Back to Journals » Blood and Lymphatic Cancer: Targets and Therapy » Volume 12
Use of BTK Inhibitors in Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL): A Practical Guidance
Authors St-Pierre F, Ma S
Received 21 March 2022
Accepted for publication 1 July 2022
Published 22 July 2022 Volume 2022:12 Pages 81—98
DOI https://doi.org/10.2147/BLCTT.S326627
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Wilson Gonsalves
Frédérique St-Pierre,1 Shuo Ma1,2
1Department of Medicine, Division of Hematology/Oncology and the Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, IL, USA; 2Department of Medicine, Division of Hematology/Oncology, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA
Correspondence: Shuo Ma, Division of Hematology-Oncology, Department of Medicine, Feinberg School of Medicine and the Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Tel +1 312-695-0990, Email [email protected]
Abstract: The treatment landscape of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) has changed significantly since the development of oral Bruton’s tyrosine kinase (BTK) inhibitors. While chemoimmunotherapy was previously the standard of care for first-line treatment, BTK inhibitors have proven to be a highly effective and safe therapeutic option for CLL/SLL, and now constitute one of the preferred first-line options. Ibrutinib, the first approved covalent BTK inhibitor in CLL/SLL, has the most long-term data supporting its efficacy in CLL/SLL treatment although is associated with increased risk of cardiovascular and hemorrhage adverse events due to off-target kinase inhibition. The second-generation covalent BTK inhibitors, including acalabrutinib and zanubrutinib, are more selective to BTK with less off-target effects. Resistance to covalent BTK inhibitors may emerge over time due to mutations in BTK and downstream kinases. Novel non-covalent BTK inhibitors currently being studied are showing promising activities to overcome such resistance. In this review, we discuss the role of BTK inhibitors in treatment of CLL/SLL, review the data that led to approval of BTK inhibitors in CLL/SLL, outline the toxicity profile of each approved BTK inhibitor and management, and give practical guidance on how to select the most appropriate agent for treatment.
Keywords: Bruton’s tyrosine kinase inhibitor, chronic lymphocytic leukemia/small lymphocytic lymphoma, ibrutinib, acalabrutinib, zanubrutinib
Introduction
The World Health Organization (WHO) classification of lymphoid neoplasms describes chronic lymphocytic leukemia (CLL/SLL) as an indolent B-cell lymphoma characterized by a leukemic course, with presence of a monoclonal B-cell population in the peripheral blood at a count of ≥ 5 × 109/L.1,2 CLL/SLL presents with a wide range of associated risk and symptomatology, from low-risk asymptomatic disease to high-risk disease with significant symptoms. Risk stratification is based on molecular and cytogenetic features of the disease, clinical presentation, and presence of organomegaly and/or cytopenia.3 Low-risk, asymptomatic disease is typically managed with observation, often for a period of many years as early treatment for asymptomatic disease has not shown any benefit in past studies.3–5 When treatment is indicated in CLL/SLL, a comprehensive evaluation of tumor characteristics and patient factors must be made in order to select the best treatment option.3 Until recently, treatment options for CLL/SLL consisted of various combinations of chemotherapy and immunotherapy with monoclonal antibodies (mAb) targeting CD20.6 In 2013, Phase I data was introduced on the safety and efficacy of a novel therapy in CLL/SLL, ibrutinib, a Bruton Tyrosine Kinase (BTK) inhibitor.7 The introduction of BTK inhibitors and other novel targeted therapies (Bcl-2 inhibitor and PI3K inhibitors), has dramatically changed the treatment landscape in CLL/SLL.3,6,8–10 The first-generation BTK inhibitor ibrutinib is associated with some off-target effects through the activation of other tyrosine kinases, leading to specific side effect profile which includes bleeding and cardiovascular toxicity.11,12 The second-generation BTK inhibitors acalabrutinib and zanubrutinib have been developed with the goal of increasing specificity to the BTK enzyme and reducing off-target effects, to improve their toxicity profile.13,14 Third-generation BTK inhibitors, the non-covalently binding inhibitors, are under investigation to overcome resistance to covalent-BTK inhibitor therapy.15 This review discusses the mechanism of action of BTK inhibitors, presents the data behind approval of the three BTK inhibitors approved in the US for treatment of B-cell malignancies, and outlines the side effect profile of each BTK inhibitor, with recommendations on how to select the best therapy for patients in an individualized manner.
Bruton’s Tyrosine Kinase (BTK)
Protein kinases are enzymes that catalyze phosphorylation of proteins, resulting in alteration of their substrate’s activity and interaction with other proteins. Aberrant protein kinase activity results in altered cellular function and is a potential driver of uncontrolled cell proliferation, which may in turn result in malignancy. BTK is a kinase that plays a crucial role in proliferation and survival of malignant B-cells.16
BTK was first identified in 1993 as the genetic cause for X-linked agammaglobulinemia, an inherited immunodeficiency disorder characterized by the absence of mature B-cells, leading to severe antibody deficiency and recurrent infections.17,18 BTK has since been established as an important component of the B-cell receptor (BCR) signaling pathway, and is required for normal B-cell development as it is involved in signaling from the pre-B-cell receptor that forms after immunoglobulin heavy-chain rearrangement.16,18
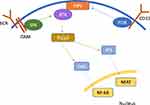
BTK belongs to the TEC family of non-receptor kinases, and is composed of 5 domains (Figure 1). It is a cytoplasmic kinase that is transiently recruited to the plasma membrane.18 BTK’s pleckstrin homology (PH) domain binds phosphatidylinositol (3,4,5)-triphosphate (PIP3), a phospholipid that resides on the plasma membrane.18,19 PIP3 is generated by phosphatidylinositol-3-kinase (PI3K), a plasma membrane-associated lipid kinase activated by the BCR co-receptor CD19. BTK is activated by a spleen tyrosine kinase (SYK) or an SRC kinase, through phosphorylation of Y551 in the BTK kinase domain. This, in turn, results in autophosphorylation in the SRC homology (SH)3 domain.18 The SH2 domain is important in the phosphorylation of phospholipase C, which cleaves phosphatidylinositol 4,5-bisphosphonate (PIP2) to generate the second messengers inositol triphosphate (IP3) and diacylglycerol (DAG).18,20 These lead to the activation of downstream transcription factors regulating B-cell signalling proteins, including nuclear factor-κB (NF-κB) and nuclear receptor of activated T cells (NFAT). The TEC homology domain (TH) contains a zinc-finger motif that optimizes stability of the protein.18 The role of BTK within BCR signalling is illustrated in Figure 2.
 |
Figure 1 BTK structure. Abbreviations: C, Carboxyl-terminus; N, Amino-terminus; PH, Pleckstrin homology; SH, SRC homology; TH, TEC homology. |
In all, BTK is a crucial component of the BCR signalling pathway and is expressed in many B-cell malignancies, including CLL/SLL. Targeting BTK therefore represents a profound opportunity to disrupt signalling and proliferation of malignant B-cells. This is the basis upon which BTK inhibitors were developed for use in CLL/SLL and other B-cell lymphomas.
