Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 12
Use of formulations for sensitive skin improves the visible signs of aging, including wrinkle size and elasticity
Authors Spada F , Lui AH, Barnes TM
Received 14 April 2019
Accepted for publication 15 May 2019
Published 6 June 2019 Volume 2019:12 Pages 415—425
DOI https://doi.org/10.2147/CCID.S212240
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Fabrizio Spada, Ada H Lui, Tanya M Barnes
Research & Development, Ego Pharmaceuticals, Braeside, Victoria 3195, Australia
Background: Sensitive skin affects an increasingly large proportion of the population and is less tolerant to frequent and prolonged use of cosmetics. This study investigates the antiaging effects of a skin care system developed for use on sensitive skin.
Methods: A total of 30 healthy Caucasian females, aged 32–72, were enrolled in this double-blind randomized placebo-controlled split-face study. A routine consisting of twice daily topical applications of the test cleanser and test moisturizer or placebo or positive control products was followed for 28 days, with parameters measured at baseline and at 7-day intervals. Objective skin assessments for hydration, transepidermal water loss (TEWL), skin surface topography, elasticity and safety assessment were conducted.
Results: Wrinkle surface, length and depth significantly improved by 34.8±4.7% (P<0.001), 19.0±3.2% (P<0.05) and 24.3±3.5% (P<0.05), respectively, after 28 days of skin care treatment with the test cleanser and test moisturizer. R2 (gross elasticity), R5 (net elasticity) and R7 (biological elasticity) significantly increased by 32.8±6.5% (P<0.001), 47.3±8.6% (P<0.001) and 50.6±5.1% (P<0.001), respectively, while R6 (viscoelastic portion) significantly decreased by 33.4±4.6% (P<0.001) after 28 days. Skin hydration was also found to increase significantly after 28 days by 42.2±8.5% (P<0.01), but there was no change in TEWL. No adverse events were reported.
Conclusions: A novel skin care routine developed for use on sensitive skin significantly improves the signs of aging including hydration, wrinkle size and elasticity without significant adverse effects.
Keywords: hydration, elasticity, wrinkles, nicotinamide, avenanthramides, vitamin E
Introduction
Skin aging is the result of both intrinsic aging due to the passage of time and extrinsic aging as a consequence of environmental damage, primarily due to ultraviolet (UV) radiation.1 Chronologically aged skin exhibits fine wrinkles, is thin, relatively flattened, dry and unblemished with some loss of elasticity.2 It exhibits a general atrophy of the extracellular matrix, which is reflected by a decrease in the number of fibroblasts.2 Furthermore, there are reduced levels of collagen and elastin, and their organization is impaired due to the decreased protein synthesis of types I and III collagen in the dermis and an increased breakdown of extracellular matrix proteins.3
Aging is accelerated in skin exposed to UV radiation, a process known as photoaging.2 The fine wrinkles and reduced elasticity which characterize intrinsically aged skin are exaggerated in photoaged skin, with the development of deep wrinkles and a marked loss of elasticity, dryness, roughness and dyspigmentation as well as an increased epidermal thickness and alterations in connective tissue organization.2,4,5 Impairment of collagen and elastin organization is typically more severe in photoaged skin compared with chronologically aged skin.2
During the last few decades, people have become increasingly concerned about their appearance, with facial skin aging being an important component.6 This has led to an ever increasing number of over-the-counter antiaging products, many of which have undergone little to no testing of their efficacy.6 Simultaneously, with the increased opportunity to try new products, there has also been a rise in the number of consumers who perceive their skin as being sensitive,7 with approximately 50% of women and 40% of men having self-diagnosed sensitive skin.8,9
Sensitive skin is characterized by subjective complaints of discomfort without visible signs of irritation, which may be triggered by hypersensitivity to a range of stimuli which may be physical, chemical, psychological, or hormonal.8–10 Symptoms of sensitive skin include itching, stinging, burning and tightness. Subjects experiencing this condition report exaggerated reactions when their skin is in contact with cosmetics, soaps and other substances, and they often report a worsening of the condition after exposure to dry and cold climate.8–10 Impaired skin barrier function has been suggested to underlie sensitive skin; however, despite extensive research, no consensus on its definition and pathomechanisms has been reached.10
We have previously reported that a gel (test moisturizer) containing a combination of moisturizing and anti-irritant ingredients, which is used to relieve the sensations of sensitive facial skin, significantly induces collagen types I and III in cultured human foreskin fibroblasts.11 The test moisturizer is therefore a candidate to improve the signs of skin aging since collagen has been shown to improve skin appearance and epidermal barrier function.
