Back to Journals » Journal of Asthma and Allergy » Volume 15
Use of Biologic Therapies in the Treatment of Asthma – A Comparative Real World Data Analysis on Healthcare Resource Utilization and Costs Before and After Therapy Initiation
Authors Hardtstock F , Krieger J , Wilke T , Lukas M, Ultsch B, Welte R, Quinzler R, Maywald U, Timmermann H
Received 23 December 2021
Accepted for publication 4 March 2022
Published 5 April 2022 Volume 2022:15 Pages 407—418
DOI https://doi.org/10.2147/JAA.S354062
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Fraence Hardtstock,1 Julia Krieger,1 Thomas Wilke,1 Marco Lukas,2 Bernhard Ultsch,2 Robert Welte,2 Renate Quinzler,3 Ulf Maywald,4 Hartmut Timmermann5
1IPAM e.V, Wismar, Germany; 2GlaxoSmithKline GmbH & Co. KG, München, Germany; 3AOK Baden-Württemberg, Stuttgart, Germany; 4AOK PLUS, Dresden, Germany; 5Schwerpunktpraxis Colonnaden, Hamburg, Germany
Correspondence: Fraence Hardtstock, Email [email protected]
Background: Asthma is one of the most common chronic diseases in Germany. While many patients achieve asthma control under standard therapies, some patients still experience exacerbations and persistent airway obstructions. Thus, further pharmacological treatment is needed, and biologics could fill this gap, as they have shown clinical benefit in patients with severe asthma. Therefore, this real-world study aimed to compare healthcare resource utilization (HCRU) and associated costs before and after biologic therapy initiation.
Methods: A retrospective claims data analysis has been conducted on adult asthma patients who initiated a long-term biologic therapy between January 2015 and June 2018. Patients were therapy-naïve to biologics for at least 12 months. HCRU and cost incurred by patients during 12 months before and after therapy initiation were compared.
Results: Overall, 571 asthma patients initiated a biologic therapy during the observational period (316 omalizumab, 232 mepolizumab, 16 benralizumab, and 7 reslizumab). Patients had a mean age of 54.86 (62.70% female), and the majority (93.70%) received at least one follow-up prescription of their index-biologic agent within one year. During baseline, patients received on average 2.75 OCS prescriptions compared to 2.17 during follow-up. Most patients received less or the same amount of OCS after therapy initiation. Moreover, hospitalizations and asthma-related sick leave days decreased significantly. The average total costs per patient were € 6618.90 during baseline and € 22,832.33 during follow-up. Biologics mainly drove the increase; however, hospitalization costs were reduced significantly (€ 2443.37 vs € 1941.93; p< 0.001).
Conclusion: Our study demonstrates an improved asthma control due to the initiation of a biologic therapy in terms of decreased hospitalization frequency, OCS consumption, and sick leave days. However, biologics are associated with high costs for healthcare providers during the first year after initiation. Therefore, short- and long-term clinical benefits and financial burden must be considered in the overall context of healthcare.
Keywords: biologics, treatment pattern, claims data, real-world evidence, Germany
Background
About 4–6% of the adult population have been diagnosed with asthma in Germany, making it one of the most common chronic diseases.1–3 Asthma is a heterogeneous disease and can be stratified across several phenotypes (ie, allergic asthma, eosinophilic asthma, intrinsic asthma, and neutrophilic asthma) and endotypes (ie, T2-high and non-T2-high groups), featured by distinct clinical and pathobiological expressions as well as by different responses to pharmacological treatments based on a biomarkers panel.4–6 Further subgroups, such as early- and late-onset allergic asthma, and severe persistent eosinophilic asthma, can be distinguished.7,8 Although a large proportion of asthma patients respond to standard therapies such as inhaled corticosteroids (ICS), long-acting beta-agonists (LABA), or tiotropium, some patients do not achieve asthma control under these therapies.8–10 These patients, who experience more frequent (≥2/year) asthma exacerbations or have persistent airway obstructions despite therapy with high-dose ICS (in combination with LABA) or treatment with oral corticosteroids (OCS) for more than six months are generally classified as severe asthma cases.11–13 Even though severe uncontrolled asthma affects only about 4% of all asthma patients, however, it increases the burden of asthma and is associated with high humanistic and economic costs.9,14,15