Mechanism of Action and Anti-Tumor Effect of BTK Inhibitors in CLL/SLL
Current FDA-approved BTK inhibitors for the treatment of CLL/SLL include the first-generation inhibitor ibrutinib, and the second-generation inhibitor acalabrutinib. Zanubrutinib, also a second-generation BTK inhibitor, is FDA-approved for the treatment of marginal zone lymphoma (MZL), mantle cell lymphoma (MCL), and Waldenstrom’s macroglobulinemia (WM), and remains under investigation for CLL/SLL.21 These three BTK inhibitors inactivate BTK by binding to cysteine 481 in the ATP-binding site of BTK, located in the kinase domain.16,22 This binding is covalent and irreversible.22 The expected “on-target” effect of BTK inhibitors is the inactivation of the BTK enzyme and disruption of BCR signalling, as illustrated in Figure 2.7 Because BCR signalling is constitutively activated in CLL/SLL, its disruption has the potential to significantly halt the progression of the disease.23 It is also hypothesized that there is an indirect effect of BCR signalling blockade on the tumor microenvironment (TME), resulting in a pro-apoptotic environment less favorable for tumor growth.24,25
Currently, FDA-approved BTK inhibitors are not fully selective for BTK and can bind to other kinases simultaneously, leading to “off-target” activity.13,22 Figure 3 illustrates the kinome map, or graphic representation of kinase-binding activity, of each FDA-approved BTK inhibitor. Although ibrutinib was associated with higher potency of inhibition of tyrosine kinases, it also was associated with lower kinase selectivity, leading to more off-target kinase activity in comparison to second-generation BTK inhibitors.13 Off-target effects are thought to be closely associated with an increase in adverse effects from the medication. For example, there are analyses that suggest that ibrutinib-associated atrial fibrillation is caused by the binding to ERBB2/HER2 and C-terminal Src kinases (CSK), which also harbor a BTK inhibitor-binding cysteine in their catalytic domain.11,26 Binding to other off-target kinases is also thought to result in higher rates of rash (Epidermal growth factor (EGFR)), diarrhea (EGFR), and bleeding (TEC).11,12,26 Contrary to ibrutinib, acalabrutinib does not inhibit other kinases with the conserved cysteine residue.27 Zanubrutinib results in some inhibition of ITK and EGFR, with lower potency of its off-target effects in comparison to ibrutinib. The better selectivity of the second-generation BTK inhibitors probably explains their improved toxicity profile. There are other covalent BTK inhibitors including spebrutinib and tirabrutinib, studied for use in some lymphomas, although these are not currently approved for use in the United States (U.S.).12
 |
Figure 3 BTK Inhibitor Kinome Map. Figure modified from Kaptein A, de Bruin G, Emmelot-van Hoek M, van de Kar B, de Jong A, Gulrajani M, et al. Potency and Selectivity of BTK Inhibitors in Clinical Development for B-Cell Malignancies. Blood. 2018;132(Supplement 1):1871. Copyright 2018, with permission from Elsevier.13 |
Covalent BTK Inhibitors - Efficacy
First-Generation Covalent BTK Inhibitor - Ibrutinib
Ibrutinib, first discovered and developed in 2007, constitutes the first generation of approved BTK inhibitors.28 Ibrutinib was first demonstrated as being efficacious and safe for relapsed/refractory CLL/SLL and for treatment-naïve CLL/SLL in the elderly in a phase Ib/II trial by O’Brien et al in 2013.29 Overall response rate (ORR) in this study was 71%, indicating a great potential for improved outcomes in elderly patients with CLL/SLL. This was followed by a Phase II study by Farooqui et al, evaluating the safety and efficacy of ibrutinib in untreated or relapsed/refractory (R/R) TP53-mutated CLL/SLL.30 Patients with TP53-mutated disease are known to have poor outcomes with high rate of refractoriness to chemotherapy, heightening the importance of studying alternate therapies in this patient population.31–33 This study also showed excellent response rates with an ORR of 97% in patients with untreated CLL/SLL, and 80% in patients with R/R disease.30 Several other phase I/II trials established the safety and efficacy of ibrutinib in the treatment of newly diagnosed and R/R CLL/SLL, including trials evaluating the combination of ibrutinib and anti-CD20 mAb therapy and the combination of ibrutinib and chemoimmunotherapy (CIT). These trials are outlined in Table 1.
 |
Table 1 Major Published Clinical Trials for Ibrutinib in CLL/SLL |
The Phase III RESONATE trial was the first to establish superior outcomes in R/R CLL/SLL with ibrutinib monotherapy in comparison to therapy with an anti-CD20 mAb (ofatumumab). The 1-year OS was 90% in the ibrutinib group compared to 80% in the ofatumumab group, and median PFS was not reached in the ibrutinib group compared to 8.1 months in the ofatumumab group.34 These results were later reproduced in a phase III trial comparing ibrutinib to the anti-CD20 mAb rituximab in R/R CLL/SLL, which found a significantly higher ORR and OS in the ibrutinib group.35 The HELIOS trial compared ibrutinib + BR (bendamustine and rituximab) to CIT with BR in R/R CLL/SLL, and found a significant PFS benefit in the ibrutinib group, with 18-month PFS of 79% vs 24% in the CIT group.36
Several phase III studies demonstrated superiority of ibrutinib over conventional immunochemotherapy. The RESONATE-2 phase III trial was the first to establish a survival outcome with ibrutinib compared to chemotherapy (chlorambucil) in untreated CLL/SLL, with a 2-year OS of 98% vs 85%, favoring the ibrutinib group.37 In the Alliance 041202 phase III study, patients 65 years of age or older with previously untreated CLL/SLL were treated with Ibrutinib with or without rituximab as compared to CIT with BR. The ibrutinib-containing arms showed a higher 2-year PFS (87% and 88% for ibrutinib and ibrutinib + rituximab arms, respectively) compared to the CIT arm (74%).38 The ECOG 1912 phase III trial studied patients 70 years of age or younger with previously untreated CLL/SLL, comparing ibrutinib plus rituximab to CIT with FCR (fludarabine, cyclophosphamide, rituximab), the previous standard of care for younger patients. A superior 3-year PFS and 3-year OS was found in the ibrutinib group compared to the FCR group (89.4% vs 72.9% and 98.8% vs 91.5% for PFS and OS, respectively).39 The iLLUMINATE phase III trial also showed superior PFS of ibrutinib plus obinutuzumab compared to CIT with chlorambucil plus obinutuzumab in treatment naïve CLL/SLL patients with comorbid conditions.40