Therefore, the aim of this study is to further investigate the antiaging effects of a novel skin care system (test cleanser and test moisturizer) developed for use on sensitive skin in healthy Caucasian women. Specifically, the biomechanical parameters of wrinkle surface, wrinkle length and wrinkle depth will be determined by photonumerical grading of the crow’s feet area at baseline and after 14 and 28 days following the skin care routine. Skin elasticity parameters R2 (gross elasticity), R5 (net elasticity), R6 (viscoelastic portion) and R7 (biological elasticity) of facial skin will be measured by cutometer at baseline and after 7, 14, 21 and 28 days. Facial skin hydration and transepidermal water loss (TEWL) will also be measured at these time points by corneometer and tewameter, respectively.
Materials and methods
Subjects
A total of 30 healthy female volunteers aged over 30 years were studied after giving written informed consent. Eligible subjects were: Caucasian females aged greater than 30 years; not taking medication or under the care of a doctor for a period of one month prior to, and throughout the entire test period; free of any dermatologica1 or systemic disorder that would interfere with test results and were available for the study duration. Exclusion criteria were: individuals under a doctor’s care; taking medication which could mask or interfere with test results; history of sensitivity to cosmetics in general and moisturizers in particular; any form of skin cancer or any other disease that could interfere with test results; diagnosed with chronic skin allergies; excessive hair on test sites; known hypersensitivity to cosmetic products; pregnant or nursing an infant.
Fifteen subjects were randomized to either Group 1 or Group 2 (Figure 1). In order to precondition test sites and keep all topical treatments constant for all subjects, subjects were required to abstain from using deodorant, soaps or cosmetic moisturizers on the test area for a period of 10 days prior to commencement and during the course of the study. At the completion of the 10-day period, subjects returned to the test facility where they were trained how to apply the skin care products.
 | Figure 1 CONSORT flow chart illustrating the flow of subjects through the study. |
Topical products
The test products were QV Face Rescue Gel (referred to as test moisturizer; Ego Pharmaceuticals Pty Ltd, Braeside, Victoria, Australia) containing glycerin, Hamamelis virginiana (witch hazel) water, disodium lauriminodipropionate tocopheryl phosphates, paraffinum liquidum, niacinamide, Avena sativa (oat) kernel extract, sodium pyrrolidone carboxylic acid (PCA), panthenol and menthol. QV Face Exfoliating Polish (referred to as test cleanser; Ego Pharmaceuticals Pty Ltd, Braeside, Victoria, Australia) containing glycerin, paraffinum liquidum, niacinamide, Carthamus tinctorius (safflower) seed oil, sodium PCA, Avena sativa (oat) kernel extract, panthenol, tocopherol, cellulose acetate, polylactic acid (and) ultramarines.
The negative control (placebo) products were QV Face Rescue Gel and QV Face Exfoliating Polish without the skin active ingredients.
The positive control products were Elucent Anti-aging Night Moisturiser (Ego Pharmaceuticals Pty Ltd, Braeside, Victoria, Australia) containing paraffinum liquidum, lactic acid, glycolic acid, niacinamide, panthenol, tocopherol, dimethicone, glycerine and Avena sativa (oat) kernel extract. Elucent Anti-aging Gentle Cleanser (Ego Pharmaceuticals Pty Ltd, Braeside, Victoria, Australia) containing glycerin, glycolic acid, lactic acid, panthenol, tocopherol, niacinamide and Avena sativa (oat) kernel extract.
Study protocol
The study design was approved by the Independent Ethics Committee of Dermatest Pty Ltd. (Study number: M08181/M08182; Rockdale, NSW, Australia) which operates in accordance with the International Conference on Harmonization (ICH) Guidelines for Good Clinical Practice.
Subjects followed a twice daily skin care routine for 28 days as outlined in Figure 1. The face was divided down the midline and Group 1 applied the placebo cleanser and placebo moisturizer to the left side of their face and the test cleanser and test moisturizer to the right side of their face. Group 2 applied the placebo cleanser and placebo moisturizer to the left side of their face and the positive control cleanser and positive control moisturizer12 to the right side of their face. Each morning the face was dampened with warm water, cleanser liberally applied to half the face including around the eye area and gently massaged, after which the skin was rinsed thoroughly and pat dry. This was repeated on the opposing side with care taken to rinse hands between product applications. A moisturizer was then liberally applied to half the face including around the eye area which was again repeated on the opposing side. This process was repeated at night. Any adverse events that were observed or reported during the entire study period were documented by the investigator.