Often, patients with severe asthma receive OCS as a short-term treatment of exacerbations but also as a controller option. However, when administrated daily for one year or longer, even if at a very low dosage (ie, ≤7.5mg/day) OCS was found to cause various complications and adverse effects, among others, various infections (eg, pneumonia and herpes zoster), bone and muscle disease, atrial fibrillation, and hypertension.16–19 Therefore, over the last decade, several innovative therapies such as mepolizumab, omalizumab, reslizumab, benralizumab, and dupilumab have been approved for use in patients with severe uncontrolled asthma.8,11,20 These biologic therapies focus on specific cytokines of the T2-high pathway, including interleukin IL-4, IL-13, and IL-5. For example, omalizumab targets IgE by using antibodies that block IgE from binding to its high-affinity receptor,21 whereas mepolizumab, benralizumab, and reslizumab are humanized monoclonal antibodies targeting the IL-5 receptor.22,23 Dupilumab is another humanized monoclonal antibody that was most recently approved for asthma in 2019 and targets the IL-4 receptor and inhibits activity in IL-4 and IL-13 receptors.20,24 For severe asthma patients, biologic therapies have shown improvements in lung capacity, reduced asthma exacerbation rates, and a decreased need for corticosteroids.16,25,26 In 2018, biologic therapies had been included in the step-by-step medication scheme of the Global Initiative for Asthma guideline and are becoming the new standard of care for patients with severe uncontrolled asthma.11,17 Although the clinical benefits of biologics for the treatment of severe asthma have been well described,17,25,27 data on the use of biologics and their impact in the real world is limited. Therefore, this study aimed to describe the usage of biologics in the treatment of asthma and compare healthcare resource utilization (HCRU) and associated direct and indirect costs before and after therapy initiation, based upon a large dataset of health insurance records from asthma patients in Germany.
Materials and Methods
Data and Study Population
This study was a retrospective, non-interventional cohort analysis of biologic-naïve asthma patients who initiated treatment with a biologic agent, utilizing anonymized routine health insurance data from two German sickness funds (AOK PLUS and AOK BW). Complete statutory insurance data of 7.7 million persons (ie, 8–9% of the total German population) on inpatient and outpatient care, including diagnoses, procedures, pharmaceutical, and non-pharmaceutical prescriptions and interventions, as well as related costs for the period from 1 January 2014 to 30 June 2019, were available. All patients with continuous enrolment to their respective sickness fund for the total observational period (death as an exception) represent the basis for the analysis (approximately 4.92 million insured persons). Because of the anonymization of the data, ethical approval from an institutional review board (IRB) was not required; however, the data owners (sickness funds) approved the project and planned analysis prior to data access.
The selection of the study population was based on diagnoses codes, documented according to the International Statistical Classification of Diseases and Related Health Problems 10. Revision, German Modification ICD-10-GM, and outpatient medication prescriptions recorded based upon the Pharmaceutical Central Number (PZN) and the Anatomical Therapeutic Chemical Classification System (ATC) for active substances. Patients with asthma (ICD-10-GM J45/J46) were included if they received either (i) at least one primary inpatient code, (ii) at least two confirmed outpatient diagnoses, and/or (iii) at least one secondary inpatient code in addition to at least one confirmed outpatient code diagnosis from any physician, between 1 January 2014 and 30 June 2018. If a patient was included due to two asthma codes, these codes must have been documented within 365 days. The index date was defined as the first prescription of a biologic medication (mepolizumab, omalizumab, reslizumab, or benralizumab) between 1 January 2015 and 30 June 2018. Patients were excluded if they received a biologic prescription between 1 January 2014 and the first observed asthma diagnosis during the observational period (therapy experienced patients) or if they were under 18 years of age at the index prescription date.
Outcomes and Statistical Analysis
Based on all included asthma patients who started treatment with one of the selected biological agents, an analysis was performed comparing medication use, hospitalizations, and sick leaves during the 12-months before and after therapy initiation with biologics. The number of patients with at least one prescription, the number of prescriptions, and the average dosage received per patient were calculated for relevant (asthma) medications such as ICS, LABA, leukotriene receptor antagonists (LTRA), OCS, and others. Each patient’s total prescription supply (measured in days) was calculated based on the number of packages received by patients, each package’s size (eg, number of tablets, capsules, or single doses), the concentration of the active substance in each package, and the defined daily doses (DDD) for medications, according to the World Health Organization (WHO). In addition, combination therapies have been observed and defined as either a prescription of a fixed-dose combination or at least one prescription of each agent class of the specified combinations prescribed within 60 days. Hospitalizations were identified based on documented overnight stays during the 12-months baseline and follow-up period.