It remains uncertain whether ibrutinib combined with an anti-CD20 mAb or CIT is superior to ibrutinib monotherapy. Of note, in the aforementioned Alliance 041202 study, the addition of rituximab to ibrutinib showed no PFS benefit over ibrutinib monotherapy.38 The phase III GENUINE trial did show a superior ORR rate in patients with R/R CLL/SLL treated with ibrutinib + anti-CD20 mAb (ublituximab) compared to ibrutinib monotherapy (83% vs 65%).41 There also appeared to be a PFS advantage to ibrutinib + ublituximab, especially in the population with 17p deletion or TP53 mutation (median PFS not reached (NR) in the combination group vs 18.9 months in the ibrutinib monotherapy group). However, no OS benefit was seen at the median follow-up time of 3.5 years.42 In a separate randomized phase II trial of ibrutinib + rituximab compared to ibrutinib monotherapy in untreated or relapsed CLL/SLL, there was no difference in PFS or OS between groups.43
Currently, ibrutinib is FDA approved for the treatment of CLL/SLL at a dose of 420 mg orally once daily (either as monotherapy, in combination with rituximab or obinutuzumab, or in combination with bendamustine and rituximab); continue until disease progression or unacceptable toxicity. Ibrutinib is listed as one of the preferred treatments for CLL/SLL in the current National Comprehensive Cancer Network (NCCN) guidelines.44
Ongoing studies are investigating the combination of ibrutinib with the novel BCL2 inhibitor venetoclax in untreated and R/R CLL/SLL with promising results, however this combination is not yet FDA approved.45,46
Second-Generation BTK Inhibitors: Acalabrutinib and Zanubrutinib
Acalabrutinib and zanubrutinib are the second-generation BTK inhibitors that are approved in the US for treatment of B-cell malignancies. Acalabrutinib is FDA-approved for CLL/SLL and MCL, while zanubrutinib is approved for treatment of WM, MZL, and MCL. Both are listed among the preferred treatments for CLL/SLL in the current NCCN guidelines.44 As discussed previously, second-generation BTK inhibitors were developed with the goal of achieving higher specificity to the BTK enzyme, resulting in less off-target kinase activation. Acalabrutinib was studied in a phase I/II trial in 2015 evaluating its safety and efficacy in R/R CLL/SLL. ORR was 95% with a tolerable toxicity profile.47 Table 2 outlines major clinical trials of second-generation BTK inhibitors. The phase III ASCEND trial compared acalabrutinib to standard of care (investigator’s choice of PI3K inhibitor idelalisib + rituximab or CIT with BR) in patients with R/R CLL/SLL. The 12-month PFS was significantly higher in the acalabrutinib arm compared to the standard of care arm (88% vs 68%).48 A phase I trial of acalabrutinib monotherapy in untreated CLL/SLL showed an ORR of 97%.49 The phase III ELEVATE-TN trial comparing acalabrutinib with or without anti-CD20 mAb (obinutuzumab) to CIT (chlorambucil + obinutuzumab) showed a significantly higher median PFS in the acalabrutinib arms (NR vs 22.6 months).50 24-month PFS was 93% (95% CI 87–96%) in the acalabrutinib + obinutuzumab group, 87% (95% CI 81–92%) in the acalabrutinib monotherapy group, and 47% (95% CI 39–55%) in the obinutuzumab-chlorambucil group. The HR for PFS between acalabrutinib-obinutuzumab and acalabrutinib monotherapy was 0.49 (95% CI 0.26–0.95, post-hoc analysis) indicating a small benefit by the addition of obinutuzumab.50
 |
Table 2 Published Clinical Trials of Second-Generation BTK Inhibitors in CLL/SLL |
Acalabrutinib was compared to ibrutinib in a non-inferiority head-to-head Elevate-RR study in patients with R/R CLL/SLL. Results indicated that acalabrutinib is non-inferior to ibrutinib with respect to survival outcomes, with a median PFS of 38.4 months in both groups. Differences in adverse events (AEs) between ibrutinib and acalabrutinib are discussed further in the next section.
The first in human Phase 1 study of zanubrutinib in B-cell malignancies demonstrated promising activity in the CLL/SLL cohort with an ORR of 96.2%.51 A phase Ib has evaluated the combination of zanubrutinib + obinutuzumab in untreated or R/R CLL/SLL. This showed an ORR of 100% in untreated CLL/SLL, and 92% in R/R CLL/SLL.52 The phase III SEQUOIA trial (NCT03336333) studied the efficacy of zanubrutinib in comparison to CIT with BR in patients with previously untreated CLL/SLL. These findings were recently presented at the American Society of Hematology annual meeting (ASH 2021). The estimated 24-month PFS was 85.5% (95% CI 80.1–89.6%) in the zanubrutinib group vs 69.5% (95% CI 62.4–75.5%) in the CIT group. There was no statistically significant difference in OS between the two groups at 24 months.53
The phase III ALPINE trial (NCT03734016) is currently investigating the safety and efficacy of zanubrutinib in comparison to ibrutinib in patients with R/R CLL/SLL.54 Results from an interim analysis at a median follow-up of 15 months (n=415 patients) showed a higher ORR with zanubrutinib compared to ibrutinib (78.3% vs 62.5%, p=0.0006). 12-month PFS was also higher in the zanubrutinib group (94.9% vs 84%, p=0.0007). Zanubrutinib demonstrated reduced cardiac toxicity compared to ibrutinib. We await the final results of the trial once the target number of events has been reached to adequately interpret these results.55
The novel irreversible BTK inhibitor orelabrutinib is also currently under investigation in R/R CLL/SLL (NCT04014205), with increased specificity to BTK.56
Covalent BTK Inhibitors - Adverse Effects
The pivotal phase III trials evaluating the safety and efficacy of ibrutinib monotherapy in CLL/SLL include the RESONATE, RESONATE-2, and Alliance trials, as well as the clinical trial by Huang et al (Table 1).34,35,37,38 The CLL/SLL-12 trial, evaluating the safety and efficacy of ibrutinib compared to placebo in low-risk CLL/SLL, can also be useful in establishing the toxicity profile of ibrutinib, although full results have not yet been published.57 The most common AEs of any grade were diarrhea (34–46%), bleeding (29–44%), fatigue (19–28%), nausea (22–26%), cough (22–25%), pyrexia (24%), anemia (15–23%), rash (23%), thrombocytopenia (16%), and neutropenia (22–26%). Most common grade ≥3 AEs were neutropenia (10–16%), anemia (2–6%), pneumonia (4–7%), thrombocytopenia (5%), hypertension (2–4%), and diarrhea (4%). Atrial fibrillation occurred in approximately 5–10% of patients treated with ibrutinib, and 3–8% of patients developed grade ≥3 atrial fibrillation.26,34,35,37,38
The phase III ELEVATE-TN and ASCEND trials have compared the safety and efficacy of acalabrutinib to CIT.48,50 The most common AEs of any grade observed were headache (22–37%), diarrhea (18–35%), neutropenia (19%), fatigue (18%), cough (15–18%), arthralgia (16%), contusion (15%), and anemia (14%). Most common grade ≥3 AEs were neutropenia (10%), anemia (7%), thrombocytopenia (3%), urinary tract infection (UTI) (2%), pneumonia (2%), dyspnea (2%), headache (1%), fatigue (1%), and back pain (1%). Atrial fibrillation occurred in 4–5% of patients (2% grade ≥3), and incidence of grade ≥3 hypertension was 2%. Bleeding occurred in up to 39% of patients.