Skin hydration, TEWL and skin elasticity parameters were measured at 0, 7, 14, 21 and 28 days, while skin surface topography parameters including wrinkle surface, wrinkle length and wrinkle depth were measured at 0, 14 and 28 days (Figure 1).
Corneometry
Hydration or water content of the epidermis (stratum corneum) was determined with a noninvasive, in vivo skin capacitance meter (Corneometer Model CM 825, Courage + Khazaka electronic GmbH, Köln, Germany) down to a depth of approximately 10–20 μm. Before each set of measurements, subjects were required to equilibrate in a closed environment with a constant temperature (20 ºC±2 ºC) and humidity (45–55% RH). Skin hydration was measured at 5 different points on the facial skin, expressed in arbitrary units.13
Transepidermal water loss
Evaluation of the water barrier function of the skin was determined with a noninvasive, in vivo tewameter (Tewameter Model TM 210, Courage + Khazaka electronic GmbH, Köln, Germany). This device uses an “open chamber” method and can detect even the slightest damage in the skin water barrier at an early stage. Before each set of measurements, subjects were required to equilibrate in a closed environment as described above. TEWL was measured at 5 different points on the facial skin, expressed in g/h/m2.14
Skin surface topography
High resolution, standardized, full face photography was performed using the VisioFace RD with inbuilt analysis software (Courage + Khazaka electronic GmbH, Köln, Germany). To ensure the reproducibility and quality of the photographs, the 3-dimensional orientation of the subjects’ faces within the photo booth were aligned with chin and head rests as well as the overlay/masking function and physical markers available in the system software. Photographs were taken using the integrated Reflex Canon 550D (18 Mpixels) camera with lighting provided by 200 white LED lights. The system software pilots the photo shooting system and color calibrates the images. Photographs were taken in relaxed conditions and the VisioFace RD analysis software was used to evaluate mean wrinkle surface in mm2, mean wrinkle length in mm and mean wrinkle depth in μm.15
Skin elasticity
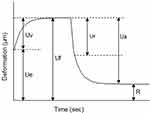
A noninvasive, in vivo skin elasticity meter (Cutometer SEM 575, Courage + Khazaka electronic GmbH, Köln, Germany) was used to measure skin firmness and elasticity of the crow’s feet area based on the suction method. The resistance of skin drawn into the probe by negative pressure (firmness) and its ability to return to its original position (elasticity) are displayed as curves which can be used to calculate the elastic and viscoelastic properties of the skin. This has been previously described in detail.12,16 Before each set of measurements, subjects were required to equilibrate in a closed environment as described above. The time/strain mode was used with a 5-sec application of a constant negative pressure of 400 mbar, followed by a 3-sec relaxation period, with one repetition. Measurements were taken at four different closely adjoining points on the face.
A typical skin deformation curve is illustrated in Figure 2. The following parameters were analyzed: R2 (Ua/Uf), gross elasticity of the skin, including viscous deformation; R5 (Ur/Ue), net elasticity of the skin without viscous deformation; R6 (Uv/Ue), the ratio of viscoelastic to elastic distension and R7 (Ur/Uf), biological elasticity, that is, the ratio of immediate retraction to total distension.
Statistical analyses
Statistical analyses were conducted using Sigmaplot version 14 (Systat Software Inc., San Jose, CA USA). To compare parameters tested over time and across the three cleansers/moisturizers tested, the percentage change from baseline for individual values of different parameters was determined by the following equation: Percentage change=[(a–b)/b]×100, where a is the individual value of hydration, TEWL, wrinkle surface, wrinkle length, wrinkle depth, R2, R5, R6 or R7 at each individual time point and b is the corresponding zero time value. A two-way ANOVA was performed and the Holm–Sidak post hoc analyses were conducted. P-values of <0.05 were considered statistically significant. For clarity, placebo results from both Group 1 and Group 2 were pooled as there was no significant difference between these two groups.