Direct costs incurred due to HCRU of patients during baseline and follow-up periods were reported descriptively and compared through Wilcoxon signed-rank test. Costs (in Euro) were measured from a societal perspective and analyzed cumulatively and separately for the following categories: (i) hospitalizations, including procedures and medication (based on cases-specific reimbursements for hospital stays), (ii) outpatient visits to physicians (based on documented treatment points per visit and respective point values (Euro) valid at that time), (iii) outpatient medication prescriptions (based on pharmacy sales prices), (iv) rehabilitation (case-specific reimbursements per rehabilitation stay), and (v) prescriptions of aids and remedies (based on prescription-individual direct costs documented in the database). Direct costs have been adjusted for inflation, using the health-specific consumer price index (CPI) stated by the German federal statistical office (reference year was 2015).28 In addition, indirect costs due to sick leaves were analyzed by the human capital approach, once for any documented sick leave and once for those sick leaves associated with an asthma diagnosis. Indirect costs due to sick leaves were approximated based on the number of days absent from work, as documented in the dataset, multiplied by the average compensation for an employee in Germany per day, calculated based on published statistics on the number of employees and employees’ compensation as published by the federal statistical office.29,30
All reported p-values were two-sided, and all analyses were carried out using Microsoft SQL Server 2019, Microsoft Excel 2019 (Microsoft Corporation, Redmond, WA), and Stata version 16.1 software (Stata Statistical Software: Release 14, StataCorp, College Station, TX).
Results
Study Sample
We identified 388,932 patients with confirmed asthma from 4.92 million continuously insured adults, which corresponds to a proportion of 7.90% within the total observed population. Of these patients, 651 (0.17%) were found to have received a biologic therapy (mepolizumab, omalizumab, reslizumab, or benralizumab) during the observational period. However, 80 patients were excluded from the analysis as they already received biologics in the 12-month baseline period or before their first observed asthma diagnosis. Thus, the final analysis sample included 571 asthma patients who initiated a biologic therapy between 1 January 2015 and 30 June 2018 and had an age of at least 18 years at the time of their first biologic prescription (index date) (Figure 1).
 |
Figure 1 Selection of patients with asthma, starting a biologic therapy. |
Patients who started therapy with biologics were on average 54.86±13.96 years old, which is similar to the age of all 388,932 asthma patients in the baseline cohort (mean 54.82±19.56). The proportion of females was slightly higher among biologic patients (62.70%) than in the general asthma cohort (59.47%). All patients were observed for 12 months before their index prescription and 12 months after their index prescription. However, five patients deceased during the follow-up period and were therefore only followed until death. Thus, the average follow-up period of the study population was 362.89 days. Overall, 27.85% of the patients who started a biologic therapy were also diagnosed with urticaria (18.39%) and/or atopic dermatitis (14.01%), two indications also known to be treated with biologics. The proportion of patients with concomitant diagnosis of urticaria and/or atopic dermatitis was highest in the group of omalizumab users (41.32%) (Table 1).
 |
Table 1 Baseline Characteristics of Observed Patients |
Omalizumab, which received a marketing authorization valid throughout the EU in 2005, was also the most common index agent observed (N=316; 55.34%), followed by mepolizumab (N=232; 40.63%), benralizumab (N=16; 2.80%), and reslizumab (N=7; 1.23%). The overall percentage of patients with each agent reflects the agents’ different market authorization dates and different market shares of these agents (Figure 2).
 |
Figure 2 Patient sample by index agent and index date (first prescription). |
Treatment
The majority of patients (535; 93.70%) received at least one follow-up prescription of their index-biologic within one year of follow-up. In addition to their index-biologic, 33 of these patients also received other biologic agents within the follow-up year. In contrast, 36 patients (6.30%) did not receive a follow-up prescription of their index-biologic. However, two of these patients received prescriptions of other biologics within the follow-up year.
During the 12 months baseline period, 526 patients (92.12%) received at least one drug prescription related to the respiratory system (R03 – drugs for obstructive airway disease). Furthermore, most of the patients received long-term non-biologic asthma therapies (523 patients, 91.59%). Of these, 474 patients (83.01%) received a combination therapy of ICS and LABA (with or without additional prescriptions of tiotropium and/or LTRA), two patients (0.35%) received ICS and LTRA in combination, and 47 patients (8.23%) received only monotherapies during the baseline year. During the follow-up period, the proportion of patients with non-biologic long-term asthma medications decreased for most agents slightly, as presented in Table 2.