The ELEVATE-RR study compared acalabrutinib to ibrutinib head-to-head in patients with R/R CLL/SLL, providing a direct comparison between these two agents. This trial showed a significantly higher rate of any-grade diarrhea (46% vs 35%), arthralgia (23% vs 16%), hypertension (23% vs 9%), contusion (18% vs 12%), atrial fibrillation (16% vs 9%), UTI (14% vs 8%), back pain (13% vs 8%), muscle spasms (13% vs 6%), and dyspepsia (12% vs 4%) in the ibrutinib group compared to the acalabrutinib group. There was a significantly higher rate of grade ≥3 hypertension (9% vs 4%) and diarrhea (5% vs 1%) in the ibrutinib group. Treatment with acalabrutinib resulted in a higher rate of any-grade headache (35% vs 20%) and cough (29% vs 21%), and a higher rate of grade ≥3 fatigue (3% vs 0%). Bleeding events were less frequent with acalabrutinib (38% vs 51%), although rate of major bleeding events was comparable (approximately 5% in each group).58
Phase III trials evaluating the safety efficacy of zanubrutinib in CLL/SLL have not been fully published yet, however results from the SEQUOIA trial were recently presented at ASH 2021, and interim results from the ALPINE trial have been released.53,55 In the SEQUOIA trial, 479 patients with untreated CLL/SLL were randomized to receive either zanubrutinib or chemoimmuntherapy with BR. The most common AEs with zanubrutinib included bleeding (45%), contusion (19%), upper respiratory tract infection (URTI) (17%), neutropenia (13%), hypertension (12%), rash (11%), constipation (10%), nausea (10%), pyrexia (7%), anemia (5%), and thrombocytopenia (4%). Grade ≥3 AEs included neutropenia (9%), hypertension (6%), bleeding (4%), thrombocytopenia (2%), URTI (0.8%), constipation (0.4%), and anemia (0.4%). Atrial fibrillation occurred in 3% of patients.53 Interim results from the ALPINE trial, comparing zanubrutinib to ibrutinib in 415 patients with R/R CLL/SLL, appears to indicate lower incidence of atrial fibrillation in the zanubrutinib group (2.5% vs 10.1%, p=0.0014).55 The statistical significance of other differences in AEs are not yet reported.
At this time, the best direct comparison that exists between zanubrutinib and ibrutinib is from the phase III ASPEN trial, which compared zanubrutinib to ibrutinib in patients with Waldenstrom macroglobulinemia (WM).59 Although this trial did not involve CLL/SLL patients, observations can still be made on the tolerability of each BTK inhibitor, as we await final results from the ALPINE trial. In this trial, there was a significantly higher rate of any-grade diarrhea (32% vs 21%), contusion (24% vs 13%), muscle spasms (24% vs 10%), peripheral edema (19% vs 9%), atrial fibrillation (15% vs 2%), and pneumonia (12% vs 2%) in the ibrutinib group compared to the zanubrutinib group. The incidence of major hemorrhage was also higher in the ibrutinib group compared to the zanubrutinib group (9% vs 6%). The rate of any-grade neutropenia was higher in the zanubrutinib group (29% vs 13%). This indicates overall better tolerability of zanubrutinib in comparison to ibrutinib.59
Comparison between incidence of AEs with ibrutinib, acalabrutinib, and zanubrutinib is outlined in Table 3, based on results from the ASPEN and ELEVATE-RR head-to-head trials.58,59
 |
Table 3 Toxicity of Approved BTK Inhibitors (Head-to-Head Trials)58,59 |
When to Treat CLL/SLL with BTK Inhibitors
Once determination has been made that a patient with CLL/SLL meets criteria for initiation of treatment, selection of the most appropriate agent becomes important. The main first-line options include BTK inhibitors, venetoclax combined with an anti-CD20 mAb, and CIT.3 As outlined previously, several large randomized trials have compared the safety and efficacy of BTK inhibitors with various CIT combinations in previously untreated CLL/SLL (Table 1), demonstrating a superior clinical outcome of BTK inhibitors in comparison to CIT. Therefore, BTK inhibitors is generally favored over CIT in the frontline setting, in particular in patients with high-risk features such as TP53 alteration or unmutated immunoglobulin heavy-chain gene (IgHV-UM). Patients with a TP53 mutation or del(17p) generally have a poor response to chemotherapy, with a historical median PFS of 9 months.3,60,61 In the phase III CLL14 trial, median PFS for TP53 mutated disease was approximately 18 months in patients treated with chlorambucil-obinutuzumab as compare to close to 4 years for venetoclax + obinutuzumab (VEN-O).62 Long-term follow-up from a Phase 2 study of ibrutinib in treatment-naïve CLL/SLL showed that 27 patients with TP53 mutated disease treated upfront with ibrutinib had a 5-year PFS of 66%.63 This suggests that in the high-risk group of CLL/SLL with TP53 alteration, BTK inhibitors should be the preferred first line of therapy.
BTK inhibitor treatment has also shown benefit in high-risk CLL/SLL with IgHV-UM status. In the ECOG-1912 phase III trial comparing ibrutinib + rituximab to FCR in 529 patients with untreated CLL/SLL, the largest benefit to ibrutinib was in the IgHV-UM group. PFS was similar in both treatment groups in patients with IgHV mutated (IgHV-M) disease.39,64 Similarly, results from the phase III Alliance 041202 trial showed significant benefit of ibrutinib over CIT with BR in the IgHV-UM cohort, and no significant difference in outcomes in the IgHV-M group.38 For this reason, in IgHV-UM CLL/SLL, BTK inhibitor is strongly favored over CIT. For patients with IgHV-M status, BTK inhibitor and CIT are comparable in efficacy and are both reasonable options.
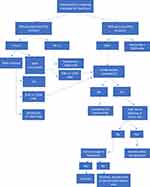
The combination of venetoclax and obinutuzumab is also an appropriate first-line option for patients with CLL/SLL, based on the CLL14 trial.62 This combination has similar efficacy to BTK inhibitors in phase II trials, and there are no completed head-to-head trials to date comparing the two regimens although ongoing clinical trials will help to address this question for the future. Choosing between VEN-O and a BTK inhibitor should be done largely based on potential side effects and logistics of administration. VEN-O requires five weeks of venetoclax dose ramp up with tumor lysis syndrome (TLS) monitoring, and four IV infusions for cycle 1 and once monthly for cycle 2–6, therefore patients must return for more frequent clinic visits.62 Given the TLS risk, patients need to have adequate renal function to be eligible for the ven-O treatment. BTK inhibitors are administered orally typically requiring less frequent clinic visits, and is thus more convenient. On the other hand, Ven-O provides a fixed duration therapy whereas BTK inhibitor treatment is continuous. Grade ≥3 neutropenia occurs in approximately 35% of patients with venetoclax, compared to 10–15% with BTK inhibitors.38,64,65 Anti-CD20 mAb should also be used with caution during the COVID-19 pandemic, as they significantly increase the risk of contracting COVID-19, and increase the risk of complications from the infection.66 Major side effects associated with BTK inhibitors are outlined in Table 3 and include GI side effects, cardiovascular toxicity (specifically hypertension and atrial fibrillation), cytopenia, bleeding, and infection. The selection of most appropriate treatment will require a thorough discussion with the patient considering the pros and cons, taking patient preference into consideration. Figure 4 proposes a treatment algorithm for selection of frontline therapy in CLL/SLL.
The use of BTK inhibitors is also approved in the R/R setting following prior treatment with CIT or venetoclax-based therapy.60 Should a BTK inhibitor treatment be discontinued due to adverse events, and alternative BTK inhibitor may be used. However, progressive CLL/SLL while being treated on a covalent BTK inhibitor would indicate likely resistance. Such patients would unlikely response to an alternative covalent (first or second generation) BTK inhibitor.12 Third generation, non-covalent binding BTK inhibitors are under investigation to overcome such resistance, as detailed in an upcoming section.