Results
Skin hydration and TEWL
Thirty subjects were enrolled in this double-blind randomized placebo-controlled split-face study. Fifteen subjects each were randomized to Group 1 (aged 50.8±2.8 years, range 32–72 years) or Group 2 (aged 47.9±2.6 years, range 32–64 years). All participants completed the study and no adverse events were observed.
The mean percentage change in hydration scores for the placebo, positive control and test cleanser/moisturizer products measured over 28 days are presented in Figure 3. The placebo cleanser/moisturizer products did not cause any significant change in skin hydration over the study period. Application of either the positive control or test cleanser/moisturizer products resulted in a significant increase of approximately 40% in skin hydration over 7 (P<0.01) and 14 days (P<0.01) respectively, compared to the placebo, which was maintained for the remainder of the study period. There was no difference observed between the test cleanser/moisturizer and positive control products.
The mean percentage change in TEWL for the placebo, positive control and test cleanser/moisturizer products determined over 28 days are presented in Figure 4. There was no significant change in TEWL found for any of the products over the study period, nor was there any difference detected between the products. This result is consistent with the fact that the test subjects did not have compromised skin.
Skin surface topography
Figure 5 shows the mean percentage change in wrinkle surface, wrinkle length and wrinkle depth for the placebo, positive control and test cleanser/moisturizer products measured over 14 and 28 days. Although there was a trend that the wrinkle size parameters were reduced following use of the test and positive control cleanser/moisturizer products for 14 days, these values were not found to be statistically significant. However, following use of the test cleanser/moisturizer products for 28 days wrinkle surface was significantly reduced by 34.8±4.7% (P<0.001), wrinkle length was significantly reduced by 19.0±3.2% (P<0.05) and wrinkle depth was significantly reduced by 24.3±3.5% (P<0.05) compared to the placebo cleanser/moisturizer. The placebo cleanser/moisturizer products did not cause any significant change in the wrinkle size parameters over the study period, and there was no difference found between the test cleanser/moisturizer and the positive control products.
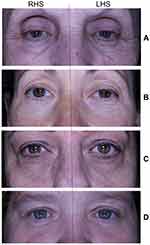
Figure 6 shows a representation of the reduction in fine lines in four subjects following the use of the test cleanser/moisturizer products for 28 days compared to the placebo products.
Skin elasticity
Figure 7 illustrates the percentage increase in R2 (gross elasticity), R5 (net elasticity) and R7 (biological elasticity), and percentage decrease in R6 (viscoelastic portion) over time. R2 significantly increased by 10.0±4.9% (P<0.001) after using the test cleanser/moisturizer for 7 days and continued to improve by 14.9±5.7% (P<0.001) after 14 days, 24.5±6.2% (P<0.001) after 21 days and 32.8±6.5% (P<0.001) after 28 days, compared to the placebo products. Similarly, R5 significantly increased by 10.3±5.4% (P<0.001) after 7 days and continued to improve by 20.1±7.5% (P<0.001) after 14 days, 33.0±6.7% (P<0.001) after 21 days and 47.3±8.6% (P<0.001) after 28 days. R6 was significantly decreased by 9.2±4.3% (P<0.001) and continued to decrease by 16.9±4.8% (P<0.001) after 14 days, 20.6±4.5% (P<0.001) after 21 days and 33.4±4.0% (P<0.001) after 28 days. R7 significantly increased by 14.2±4.5% (P<0.001) after 7 days and continued to improve by 19.3±5.3% (P<0.001) after 14 days, 31.8±4.1% (P<0.001) after 21 days and 50.6±5.1% (P<0.001) after 28 days.
The placebo cleanser/moisturizer products did not cause any significant change in any of the elasticity parameters over the study period, and there was no difference found between the test cleanser/moisturizer and the positive control products.
Discussion
In this study, a novel skin care routine developed for use on sensitive skin11 was further investigated for its ability to improve the signs of aging. Specifically, skin hydration/moisturization was found to significantly improve by 42.2% following the test cleanser/moisturizer routine for 28 days compared to baseline. There was also an improvement in fine lines and wrinkles of the crow’s feet area as demonstrated by a significant reduction in mean wrinkle surface (34.8%), mean wrinkle length (19.0%) and mean wrinkle depth (24.3%). This improvement is characteristic of products that tighten the skin, resulting in the improvement of the edge of wrinkles and fine lines as seen in the photographs (Figure 6).