 |
Table 2 Medication Prescriptions During 12-Months Baseline and Follow-Up Period |
Moreover, 437 patients (76.53%) received at least one OCS prescription (ATC H02-, oral administration forms only) during the baseline period. The mean number of OCS prescriptions per patient was 2.75±2.77, and patients with at least one OCS prescription received on average OCS to cover 235±219 days at the DDD. After therapy initiation with biologics, the proportion of patients with OCS decreased from 76.53% to 60.60%, and the number of respective prescriptions per patient was reduced to 2.17±2.70. In total, 295 patients (51.66%) received OCS during the baseline and follow-up periods (Table 3). Of these patients, 63.05% received less or the same amount of OCS during the follow-up period (mean: 332.31±256.18), as compared to the baseline period (mean: 167.49±165.28), and 36.95% received more OCS supply after starting atherapy with biologics (+176 days of medication supply).
 |
Table 3 OCS Supply During 12-Months Baseline and Follow-Up Period |
Hospitalizations and Sick Leaves
After starting treatment with a biologic agent, the proportion of patients experiencing hospitalizations decreased significantly from 42.38% during the baseline period to 31.87%. Moreover, the number of hospital days decreased significantly, from 6.95±14.74 days on average per patient during the baseline period to 5.07±13.92 days in the follow-up period. The same applied to asthma-related hospitalizations, where a significant reduction was found within the patient population, as described in Table 4. For sick leave days related to any diagnosis, no significant change in the total number of patients with at least one sick leave day was observed (p=0.180). However, there was a tendency (although not significant) towards fewer sick leave days per patient in the follow-up period (54±95.47 days versus 68±111.74 days in the baseline period). In contrast, in the analysis of asthma-related sick leave days, the number of patients with at least one sick leave day decreased from 28.07% to 20.14% (p<0.001), but the average number of sick leave days of affected patients remained at a stable level (73 days during both periods; p=0.409).
 |
Table 4 Hospitalizations and Sick Leave Days During Baseline and Follow-Up Period |
Healthcare Cost
The average all-cause direct costs generated by a patient who initiated a therapy with biologics were €6618.90 ± €9712.38 in the baseline period and €22,832.33 ± €13,717.74 in the follow-up period (Figure 3). Whereas hospitalization costs were the main cost driver during the baseline period (36.92% of the incurred costs), costs associated with outpatient medication contributed to a substantial increase of total costs in the follow-up period. However, the observed increase in costs due to outpatient medication prescription between the baseline and follow-up period was mainly driven by biologics (€0 during baseline versus €16,243.20 per patient during follow-up). There was no significant change in the cost due to non-biologic asthma-related prescriptions (any ATC- R03 (without biologics) and OCS), with average costs of €979.18 per patient in the baseline period and €985.75 during the follow-up period (p>0.1). Moreover, costs incurred due to other medication (not related to asthma) slightly increased from €1248.28 to €1532.07€ per patient (p<0.001).
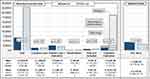 |
Figure 3 All-cause healthcare expenditures per patient during 12-month baseline and follow-up period. |
The total hospitalization costs per patient were reduced significantly (p<0.001) from €2443.37±€5870.49 during the baseline period to €1941.93±€5776.12 during the follow-up period. Furthermore, sick leave related indirect costs due to loss of productivity decreased from €4282.59±€12,176.02 per patient during the baseline period (€2538.44 due to asthma-related sick leaves), to €3170.32 ± €9948.36 per patient during the follow-up period (€1750.14 due to asthma-related sick leaves). There were no statistically significant differences observed in costs due to outpatient physician visits and provided care (p=0.197) and costs related to rehabilitations (p=0.511). In contrast, expenses related to medication prescriptions of (any kinds of) aids and remedies have slightly increased during the post-index period (p=0.033). However, these represent only a small proportion of the total incurred costs.
Discussion
This real-world study used a large representative database to describe the pharmacological treatment of asthma patients who initiated therapy with a biologic agent and compare HCRU and incurred healthcare expenditures before and after treatment. Since claims data from different regions in Germany were used in the analysis, ultimately representing approximately 8–9% of the overall German population, study site or patient selection bias could be avoided, and complete data on patients’ outpatient and inpatient care was available. The data, primarily collected for billing and reimbursement purposes, includes complete information on patients’ HCRU across inpatient and outpatient healthcare facilities and resulting expenditures of the statutory German health care system.