Selecting the Appropriate BTK Inhibitor
As outlined previously, ibrutinib and acalabrutinib are the two currently FDA-approved BTK inhibitors for the treatment of CLL/SLL in the first-line and R/R setting. Once the decision is made to treat with a BTK inhibitor, a selection must be made among the available agents. The head-to-head ELEVATE-RR trial between ibrutinib and acalabrutinib showed non-inferiority of acalabrutinib in clinical efficacy, and improved toxicity profiles as discussed above.58 Selection of the most appropriate BTK inhibitor should be made based on patient comorbidities, expected side effect profile, and cost. In patients with a cardiovascular history, particularly those who are at risk for developing poorly controlled hypertension or atrial fibrillation, acalabrutinib is a preferred choice over ibrutinib. Patients with a higher bleeding risk might also benefit from acalabrutinib. Acalabrutinib is associated with a higher risk of headaches and cough, and therefore patients with a history of migraine or chronic cough who do not have a significant cardiovascular or bleeding risk may experience less debilitating side effects with ibrutinib.58 In addition, due to the acidic environment needed for acalabrutinib absorption, it should not be used concurrently with a proton pump inhibitor (PPI). Patients who require long-term PPI use would not be appropriate for acalabrutinib. A new formulation of acalabrutinib is being developed which may ultimately overcome this problem. The side effect profile of first- and second-generation BTK inhibitors is outlined in Table 3. In patients who have no significant comorbidities, initiating treatment with either ibrutinib or acalabrutinib in the first-line setting may be most appropriate. If ibrutinib is not well tolerated, consideration can be made to switch to a second-generation BTK inhibitor. Zanubrutinib, although not yet FDA-approved in CLL/SLL, is associated with a significantly lower cardiovascular toxicity compared to ibrutinib based on the Alpine study discussed above, and is another reasonable option especially for patients with cardiac risk factors.54,59 Zanubrutinib’s absorption is not impacted by the concurrent use of anti-acid agents. There does appears to be a higher risk for neutropenia with zanubrutinib compared to ibrutinib.59 Zanubrutinib will likely become another important option in CLL/SLL if and when it is approved, pending results from ongoing trials.
Managing Toxicities of BTK Inhibitors
Cardiovascular Toxicity
Important cardiovascular toxicities of BTK inhibitors include arrhythmia, particularly atrial fibrillation, and hypertension. Rare cardiac complications such as ventricular arrhythmia and congestive heart failure have also been reported. Although atrial fibrillation is more common with ibrutinib, it can occur with any of the currently FDA-approved BTK inhibitors. It is recommended that prior to initiation of therapy, a baseline cardiovascular risk assessment be performed.67 In a retrospective review of 168 patients treated with ibrutinib, we have reported that risk factors associated with the development of atrial fibrillation during treatment include pre-existing heart failure and left atrial abnormality on electrocardiogram (ECG). These factors were independently associated with higher risk of arrhythmia in patients treated with ibrutinib.68 Therefore, it is recommended that a baseline ECG and echocardiogram should be obtained prior to initiation of a BTK inhibitor.67,68 Other general risk factors for the development of atrial fibrillation include age > 65, hypertension, and prior exposure to cardiotoxic chemotherapy, and these factors should also be considered in clinical decision-making.69
Patients who have pre-existing well-controlled atrial fibrillation with CHA2DS2-VASc score of 0–1 and patients who are high-risk by cardiac risk assessment can still be considered for initiation of therapy with a BTK inhibitor, although second-generation BTK inhibitor would be preferred over ibrutinib in these situations.67 In the setting of new atrial fibrillation while receiving treatment with a BTK inhibitor, a risk-benefit assessment of continuation of therapy must be performed. The approach for management of atrial fibrillation should be multidisciplinary and involve both the treating oncologist and cardiologist. If the newly diagnosed atrial fibrillation is well controlled on rate or rhythm control, and the patient low-risk (CHA2DS2-VASc score 0–1), BTK inhibitor may be continued. In higher risk patients, especially those with symptomatic atrial fibrillation, BTK inhibitor treatment should be held until the atrial fibrillation is controlled, or discontinued completely, depending on risk and severity of the event.67 Generally, beta-blockers are preferred for rate control in the first-line setting because they are typically well tolerated in conjunction with BTK inhibitors. Calcium channel blockers have a CYP3A4 inhibitory effect, which interacts with ibrutinib by increasing its circulating level, placing the patient at risk for higher toxicity from the drug. Digoxin also interacts with ibrutinib through P-glycoprotein interactions and therefore should be avoided when possible.70 With respect to anticoagulation, the decision is made based on clotting risk (CHA2DS2-Vasc score) and bleeding risk (HAS-BLED score), similarly to management in patients who are not being treated with a BTK inhibitor. The bleeding risk associated with BTK inhibitors should be considered as well. A phase I trial of ibrutinib in CLL/SLL reported a fatal subdural hematoma in a patient who was also receiving warfarin, and subsequent BTK inhibitor trials generally excluded patients on warfarin. Therefore, the use of vitamin K antagonists in these patients is usually avoided.67,71 Direct oral anticoagulants (DOACs) are generally preferred in this setting.70
Hypertension is a reported side effect with the three FDA-approved covalent BTK inhibitors and may develop at any time during the treatment. Optimizing pharmacotherapy for control of baseline hypertension prior to treatment initiation and close blood pressure monitoring during treatment is crucial.67 A retrospective study of 247 patients at 3 institutions treated with ibrutinib identified common practices for treatment of hypertension in patients on ibrutinib. Agents that were used successfully with minimal interaction included beta-blockers, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers. Calcium channel blockers were used occasionally, although their interaction with CYP3A4 should be considered.72
Bleeding
Bleeding is a toxicity common to all available covalent BTK inhibitors, although does appear to occur more frequently with ibrutinib in comparison to the second-generation BTK inhibitors. A comprehensive bleeding risk assessment should be performed prior to initiation of therapy, with efforts concentrated on risk reduction and prevention. Patients should be cautioned against the use of NSAIDs and inadvertent use of aspirin-containing products. Dual antiplatelet therapy (DAPT) increases the risk of major bleeding by 40–50% compared to single antiplatelet therapy, and therefore the use of BTK inhibitor in this setting is not advised. Patients with a recent coronary artery stent requiring DAPT may benefit from delaying the start of BTK inhibitors, if possible, until DAPT is no longer required. Low-dose aspirin should also be used judiciously in these patients. Patients with low or moderate cardiovascular risk who are on a prophylactic low-dose aspirin should consider discontinuing aspirin if starting treatment with a BTK inhibitor. For patients with high cardiovascular risk, single antiplatelet therapy with low-dose aspirin is acceptable. For patients requiring concurrent anticoagulation, as discussed above, vitamin K antagonists are generally avoided, and DOACs or low-molecular weight heparin is preferred. In the event of a planned invasive procedure, evaluate the risks and benefits of withholding ibrutinib for 3 to 7 days prior to and after the surgery, depending on the procedure type and risk of bleeding. In the case of unplanned procedures, if the bleeding risk is high, preventative perioperative platelet transfusion may be considered.73
Low-grade bleeding (grade 0–2) can be managed with supportive care and by temporarily holding ibrutinib. Patients with high-grade bleeding (grade 3–4) requiring hospitalization and blood transfusion should have their BTK inhibitor held, and consider platelet transfusion. It should be noted that ibrutinib may still inhibit transfused platelets if given within 3–4 hours of transfusion, reinforcing the importance of holding the drug in the case of severe bleeding.73
Other Common Toxicities
Other common toxicities include infection, cytopenia, and diarrhea. Diarrhea is generally mild and transient, and is managed with supportive care and antimotility agents. Evening dosing of ibrutinib may also be helpful to mitigate symptoms. Ibrutinib should be held in the event of grade ≥3 diarrhea. In the case of a severe infection, the BTK inhibitor should be held until the start of clinical improvement, and typically can be safely resumed once the infection has resolved. Cytopenias may occur with BTK inhibitor treatment and therefore blood counts should be regularly monitored. Dose reduction should be considered for recurrent treatment-related adverse events according to product information.