Improvements in skin elasticity of facial skin were also found in this study, including gross elasticity (R2), net elasticity (R5) and biological elasticity (R7) which were significantly improved by 32.8%, 33.4% and 50.6%, respectively. R2 measures the total skin stretch including viscous deformation which represents the perceived stretchiness of hydrated skin.17 R5 is independent of viscous deformation and is used to characterize the mechanism of skin movement, testing specifically the tensioned nature of the skin.18 R7 is measured after suction has terminated. Further, the viscoelastic portion (R6) was significantly decreased by 33.4%. R6 is measured while the skin is at maximum distension and represents a time-dependent parameter that measures the ratio of softness versus tensioning.19 Overall, these results indicate that the test products have successfully tensioned the skin.
The novel ingredients in the test cleanser and test moisturizer are likely to contribute to their positive effects on skin aging. Skin moisturization is enhanced by the presence of glycerin, paraffinium liquidum, sodium PCA and panthenol in the test products. Glycerin is a humectant and emollient commonly used to moisturize dry skin by drawing transepidermal water to the surface of the skin.20 Paraffinum liquidum provides an emollient film that reduces TEWL from the stratum corneum.21 PCA is a humectant and forms part of the skins natural moisturizing factor (NMF), enhancing the skins ability to hold water.22 Finally, panthenol is used as a humectant and emollient to improve stratum corneum hydration, to reduce TEWL and to maintain skin softness and elasticity.23 It can also promote lipid synthesis to improve skin barrier function.23
The test products also contain niacinamide, also known as nicotinamide or nicotinic acid amide, which is a water-soluble, active form of vitamin B3 and an integral part of the coenzymes nicotinamide adenine dinucleotide (NAD) and NAD phosphate (NADPH).24 In clinical trials, a significant improvement in skin appearance, including reductions in fine lines and wrinkles, hyperpigmented spots, red blotchiness and skin sallowness, as well as improved elasticity was found following application of 5% niacinamide for 12 weeks in Caucasian women.25,26 In another clinical trial, application of 4% niacinamide in Japanese women for 8 weeks resulted in a significant decrease in wrinkles and skin roughness.27
The antiwrinkling effects of niacinamide may be explained by the fact that niacinamide increases collagen production,24 an effect that is also relevant to other antiwrinkle agents, such as all-trans retinoic acid.28 Niacinamide reduces the increase in excess dermal glycosaminoglycans that is a characteristic of photodamaged or wrinkled skin.29 Niacinamide also modulates yellowing (sallowness) of the skin by an inhibition of protein glycation via antioxidant effects.25,26 Niacinamide increases both the lipid (ceramide) and protein (keratin, involucrin, filaggrin) components of the skin barrier and enhances the skin barrier properties, which may improve red blotchiness.30,31 Further, niacinamide has been demonstrated to inhibit melanosome transfer from melanocytes to keratinocytes and promote skin lightening in vivo.32 The mechanism by which elasticity improves is not defined, but dermal remodeling (as evidenced by the observed increases in skin collagen) and prevention of dermal matrix glycation may be contributory factors.25,26
Other ingredients present in the test products for their anti-inflammatory properties which help to soothe sensitive skin and for their potential to combat skin aging include Avena sativa (oat) kernel extract, disodium lauriminodipropionate tocopheryl phosphates (a form of vitamin E) and Hamamelis virginiana (witch hazel) water. Oat avenanthramides are known to suppress histamine release at very low doses, helping to plump up the skin, reduce wrinkles, and restore the skin natural barrier.33 They are also the main group of active polyphenolic antioxidants responsible for oat anti-inflammatory, antierythema, antipruritic and antihistaminic properties.33 Another antiaging benefit of oat avenanthramides is their antigenotoxic activity, which can protect the DNA of epidermal cells against environmental insults, including UV radiation.34 Vitamin E is a lipophilic antioxidant and has a primary role in protecting cell membranes against oxidative stress and maintaining the collagen network in the skin.35 Its levels are rapidly depleted after UV exposure which promotes skin aging.35 Hamamelis virginiana has been shown to be beneficial in reducing UV induced erythema.36
In conclusion, this study has demonstrated that a novel skin care system which is suitable for use on sensitive skin has positive effects on several signs of aging including skin moisturization, skin elasticity and wrinkle size, without any adverse effects over 28 days. Further improvement of these parameters would be expected with continued use.
Acknowledgments
The authors would like to thank Dermatest Pty. Ltd. for assistance in performing these studies.
