi:10.5863/1551-6776-17.4.340
5. Isabella R, Rebecca L, Ricardo DDC, Paulo FB, Ana V. Oral mucositis in pediatric patients in treatment for acute lymphoblastic leukemia. Int J Env Res Pub He. 2017;14(12):1468. doi:10.3390/ijerph14121468
6. Akram FQ, Sumant G, Tamas R, Richard ML, Dorothy K. Prevention of oral mucositis in children receiving cancer therapy: a systematic review and evidence-based analysis. Oral Oncol. 2013;49(2):102–107. doi:10.1016/j.oraloncology.2012.08.008
7. Peterson DE, Srivastava R, Lalla RV. Oral mucosal injury in oncology patients: perspectives on maturation of a field. Oral Dis. 2015;21(2):133–141. doi:10.1111/odi.12167
8. Friend A, Rubagumya F, Cartledge P. Global health journal club: is honey effective as a treatment for chemotherapy-induced mucositis in paediatric oncology patients? J Trop Pediatrics. 2018;64(2):162–168. doi:10.1093/tropej/fmx092
9. Patel P, Robinson PD, Baggott C, et al. Clinical practice guideline for the prevention of oral and oropharyngeal mucositis in pediatric cancer and hematopoietic stem cell transplant patients: 2021 update. Eur J Cancer. 2021;154:92–101. doi:10.1016/j.ejca.2021.05.013
10. Nur O. Complementary therapies in the management of induced oral mucositis during cancer treatment. J Educ Res Nursing. 2017;14(4):304–311.
11. Marcela B, Lucia J, Valeria J, et al. Antibacterial activity of different blossom honeys: new findings. Molecules. 2019;24(8):1573. doi:10.3390/molecules24081573
12. Singh R, Sharma S, Kaur S, Medhi B, Trehan A, Bijarania SK. Effectiveness of topical application of honey on oral mucosa of children for the management of oral mucositis associated with chemotherapy. Indian J Pediatr. 2019;86(3):224–228. doi:10.1007/s12098-018-2733-x
13. Liu T, Luo Y, Tam K, Lin C, Huang T. Prophylactic and therapeutic effects of honey on radiochemotherapy-induced mucositis: a meta-analysis of randomized controlled trials. Support Care Cancer. 2019;27(7):2361–2370. doi:10.1007/s00520-019-04722-3
14. Eteraf-Oskouei T, Najafi M. Traditional and modern uses of natural honey in human diseases: a review. Iran J Basic Med Sci. 2013;16(6):731.
15. Bergman A, Yanai J, Weiss J, Bell D, David MP. Acceleration of wound healing by topical application of honey: an animal model. Am J Surgery. 1983;145(3):374–376. doi:10.1016/0002-9610(83)90204-0
16. Van der Weyden EA. The use of honey for the treatment of two patients with pressure ulcers. Br J Community Nurs. 2003;8(12):S14–S20. doi:10.12968/bjcn.2003.8.Sup6.12553
17. Wardill HR, Bowen JM, Gibson RJ. New pharmacotherapy options for chemotherapy-induced alimentary mucositis. Expert Opin Biol Th. 2014;14(3):347–354. doi:10.1517/14712598.2014.874412
18. Bardy J, Molassiotis A, Ryder WD, et al. A double-blind, placebo-controlled, randomised trial of active manuka honey and standard oral care for radiation-induced oral mucositis. Br J Oral Maxillofac Surg. 2012;50(3):221–226. doi:10.1016/j.bjoms.2011.03.005
19. Munstedt K, Momm F, Hubner J. Honey in the management of side effects of radiotherapy- or radio/chemotherapy-induced oral mucositis. A systematic review. Complement Ther Clin Pract. 2019;34:145–152. doi:10.1016/j.ctcp.2018.11.016
20. Hawley P, Hovan A, Mcgahan CE, Saunders D. A randomized placebo-controlled trial of manuka honey for radiation-induced oral mucositis. Support Care Cancer. 2014;22(3):751–761. doi:10.1007/s00520-013-2031-0
21. Emma P, Aubrey B, Patries H. Manuka honey mouthwash does not affect oral mucositis in head and neck cancer patients in New Zealand. J Radiother Pract. 2012;11(4):249–256. doi:10.1017/S1460396911000410
22. Karsten M, Heidrun M, Lesław J. Using bee products for the prevention and treatment of oral mucositis induced by cancer treatment. Molecules. 2019;24(17):3023. doi:10.3390/molecules24173023
23. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Commun H. 1998;52(6):377–384. doi:10.1136/jech.52.6.377
24. Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer Am Cancer Soc. 2004;100(9 Suppl):1995–2025.
25. Centre for Reviews and Dissemination. Systematic Review: CRD’s Guidance for Undertaking Reviews in Health Care. Centre for Reviews and Dissemination, University of York; 2009.
26. Kobya BH, Guducu TF. Honey prevents oral mucositis in children undergoing chemotherapy: a quasi-experimental study with a control group. Complement Ther Med. 2016;29:132–140. doi:10.1016/j.ctim.2016.09.018
27. Abdulrhman M, Elbarbary NS, Ahmed AD, Saeid ER. Honey and a mixture of honey, beeswax, and olive oil-propolis extract in treatment of chemotherapy-induced oral mucositis: a randomized controlled pilot study. Pediatr Hemat Oncol. 2012;29(3):285–292. doi:10.3109/08880018.2012.669026
28. Al JSK, Al MMS, Hussein A, et al. Effects of honey on oral mucositis among pediatric cancer patients undergoing chemo/radiotherapy treatment at King Abdulaziz University Hospital in Jeddah, Kingdom of Saudi Arabia. Evid Based Complement Alternat Med. 2017;2017:1–7.
29. Mishra L, Nayak G. Effect of flavoured (honey and tulsi) ice chips in reduction of oral mucositis among children receiving chemotherapy. Int J Pharm Sci Rev Res. 2017;43(107):25–28.
30. Hróbjartsson A, Thomsen ASS, Emanuelsson F, et al. Observer bias in randomized clinical trials with time-to-event outcomes: systematic review of trials with both blinded and non-blinded outcome assessors. Int J Epidemiol. 2014;43(3):937–948. doi:10.1093/ije/dyt270
31. Ravleen N, Deepa JP, Supreet J, Shashikant S. Natural agents in the management of oral mucositis in cancer patients-systematic review. J Oral Biol Craniofacial Res. 2017;8(3):245–254. doi:10.1016/j.jobcr.2017.12.003
32. Yusof HM, Manan MA, Sarbon NM, et al. Gender differences on the effects of honey and black seed mixture supplementation. J Sustainability Sci Management. 2017;1(3):119–134.
33. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