Pseudo-Richter Transformation
Although not technically a toxicity, there is a reported phenomenon referred to as Pseudo-Richter transformation that can occur upon temporary holding of ibrutinib for surgery or acute illness. Clinicians should be aware that apparent clinical progression with lymphadenopathy, lymphocytosis, or progressive cytopenia may occur upon holding ibrutinib. In some cases, tissue biopsy may show evidence of large cell lymphoma suggesting Richter transformation. We reported a case series of such phenomenon when upon resuming ibrutinib, clinical signs and symptoms resolved, and the pseudo-large cell transformation regressed on repeat tissue biopsy.74 Therefore, when Richter transformation developed while being temporarily off BTK inhibitor treatment, resumption of BTK inhibitor treatment should be attempted first to see if the transformation may regress, avoiding the need for conventional intensive chemotherapy.
Overcoming Resistance: Third Generation, Non-Covalent-Binding BTK Inhibitors
Despite overall improved outcomes with BTK inhibitors in CLL/SLL, patients may eventually develop resistance to covalent BTK inhibition, and outcomes for these patients were historically poor in the relapsed/refractory setting.75 Resistance can occur through multiple mechanism, although the most common is a BTK mutation substituting the cysteine 481 (C481) residue with an alternative amino acid, commonly serine. This leads to loss of the covalent bond between the covalent BTK inhibitor and the BTK kinase domain.12 Therefore, resistance to one covalent BTK inhibitor confers resistance to all other covalent BTK inhibitors, and there is no rationale to switch between first- and second-generation BTK inhibitors after progression of disease on treatment. Novel third generation, non-covalent binding BTK inhibitors are being developed with the goal of overcoming resistance to covalent BTK inhibitors. The non-covalent BTK inhibitors do not require stabilization from the C481 amino acid with the ATP binding domain, and therefore these BTK inhibitors can continue to inhibit the kinase in the presence of a BTK C481 mutation.12 Pirtobrutinib is a novel non-covalent BTK inhibitor currently being evaluated for the treatment of R/R CLL/SLL after a first- or second-generation BTK inhibitor.76 Updated results from the phase I/II BRUIN study of pirtobrutinib in 323 patients with R/R CLL/SLL were recently presented at ASH 2021. 86% of patients had received therapy with a prior BTK inhibitor, and all patients had received at least two prior lines of therapy. At a median follow-up time of 6 months, the ORR was 63%, and responses appeared to deepen over time, with an ORR of 86% among patients with at least 10 months of follow-up. Toxicity profile interesting appears quite favorable, with no significant cardiac or bleeding events reported.76 ARQ 531 is another non-covalent BTK inhibitor under investigation in R/R lymphoid malignancy including CLL, with phase I data suggesting anti-tumor activity as single-agent therapy, and a manageable safety profile.77 Further data will be necessary to confirm long-term efficacy and safety, however there is good initial evidence that novel non-covalent agents may represent an important therapeutic option in patients who develop resistance to covalent BTK inhibitors.
Conclusion
BTK inhibitors have become an important treatment option in the frontline and R/R setting in CLL/SLL. Determining which patients will benefit most from BTK inhibitors and selecting the most appropriate agent in an individualized manner requires careful considerations of disease and patient comorbidities. Monitoring and management of potential adverse effects is important for the safe long-term use of BTK inhibitors. Resistance to covalent BTK inhibitors might be overcome by non-covalent novel BTK inhibitors. Novel combinations of BTK inhibitors + venetoclax +/- anti-CD20 mAb are currently under investigation and may further expand the role of BTK inhibitors in the treatment of CLL/SLL in the future.78–80
Funding
There is no funding to report.
Disclosure
Dr. Shuo Ma received research funding from Abbvie, AstraZeneca, BeiGene, Janssen, Juno, Loxo, Pharmacyclics, TG Therapeutics; and honorarium from Abbvie, AstraZeneca, BeiGene, Genentech, Janssen, Pharmacyclics, TG Therapeutics. The authors report no other conflicts of interest in this work.
References
1. Catovsky DM, Montserrat E, Harris NL. B-cell prolymphocytic leukaemia. In: World Health Organization Classification of Tumours Pathology and Genetrics of Tumours of Haematopoietic and Lymphoid Tissues. Press I, ed. IARC Press; 2001:131–132.
2. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi:10.1182/blood-2016-01-643569
3. Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. doi:10.1182/blood-2017-09-806398
4. CLL Trialists’ Collaborative Group. Chemotherapeutic options in chronic lymphocytic leukemia: a meta-analysis of the randomized trials. J Natl Cancer Inst. 1999;91(10):861–868. doi:10.1093/jnci/91.10.861
5. Ma S, Platanias LC. Can early intervention with pharmacotherapy reduce the morbidity and mortality of chronic lymphocytic leukemia? Expert Opin Pharmacother. 2018;19(11):1171–1175. doi:10.1080/14656566.2018.1498844
6. Rai KR, Jain P. Chronic lymphocytic leukemia (CLL)—then and now. Am J Hematol. 2016;91(3):330–340. doi:10.1002/ajh.24282
7. Woyach JA, Bojnik E, Ruppert AS, et al. Bruton’s tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL). Blood. 2014;123(8):1207–1213. doi:10.1182/blood-2013-07-515361
8. Small S, Ma S. Frontline treatment for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL): targeted therapy vs. chemoimmunotherapy. Curr Hematol Malig Rep. 2021;16(4):325–335. doi:10.1007/s11899-021-00637-1
9. Scheffold A, Jebaraj BMC, Stilgenbauer S. Venetoclax: targeting BCL2 in hematological cancers. Recent Results Cancer Res. 2018;212:215–242. doi:10.1007/978-3-319-91439-8_11
10. Moia R, Diop F, Favini C, Kodipad AA, Gaidano G. Potential of BCL2 as a target for chronic lymphocytic leukemia treatment. Expert Rev Hematol. 2018;11(5):391–402. doi:10.1080/17474086.2018.1456332
11. Estupiñán HY, Berglöf A, Zain R, Smith CIE. Comparative analysis of BTK inhibitors and mechanisms underlying adverse effects. Review. Front Cell Dev Biol. 2021;9. doi:10.3389/fcell.2021.630942
12. Ahn IE, Brown JR. Targeting Bruton’s tyrosine kinase in CLL. Review. Front Immunol. 2021;12. doi:10.3389/fimmu.2021.687458
13. Kaptein A, de Bruin G, Emmelot-van Hoek M, et al. Potency and selectivity of BTK inhibitors in clinical development for B-cell malignancies. Blood. 2018;132(Supplement 1):1871. doi:10.1182/blood-2018-99-109973
14. Smolej L. On the road to optimized BTK inhibition in CLL. Blood. 2021;137(24):3313–3314. doi:10.1182/blood.2021011462
15. Yang G, Wang J, Tan L, et al. The HCK/BTK inhibitor KIN-8194 is active in MYD88-driven lymphomas and overcomes mutated BTKCys481 ibrutinib resistance. Blood. 2021;138(20):1966–1979. doi:10.1182/blood.2021011405
16. Pal Singh S, Dammeijer F, Hendriks RW. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol Cancer. 2018;17(1):57. doi:10.1186/s12943-018-0779-z
17. Weber ANR, Bittner Z, Liu X, Dang T-M, Radsak MP, Brunner C. Bruton’s Tyrosine kinase: an emerging key player in innate immunity. Mini review. Front Immunol. 2017;8. doi:10.3389/fimmu.2017.01454
18. Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat Rev Cancer. 2014;14(4):219–232. doi:10.1038/nrc3702
19. Traynor-Kaplan AE, Harris AL, Thompson BL, Taylor P, Sklar LA. An inositol tetrakisphosphate-containing phospholipid in activated neutrophils. Nature. 1988;334(6180):353–356. doi:10.1038/334353a0
20. Tzeng SR, Pai MT, Lung FD, et al. Stability and peptide binding specificity of Btk SH2 domain: molecular basis for X-linked agammaglobulinemia. Protein Sci. 2000;9(12):2377–2385. doi:10.1110/ps.9.12.2377
21. Maggie LS. Second-generation BTK inhibitors hit the treatment bullseye with fewer off-target effects. Evid Based Oncol. 2020;26(7):SP226–SP227.