Disclosure
Fabrizio Spada, Ada H Lui and Tanya M Barnes are employed by Ego Pharmaceuticals, the sponsor of the study and manufacturer of the QV Face product range.
Fabrizio Spada and Ada H Lui report that they have the patent AU2014386711 pending. The authors report no other conflicts of interest in this work.
References
1. Langton AK, Sherratt MJ, Griffiths CEM, Watson REB. A new wrinkle on old skin: the role of elastic fibres in skin ageing. Int J Cosmet Sci. 2010;32(5):330–339. doi:10.1111/j.1468-2494.2010.00574.x
2. Callaghan TM, Wilhelm KP. A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part 2: clinical perspectives and clinical methods in the evaluation of ageing skin. Int J Cosmet Sci. 2008;30(5):323–332. doi:10.1111/j.1468-2494.2008.00455.x
3. Brenneisen P, Sies H, Scharfetter-Kochanek K. Ultraviolet B irradiation and matrix metalloproteinases: from induction via signaling to initial events. Ann N Y Acad Sci. 2002;973:31–43.
4. Baswan SM, Leverett J, Pawelek J. Clinical evaluation of the lightening effect of cytidine on hyperpigmented skin. J Cosmet Dermatol. 2019;18(1):278–285. doi:10.1111/jocd.12784
5. Rokhsar CK, Lee S, Fitzpatrick RE. Review of photorejuvenation: devices, cosmeceuticals, or both? Dermatol Surg. 2005;31(9 Pt 2):1166–1178.
6. Darland AM, Chubb HA, Sachs DL, Helfrich YR. Patient interest in and familiarity with anti-aging therapies: a survey of the general dermatology clinic population. J Cosmet Dermatol. 2018;17(3):403–409. doi:10.1111/jocd.12386
7. Farage MA. How do perceptions of sensitive skin differ at different anatomical sites? An epidemiological study. Clin Exp Dermatol. 2009;34(8):e521–e530. doi:10.1111/j.1365-2230.2009.03487.x
8. Misery L, Sibaud V, Merial-Kieny C, Taieb C. Sensitive skin in the American population: prevalence, clinical data, and role of the dermatologist. Int J Dermatol. 2011;50(8):961–967. doi:10.1111/j.1365-4632.2011.04884.x
9. Inamadar AC, Palit A. Sensitive skin: an overview. Indian J Dermatol Venereol Leprol. 2013;79(1):9–16. doi:10.4103/0378-6323.104664
10. Berardesca E, Farage M, Maibach H. Sensitive skin: an overview. Int J Cosmet Sci. 2013;35(1):2–8. doi:10.1111/j.1468-2494.2012.00754.x
11. Heinicke IR, Adams DH, Barnes TM, Greive KA. Evaluation of a topical treatment for the relief of sensitive skin. Clin Cosmet Investig Dermatol. 2015;8:405–412. doi:10.2147/CCID.S87509
12. Tran D, Townley JP, Barnes TM, Greive KA. An antiaging skin care system containing alpha hydroxy acids and vitamins improves the biomechanical parameters of facial skin. Clin Cosmet Investig Dermatol. 2014;8:9–17. doi:10.2147/CCID.S75439
13. Courage W. Hardware and measuring principle: corneometer. In: Elsner P, Berardesca E, Maibach HI, editors. Bioengineering of the Skin: Water and the Stratum Corneum, Volume I. Philadelphia: CRC Press; 1994:171–176.
14. Distante F, Berardesca E. Transepidermal water loss. In: Berardesca E, Elsner P, Wilhelm KP, Maibach HI, editors. Bioengineering of the Skin: Methods and Instrumentation, Volume III. Philadelphia: CRC Press; 1995:1–4.
15. Hamer MA, Jacobs LC, Lall JS, et al. Validation of image analysis techniques to measure skin aging features from facial photographs. Skin Res Technol. 2015;21(4):392–402. doi:10.1111/srt.12205
16. O’goshi K. Suction cup method for measurement of skin mechanical properties: the cutometer. In: Serup J, Jemec GBE, editors. Handbook of Non-Invasive Methods and the Skin. Florida: CRC Press; 1995:579–582.
17. Dobrev H. In vivo study of skin mechanical properties in Raynaud’s phenomenon. Skin Res Technol. 2007;13(1):91–94. doi:10.1111/j.1600-0846.2007.00197.x
18. Koch RJ, Cheng ET. Quantification of skin elasticity changes associated with pulsed carbon dioxide laser skin resurfacing. Arch Facial Plast Surg. 1999;1(4):272–275.