Based on 4.92 million continuously insured adults in our database, a proportion of 7.90% with confirmed asthma has been identified, which is in line with previous estimations for Germany.1,2 Within the total asthma population (N=388,932), and over a period of 3.5 years, only 651 patients (0.17%) received a biological therapy approved for severe asthma, indicating a low proportion of patients receiving this type of therapy. However, biologics are only approved for use in a particular patient population, namely those with severe asthma, who fail to achieve disease control under (standard) treatment with other long-term therapies such as high-dose ICS in combination with LABA. Previous publications reported a wide range in terms of the proportion of patients with severe asthma among all asthma patients, ranging from 1% to more than 30%.15,31–33 This reflects the uncertainty around the identification of this population in a real-world setting. Our study indicates an increase in the proportion of patients treated with biologics over time, and further growth is expected, as some of the observed biologic therapies have been approved recently, and more therapies are expected to be approved. Furthermore, the study relies on the assumption that the ICD-10-GM codes J45 and J46 accurately identify asthma and are correctly used by physicians. Since German claims databases contain data from routine practice, data may be missing or subject to coding errors regarding outpatient diagnoses. However, the coding of the database is generally considered to be of high quality.43,44 Furthermore, the specific biologic asthma medication used to identify patients starting with biologic therapies may reflect German-specific clinical practices only, maybe limiting the generalizability of findings to other countries. However, uniform healthcare regulations, reimbursement conditions, data entry requirements, access to health resources, and treatment of patients are not expected to diverge strongly across different sickness funds and regions, which increases the generalizability in Germany.
The analysis confirmed that biologics can effectively reduce the number of asthma-related hospital admissions, which can be considered a proxy of severe exacerbations.9 After therapy initiation, the proportion of patients experiencing asthma-related hospitalizations decreased significantly from 16.99% in the baseline to 7.18% in the follow-up period. Also, the number of hospitalization days per patient strongly decreased by 56% after therapy initiation (1.15 days during follow-up versus 2.06 days during baseline). In line with our results, previous clinical trials and observational studies showed a reduction in exacerbation rates under treatment with biologics.14 For example randomized controlled trials (RCTs) showed that benralizumab reduces the exacerbation frequency by 29–70% (compared to placebo),34–36 and mepolizumab by 39–52%.23,37 Moreover, a recent real-world analysis that described the rate of severe exacerbations (requiring hospitalization) before and after therapy initiation with mepolizumab reported a reduction by 69%.38 Besides a decrease in the hospitalization frequency, our study also revealed a significant reduction in asthma-related sick leaves, with 28.07% of patients with at least one sick leave day during the baseline period compared to 20.14% during the follow-up period. However, sick leave days of those with asthma-related sick leaves remained high (73 days per patient per year).
Due to the reduced hospitalization rate and sick leave days, also costs related to inpatient care (€2443.37 per patient during baseline vs €1941.93 during follow-up) as well as indirect costs due to sick leaves (€4282.59 vs €3170.32) were significantly lower after biologic therapy initiation in our study. This contrasts with the costs incurred due to the biologic agents themselves, as presented in this analysis (€0 during baseline vs €16,243.20 during follow-up), leading to a substantial increase in overall healthcare expenditures. Therefore, in a short-term view of only one year, the initiation of biologics therapy can only partly proof its comprehensive positive effect. Thus, higher costs associated with such treatment must be weighed against positive short-term and long-term effects on clinical outcomes, HCRU, and work productivity. Importantly, biological therapy for treating severe asthma has been shown to improve “patients” health-related quality of life.39,40 Further health economic studies that compared biologic treatment with other non-biologic treatment (ie ICS and OCS) were able to show the cost-effectiveness of biologics considering that the costs per quality of life gained being below the threshold of €50,000.1,2 Moreover, our study confirmed that severe asthma patients require less OCS after initiating a biologic therapy, which shall translate into a lower frequency of long-term side effects and OCS-associated complications, as described in previous literature.16 This observation is in line with previous studies that reported a decrease in OCS consumption (besides the general reduction of other asthma medications) based upon the example of omalizumab users.41,42 Further analysis should primarily focus on these long-term aspects of treatment with biologics and patient-centered aspects that need to be considered in the treatment decision process.