22. Palma M, Mulder TA, Österborg A. BTK inhibitors in chronic lymphocytic leukemia: biological activity and immune effects. Front Immunol. 2021;12:686768. doi:10.3389/fimmu.2021.686768
23. Guarini A, Chiaretti S, Tavolaro S, et al. BCR ligation induced by IgM stimulation results in gene expression and functional changes only in IgV H unmutated chronic lymphocytic leukemia (CLL) cells. Blood. 2008;112(3):782–792. doi:10.1182/blood-2007-12-127688
24. Herishanu Y, Pérez-Galán P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117(2):563–574. doi:10.1182/blood-2010-05-284984
25. Chen SS, Chang BY, Chang S, et al. BTK inhibition results in impaired CXCR4 chemokine receptor surface expression, signaling and function in chronic lymphocytic leukemia. Leukemia. 2016;30(4):833–843. doi:10.1038/leu.2015.316
26. O’Brien SM, Brown JR, Byrd JC, et al. Monitoring and managing BTK inhibitor treatment-related adverse events in clinical practice. Perspective. Front Oncol. 2021;11. doi:10.3389/fonc.2021.720704
27. Sun C, Nierman P, Kendall EK, et al. Clinical and biological implications of target occupancy in CLL treated with the BTK inhibitor acalabrutinib. Blood. 2020;136(1):93–105. doi:10.1182/blood.2019003715
28. Pan Z, Scheerens H, Li S-J, et al. Discovery of selective irreversible inhibitors for Bruton’s Tyrosine kinase. ChemMedChem. 2007;2(1):58–61. doi:10.1002/cmdc.200600221
29. O’Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15(1):48–58. doi:10.1016/S1470-2045(13)70513-8
30. Farooqui MZH, Valdez J, Martyr S, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol. 2015;16(2):169–176. doi:10.1016/S1470-2045(14)71182-9
31. Schnaiter A, Stilgenbauer S. 17p deletion in chronic lymphocytic leukemia: risk stratification and therapeutic approach. Hematol Oncol Clin North Am. 2013;27(2):289–301. doi:10.1016/j.hoc.2013.01.008
32. Moia R, Boggione P, Mahmoud AM, et al. Targeting p53 in chronic lymphocytic leukemia. Expert Opin Ther Targets. 2020;24(12):1239–1250. doi:10.1080/14728222.2020.1832465
33. Al-Sawaf O, Fischer K. TP53 mutations in CLL: does frequency matter? Blood. 2021;138(25):2600–2601. doi:10.1182/blood.2021012343
34. Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. doi:10.1056/NEJMoa1400376
35. Huang X, Qiu L, Jin J, et al. Ibrutinib versus rituximab in relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma: a randomized, open-label Phase 3 study. Cancer Med. 2018;7(4):1043–1055. doi:10.1002/cam4.1337
36. Chanan-Khan A, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17(2):200–211. doi:10.1016/S1470-2045(15)00465-9
37. Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437. doi:10.1056/NEJMoa1509388
38. Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–2528. doi:10.1056/NEJMoa1812836
39. Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432–443. doi:10.1056/NEJMoa1817073
40. Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(1):43–56. doi:10.1016/S1470-2045(18)30788-5
41. Sharman JP, Brander DM, Mato AR, et al. Ublituximab plus ibrutinib versus ibrutinib alone for patients with relapsed or refractory high-risk chronic lymphocytic leukaemia (GENUINE): a phase 3, multicentre, open-label, randomised trial. Lancet Haematol. 2021;8(4):e254–e266. doi:10.1016/S2352-3026(20)30433-6
42. Sharman JP, Brander DM, Mato AR, et al. Effect of adding ublituximab to ibrutinib on PFS, ORR, and MRD negativity in previously treated high-risk chronic lymphocytic leukemia: final results of the GENUINE phase III study. J Clin Oncol. 2020;38(15_suppl):8022. doi:10.1200/JCO.2020.38.15_suppl.8022
43. Burger JA, Sivina M, Jain N, et al. Randomized trial of ibrutinib vs ibrutinib plus rituximab in patients with chronic lymphocytic leukemia. Blood. 2019;133(10):1011–1019. doi:10.1182/blood-2018-10-879429
44. National Comprehensive Cancer Network. Chronic lymphocytic leukemia/small lymphocytic lymphoma. National Comprehensive Cancer Network. Available from: https://www.nccn.org/professionals/physician_gls/pdf/cll.pdf.