19. Dobrev H. Use of Cutometer to assess epidermal hydration. Skin Res Technol. 2000;6(4):239–244. doi:10.1034/j.1600-0846.2000.006004239.x
20. Bissett DL, McBride JF. Skin conditioning with glycerol. J Soc Cosmet Chem. 1984;35(7):345–350.
21. Taylor EA. Cutaneous adsorption of bath oils. Arch Dermatol. 1963;87:369–371.
22. Fowler J. Understanding the role of natural moisturizing factor in skin hydration. Prac Dermatol. 2012.
23. Ebner F, Heller A, Rippke F, Tausch I. Topical use of dexpanthenol in skin disorders. Am J Clin Dermatol. 2002;3(6):427–433. doi:10.2165/00128071-200203060-00005
24. Matts PJ, Oblong JE, Bissett DL. A review of the range of effects of niacinamide in human skin. Int Fed Soc Cosmet Chem Mag. 2002;5(4):285–289.
25. Bissett DL, Miyamoto K, Sun P, Li J, Berge CA. Topical niacinamide reduces yellowing, wrinkling, red blotchiness, and hyperpigmented spots in aging facial skin. Int J Cosmet Sci. 2004;26(5):231–238. doi:10.1111/j.1467-2494.2004.00228.x
26. Bissett DL, Oblong JE, Berge CA. Niacinamide: a B vitamin that improves aging facial skin appearance. Dermatol Surg. 2005;31(7 Pt 2):860–865.
27. Kawada A, Konishi N, Oiso N, Kawara S, Date A. Evaluation of anti-wrinkle effects of a novel cosmetic containing niacinamide. J Dermatol. 2008;35(10):637–642. doi:10.1111/j.1346-8138.2008.00537.x
28. Varani J, Fisher GJ, Kang S, Voorhees JJ. Molecular mechanisms of intrinsic skin aging and retinoid-induced repair and reversal. J Investig Dermatol Symp Proc. 1998;3(1):57–60.
29. Kang S, Duell EA, Fisher GJ, et al. Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid binding proteins characteristic of retinoic acid but without measurable retinoic acid levels of irritation. J Invest Dermatol. 1995;105(4):549–556.
30. Bissett DL, Oblong JE, Saud A, et al. Topical niacinamide provides skin aging appearance benefits while enhancing barrier function. In: Elsner P, Maibach HI, editors. Cosmeceuticals and Active Cosmetics.
31. Bissett DL. Topical niacinamide and barrier enhancement. Cutis. 2002;70(6 Suppl):8–12.
32. Hakozaki T, Minwalla L, Zhuang J, et al. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br J Dermatol. 2002;147(1):20–31.
33. Perrelli A, Goitre L, Salzano AM, Moglia A, Scaloni A, Retta SF. Biological activities, health benefits, and therapeutic properties of avenanthramides: from skin protection to prevention and treatment of cerebrovascular diseases. Oxid Med Cell Longev. 2018;2018:6015351. doi:10.1155/2018/6015351
34. Chen CY, Milbury PE, Collins FW, Blumberg JB. Avenanthramides are bioavailable and have antioxidant activity in humans after acute consumption of an enriched mixture from oats. J Nutr. 2007;137(6):1375–1382. doi:10.1093/jn/137.6.1375
35. Al-Niaimi F, Chiang NYZ. Topical vitamin C and the skin: mechanisms of action and clinical applications. J Clin Aesthet Dermatol. 2017;10(7):14–17.
36. Hughes-Formella BJ, Filbry A, Gassmueller J, Rippke F. Anti-inflammatory efficacy of topical preparations with 10% Hamamelis distillate in a UV erythema test. Skin Pharmacol App Skin Physiol. 2002;15(2):125–132. doi:10.1159/000049400
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




 ) positive control, n=15 subjects and (
) positive control, n=15 subjects and ( ) test cleanser/moisturizer, n=15 subjects. Results are presented as mean ± SEM. *P<0.001 versus placebo, †P<0.01 versus placebo, #P<0.05 versus placebo.
) test cleanser/moisturizer, n=15 subjects. Results are presented as mean ± SEM. *P<0.001 versus placebo, †P<0.01 versus placebo, #P<0.05 versus placebo.