With respect to the medications examined in this analysis, it should be noted that while data on outpatient medication prescriptions are considered complete, there may be a lack of reporting regarding pharmacological interventions applied within the inpatient setting and other interventions which do not qualify for medical claims (ie, over-the-counter medicines). However, most of the agents used to treat asthma require a prescription, and asthma patients usually only undergo inpatient treatment in the event of an exacerbation and are otherwise treated in an outpatient setting.45 It should be noted that dupilumab could not be considered for comparing figures of baseline and follow-up period, as it was only recently approved to treat asthma. Furthermore, it cannot be precisely determined whether the investigated biologic agents were prescribed to treat asthma or another present indication for which biologics are also approved (ie, urticaria, atopic dermatitis). This is particularly noticeable due to comparatively high rates of urticaria and atopic dermatitis within our population and a small proportion of patients who did not receive other long-term asthma medications before the initiation of a biologic. Characteristics and medication of affected patients were examined in more detail in a post-hoc analysis to quantify a possible bias, with the result that only twelve patients (26.67%) from the sample received diagnoses of urticaria or atopic dermatitis and no asthma medications before the initiation of biologic therapy. In addition to these considerations, it should be noted that the study results might be driven by other underlying comorbidities of the patient population that were not observed. This is particularly relevant given the multiple uses of some of the observed medications (ie, OCS) and should be further investigated in future studies.
Moreover, some cost-relevant aspects might be unobserved within this study, ie, out-of-pocket expenses for patients. However, due to the German reimbursement system, these are generally very low and therefore negligible in the overall context. In addition, the reimbursement of inpatient care in Germany is based on the DRG system, which provides for a flat-rate payment per hospital stay within certain DRG groups. However, certain cost-relevant aspects of the individual stays are not necessarily considered and are therefore not depicted.
Finally, it should be noted that the reported costs may leave some aspects unconsidered. For example, the analyzed sick leave days do not consider undocumented absence days and reduced productivity due to symptoms. In addition, early retirement attributable to severe asthma was not considered in the analysis. Thus, the actual impact on work loss and productivity might be higher.
Conclusions
Despite their effectiveness, which has been proven in several previous studies, biologics are only prescribed in a small proportion of patients with asthma. However, improved asthma control due to biologics can effectively decrease hospitalizations, consumption of OCS, and the number of sick leave days. Since a considerable level of investment is required by health systems in relation to the use of biologic therapy, a precise selection of asthma patients who require biologics to achieve asthma control is essential.
Abbreviations
ATC, anatomical therapeutic chemical classification system; CPI, consumer price index; DDD, defined daily doses; HCRU, healthcare resource utilization; ICS, inhaled corticosteroids; ICD-10, International Statistical Classification of Diseases and Related Health Problems 10; LABA, long-acting beta-agonists; LTRA, leukotriene receptor antagonists; OCS, oral corticosteroids; PZN, pharmaceutical central number; RCTs, randomized controlled trials; WHO, World Health Organization.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are not publicly available since the findings of this study are extracted from individual patient records. Data were available for research purposes from the sickness fund upon request, in an anonymized form. Due to restrictions around revealing “patients” confidential information, data were used under license for the current study, and so are neither publicly available nor can be shared further.
Ethics Approval
This study involved the use of two anonymized health insurance claims datasets provided free of charge by AOK PLUS and AOK BW under formal agreement and legal basis of §75, Tenth Book of the Social Code (SGB X). No ethical approval from an institutional review board was required to implement this study, as a result of the anonymization of all patient data provided by the sickness funds.
Acknowledgments
Funding for this study was provided by GlaxoSmithKline.
Author Contributions
F.H., J.K. and T.W. conducted the analysis based upon the anonymized datasets that were provided free of charge by the sickness funds to support this research. Only IPAM had access to the anonymized datasets, which only captured information relevant to the project and specified within the research protocol. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Fränce Hardtstock and Julia Krieger participated in this study as staff members of IPAM; the work of IPAM in this study was financed by GlaxoSmithKline GmbH & Co. KG. Thomas Wilke is the founder of IPAM e.V., and has received honoraria from various pharmaceutical companies, including Novo Nordisk, Janssen Pharmaceutica, Boehringer Ingelheim Pharma, Bayer Health Care, Novartis, Sanofi, Pfizer and GlaxoSmithKline. Marco Lukas, Bernhard Ultsch and Robert Welte are GlaxoSmithKline employees and shareholders. Renate Quinzler is an employee of AOK Baden-Württemberg. Ulf Maywald is an employee of AOK PLUS. Hartmut Timmermann is affiliated with Schwerpunktpraxis Colonnaden and has received consultancy fees and grants from several pharmaceutical companies, including AstraZenica, Takeda, Bayer Health Care, Almirall, Astrellas Pharma, Novartis, Sanofi, Leti Pharma, Boehringer Ingelheim Pharma, Meda Pharmaceuticals, Mundipharma, Pfizer, Nycomed, Berlin-Chemie, Teva and GlaxoSmithKline. The authors report no other conflicts of interest in this work.
References
1. Steppuhn H, Kuhnert R, Scheidt-Nave C. 12-month prevalence of asthma among adults in Germany. J Health Monit. 2017;2(3):34–42.