45. Jain N, Keating M, Thompson P, et al. Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med. 2019;380(22):2095–2103. doi:10.1056/NEJMoa1900574
46. Rogers KA, Huang Y, Ruppert AS, et al. Phase II study of combination obinutuzumab, ibrutinib, and venetoclax in treatment-naïve and relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(31):3626–3637. doi:10.1200/JCO.20.00491
47. Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323–332. doi:10.1056/NEJMoa1509981
48. Ghia P, Pluta A, Wach M, et al. ASCEND: phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2849–2861. doi:10.1200/JCO.19.03355
49. Byrd JC, Woyach JA, Furman RR, et al. Acalabrutinib in treatment-naive chronic lymphocytic leukemia. Blood. 2021;137(24):3327–3338. doi:10.1182/blood.2020009617
50. Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE-TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278–1291. doi:10.1016/S0140-6736(20)30262-2
51. Tam CS, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851–859. doi:10.1182/blood.2019001160
52. Tam CS, Quach H, Nicol A, et al. Zanubrutinib (BGB-3111) plus obinutuzumab in patients with chronic lymphocytic leukemia and follicular lymphoma. Blood Adv. 2020;4(19):4802–4811. doi:10.1182/bloodadvances.2020002183
53. Tam CS, Giannopoulos K, Jurczak W, et al. SEQUOIA: results of a phase 3 randomized study of zanubrutinib versus Bendamustine + Rituximab (BR) in patients with Treatment-Naïve (TN) Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL). Blood. 2021;138(Supplement1):396. doi:10.1182/blood-2021-148457
54. Hillmen P, Brown JR, Eichhorst BF, et al. ALPINE: zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Future Oncol. 2020;16(10):517–523. doi:10.2217/fon-2019-0844
55. Peter Hillmen BE, Brown JR, Lamanna N, et al. First interim analysis of ALPINE study: results of a phase 3 randomized study of zanubrutinib vs. ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. European Hematology Association (EHA) Annual Meeting 2021. Available from: https://library.ehaweb.org/eha/2021/eha2021-virtual-congress/330170/peter.hillmen.first.interim.analysis.of.alpine.study.results.of.a.phase.3.html.
56. Xu W, Song Y, Li Z, et al. Safety, tolerability and efficacy of orelabrutinib, once a day, to treat Chinese patients with relapsed or refractory chronic lymphocytic leukemia/small cell leukemia. Blood. 2019;134(Supplement_1):4319. doi:10.1182/blood-2019-123331
57. Langerbeins P, Zhang C, Robrecht S, et al. The CLL12 trial: ibrutinib vs placebo in treatment-naïve, early-stage chronic lymphocytic leukemia. Blood. 2022;139(2):177–187. doi:10.1182/blood.2021010845
58. Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;39(31):3441–3452. doi:10.1200/jco.21.01210
59. Tam CS, Opat S, D’Sa S, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood. 2020;136(18):2038–2050. doi:10.1182/blood.2020006844
60. Patel K, Pagel JM. Current and future treatment strategies in chronic lymphocytic leukemia. J Hematol Oncol. 2021;14(1):69. doi:10.1186/s13045-021-01054-w
61. Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910–1916. doi:10.1056/nejm200012283432602
62. Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225–2236. doi:10.1056/NEJMoa1815281
63. Sivina M, Jain N, Kim E, et al. Ibrutinib induces durable remissions in treatment-naïve CLL patients with 17p deletion/TP53 mutations: five year follow-up from a phase 2 study. Blood. 2020;136(Supplement1):22–23. doi:10.1182/blood-2020-141014
64. Lévy V, Delmer A, Cymbalista F. Frontline treatment in CLL: the case for time-limited treatment. Hematology. 2021;2021(1):59–67. doi:10.1182/hematology.2021000233
65. Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15(10):1090–1099. doi:10.1016/S1470-2045(14)70335-3
66. Levavi H, Lancman G, Gabrilove J. Impact of rituximab on COVID-19 outcomes. Ann Hematol. 2021;100(11):2805–2812. doi:10.1007/s00277-021-04662-1
67. Lipsky A, Lamanna N. Managing toxicities of Bruton tyrosine kinase inhibitors. Hematology. 2020;2020(1):336–345. doi:10.1182/hematology.2020000118
68. Lentz R, Feinglass J, Ma S, Akhter N. Risk factors for the development of atrial fibrillation on ibrutinib treatment. Leuk Lymphoma. 2019;60(6):1447–1453. doi:10.1080/10428194.2018.1533129
69. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(36):2768–2801. doi:10.1093/eurheartj/ehw211
70. Essa H, Lodhi T, Dobson R, Wright D, Lip Gregory YH. How to manage atrial fibrillation secondary to ibrutinib. JACC. 2021;3(1):140–144. doi:10.1016/j.jaccao.2020.11.016
71. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi:10.1056/NEJMoa1215637
72. Roeker LE, Sarraf Yazdy M, Rhodes J, et al. Hypertension in patients treated with ibrutinib for chronic lymphocytic leukemia. JAMA Netw Open. 2019;2(12):e1916326–e1916326. doi:10.1001/jamanetworkopen.2019.16326
73. Shatzel JJ, Olson SR, Tao DL, McCarty OJT, Danilov AV, DeLoughery TG. Ibrutinib-associated bleeding: pathogenesis, management and risk reduction strategies. J Thromb Haemost. 2017;15(5):835–847. doi:10.1111/jth.13651
74. Barnea Slonim L, Ma S, Behdad A, Chen Q. Pseudo-Richter transformation of chronic lymphocytic leukaemia/small lymphocytic lymphoma following ibrutinib interruption: a diagnostic pitfall. Br J Haematol. 2020;191(1):e22–e25. doi:10.1111/bjh.16948
75. Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125(13):2062–2067. doi:10.1182/blood-2014-09-603670
76. Mato AR, Pagel JM, Coombs CC, et al. Pirtobrutinib, a next generation, highly selective, non-covalent BTK inhibitor in previously treated CLL/SLL: updated results from the phase 1/2 BRUIN study. Blood. 2021;138(Supplement1):391. doi:10.1182/blood-2021-147599
77. Woyach J, Stephens DM, Flinn IW, et al. Final results of phase 1, dose escalation study evaluating ARQ 531 in patients with relapsed or refractory B-cell lymphoid malignancies. Blood. 2019;134(Supplement_1):4298. doi:10.1182/blood-2019-127260
78. Wierda WG, Allan JN, Siddiqi T, et al. Ibrutinib plus venetoclax for first-line treatment of chronic lymphocytic leukemia: primary analysis results from the minimal residual disease cohort of the randomized phase II CAPTIVATE study. J Clin Oncol. 2021;39(34):3853–3865. doi:10.1200/jco.21.00807
79. Davids MS, Lampson BL, Tyekucheva S, et al. Acalabrutinib, venetoclax, and obinutuzumab as frontline treatment for chronic lymphocytic leukaemia: a single-arm, open-label, phase 2 study. Lancet Oncol. 2021;22(10):1391–1402. doi:10.1016/S1470-2045(21)00455-1
80. Julia VT, Carsten N, Kater AP, et al. The GAIA (CLL13) trial: an international intergroup phase III study for frontline therapy in chronic lymphocytic leukemia (CLL). J Clin Oncol. 2018;36(15_suppl):TPS7582–TPS7582. doi:10.1200/JCO.2018.36.15_suppl.TPS7582
81. Brown JR, Barrientos JC, Barr PM, et al. The Bruton tyrosine kinase inhibitor ibrutinib with chemoimmunotherapy in patients with chronic lymphocytic leukemia. Blood. 2015;125(19):2915–2922. doi:10.1182/blood-2014-09-585869
82. Jaglowski SM, Jones JA, Nagar V, et al. Safety and activity of BTK inhibitor ibrutinib combined with ofatumumab in chronic lymphocytic leukemia: a phase 1b/2 study. Blood. 2015;126(7):842–850. doi:10.1182/blood-2014-12-617522
83. Davids MS, Brander DM, Kim HT, et al. Ibrutinib plus fludarabine, cyclophosphamide, and rituximab as initial treatment for younger patients with chronic lymphocytic leukaemia: a single-arm, multicentre, phase 2 trial. Lancet Haematol. 2019;6(8):e419–e428. doi:10.1016/S2352-3026(19)30104-8
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.