2. Stock S, Redaelli M, Luengen M, Wendland G, Civello D, Lauterbach KW. Asthma: prevalence and cost of illness. Eur Respir J. 2005;25(1):47–53. doi:10.1183/09031936.04.00116203
3. Khan A, Sternbach N, Kamat S, Annunziata K, Jaffe D, Gouia I. Prevalence of asthma in France, Germany, Italy, Spain, and the United Kingdom, based on the 2018 European National Health and Wellness Survey. Chest. 2020;158(4):A27. doi:10.1016/j.chest.2020.08.067
4. Agache I, Akdis C, Jutel M, Virchow JC. Untangling asthma phenotypes and endotypes. Allergy. 2012;67(7):835–846. doi:10.1111/j.1398-9995.2012.02832.x
5. De Groot JC, Storm H, Amelink M, et al. Clinical profile of patients with adult-onset eosinophilic asthma. ERJ Open Res. 2016;2(2):1–8. doi:10.1183/23120541.00100-2015
6. Kuruvilla ME, Lee FEH, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56(2):219–233. doi:10.1007/s12016-018-8712-1
7. Verhamme KM, Engelkes M, de Ridder M, et al. Characteristics of adult onset vs. late onset asthma - a multinational database cohort study. Eur Respir J. 2017;50:OA316. European Respiratory Society (ERS)
8. Buhl R, Humbert M, Bjermer L, et al. Severe eosinophilic asthma: a roadmap to consensus. Eur Respir J. 2017;49(5):1700634. doi:10.1183/13993003.00634-2017
9. Peters SP, Ferguson G, Deniz Y, Reisner C. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med. 2006;100(7):1139–1151. doi:10.1016/j.rmed.2006.03.031
10. Numata T, Miyagawa H, Nishioka S, et al. Efficacy of benralizumab for patients with severe eosinophilic asthma: a retrospective, real-life study. BMC Pulm Med. 2020;20(1):1–10. doi:10.1186/s12890-020-01248-x
11. Global Initiative for Asthma. Pocket Guide for Asthma Management and Prevention. Global Initiative for Asthma; 2019:1–32.
12. Hamelmann E. Managing severe asthma: a role for the long-acting muscarinic antagonist tiotropium. Biomed Res Int. 2018;2018:7–9. doi:10.1155/2018/7473690
13. Global Initiative for Asthma. Difficult-To-Treat and Severe Asthma. 2018.
14. Chen S, Golam S, Myers J, Bly C, Smolen H, Xu X. Systematic literature review of the clinical, humanistic, and economic burden associated with asthma uncontrolled by GINA steps 4 or 5 treatment. Curr Med Res Opin. 2018;34(12):2075–2088. doi:10.1080/03007995.2018.1505352
15. Caminati M, Senna G. Uncontrolled severe asthma: starting from the unmet needs. Curr Med Res Opin. 2019;35(2):175–177. doi:10.1080/03007995.2018.1528218
16. Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. 2018;52(4):1800703. doi:10.1183/13993003.00703-2018
17. Cataldo D, Louis R, Michils A, et al. Severe asthma: oral corticosteroid alternatives and the need for optimal referral pathways. J Asthma. 2021;58(4):448–458. doi:10.1080/02770903.2019.1705335
18. Dalal AA, Duh MS, Gozalo L, et al. Dose-response relationship between long-term systemic corticosteroid use and related complications in patients with severe asthma. J Manag Care Spec Pharm. 2016;22(7):833–847. doi:10.18553/jmcp.2016.22.7.833
19. Bloechliger M, Reinau D, Spoendlin J, et al. Adverse events profile of oral corticosteroids among asthma patients in the UK: cohort study with a nested case-control analysis. Respir Res. 2018;19(75). doi:10.1186/s12931-018-0742-y
20. Manka A, Wechsler E. Selecting the right biologic for your patients with severe asthma. Ann Allergy Asthma Immunol. 2018;121(4):406–413. doi:10.1016/j.anai.2018.07.033
21. Thomson NC, Chaudhuri R. Omalizumab: clinical use for the management of asthma. Clin Med Insights Circ Respir Pulm Med. 2011;6(1):27–40.
22. Hashimoto S, Bel EH. Targeting IL-5 in severe asthma: a DREAM come true? Lancet. 2012;380:9842. doi:10.1016/S0140-6736(12)61132-5
23. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi:10.1016/S0140-6736(12)60988-X
24. European Medicines Agency. New add-on treatment for patients with severe asthma; 2019. Available from: https://www.ema.europa.eu/en/news/new-add-treatment-patients-severe-asthma.
25. Mcgregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433–445. doi:10.1164/rccm.201810-1944CI
26. Agache I, Beltran J, Akdis C, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI guidelines - recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1023–1042. doi:10.1111/all.14221
27. Yancey SW, Keene ON, Albers FC, et al. Biomarkers for severe eosinophilic asthma. J Allergy Clin Immunol. 2017;140(6):1509–1518. doi:10.1016/j.jaci.2017.10.005
28. Federal Statistical Office. Consumer price index for Germany; 2021. Available from: https://www.destatis.de/DE/Themen/Wirtschaft/Konjunkturindikatoren/Basisdaten/vpi002a.html;jsessionid=40B929A485D0E5D39868A57953A14C13.live741?view=main%5BPrint%5D.
29. Federal Statistical Office. Statistical yearbook 2019, National accounts; 2019. Available from: https://www.destatis.de/DE/Themen/Querschnitt/Jahrbuch/jb-vgr.pdf?__blob=publicationFile.
30. Federal Statistical Office. National accounts, major macroeconomic variables in billions of Euros, rate of change in gross domestic product (GDP); 2021. Available from: https://www.destatis.de/DE/Themen/Wirtschaft/Volkswirtschaftliche-Gesamtrechnungen-Inlandsprodukt/Tabellen/inlandsprodukt-gesamtwirtschaft.html.
31. Caminati M, Vaia R, Furci F, Guarnieri G, Senna G. Uncontrolled asthma: unmet needs in the management of patients. J Asthma Allergy. 2021;14:457–466. doi:10.2147/JAA.S260604
32. Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma Worldwide: data from the International severe asthma registry. Chest. 2020;157(4):790–804. doi:10.1016/j.chest.2019.10.053
33. Breekveldt-Postma NS, Erkens JA, Aalbers R, Van De Ven MJT, Lammers JWJ, Herings RMC. Extent of uncontrolled disease and associated medical costs in severe asthma - A PHARMO study. Curr Med Res Opin. 2008;24(4):975–983. doi:10.1185/030079908X280518
34. Castro M, Wenzel SE, Bleecker ER, et al. Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med. 2014;2(11):879–890. doi:10.1016/S2213-2600(14)70201-2
35. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled Phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi:10.1016/S0140-6736(16)31324-1
36. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi:10.1016/S0140-6736(16)31322-8
37. Ortega HG, Yancey SW, Mayer B, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4(7):549–556. doi:10.1016/S2213-2600(16)30031-5
38. Harrison T, Canonica GW, Chupp G, et al. Real-world mepolizumab in the prospective severe asthma REALITI-A study: initial analysis. Eur Respir J. 2020;56(4):2000151. doi:10.1183/13993003.00151-2020
39. Corren J, Castro M, Chanez P, et al. Dupilumab improves symptoms, quality of life, and productivity in uncontrolled persistent asthma. Ann Allergy Asthma Immunol. 2019;122(1):41–49.e2. doi:10.1016/j.anai.2018.08.005
40. Hossny E, Caraballo L, Casale T, El-Gamal Y, Rosenwasser L. Severe asthma and quality of life. World Allergy Organ J. 2017;10(1):28. doi:10.1186/s40413-017-0159-y
41. Niven RM, Saralaya D, Chaudhuri R, et al. Impact of omalizumab on treatment of severe allergic asthma in UK clinical practice: a UK multicentre observational study (the APEX II study). BMJ Open. 2016;6(8):e011857. doi:10.1136/bmjopen-2016-011857
42. Britton M, Howes T, Saralaya D, et al. Long-term effectiveness of omalizumab in patients with severe persistent allergic (IgE-mediated) asthma: real-life data from 3 UK centres. Eur Respir J. 2012;40(Suppl 56).P227.
43. Langner I, Ohlmeier C, Zeeb H, Haug U, Riedel O. Individual mortality information in the German Pharmacoepidemiological Research Database (GePaRD): a validation study using a record linkage with a large cancer registry. BMJ Open. 2019;9(7):1–7. doi:10.1136/bmjopen-2018-028223
44. Hartmann J, Weidmann C, Biehle R. Validierung von GKV-Routinedaten am Beispiel von geschlechtsspezifischen Diagnosen [Validation of SHI Claims Data Exemplified by Gender-specific Diagnoses]. Das Gesundheitswesen. 2016;78(10):e162–e167. German.
45. Globale Initiative für Asthma. Global Strategy for Asthma Management and Prevention; 2016.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
