Back to Journals » Clinical Interventions in Aging » Volume 9
Use of basal insulin and the associated clinical outcomes among elderly nursing home residents with type 2 diabetes mellitus: a retrospective chart review study
Authors Davis KL, Wei W, Meyers J, Kilpatrick B, Pandya N
Received 3 April 2014
Accepted for publication 20 June 2014
Published 23 October 2014 Volume 2014:9 Pages 1815—1822
DOI https://doi.org/10.2147/CIA.S65411
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Keith L Davis,1 Wenhui Wei,2 Juliana L Meyers,1 Brett S Kilpatrick,3 Naushira Pandya4
1RTI Health Solutions, Research Triangle Park, NC, USA; 2Sanofi US, Inc, Bridgewater, NJ, USA; 3AnalytiCare, LLC, Glenview, IL, USA; 4Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, FL, USA
Background: The management of type 2 diabetes mellitus in long-term care (LTC) settings can be complex as a result of age-related complications. Despite guideline recommendations, sliding scale insulin remains commonplace in the LTC setting and data on basal insulin use are lacking.
Methods: This retrospective study used medical chart data and the Minimum Data Set from elderly LTC facility patients who received basal insulin (insulin glargine, insulin detemir, or neutral protamine Hagedorn insulin) for the treatment of diabetes, to investigate the practice patterns and associated clinical outcomes.
Results: A total of 2,096 elderly, insulin-treated patients in LTC were identified, with 59.5% of them (N=1,247) receiving basal insulin. Of these, more than 50% of patients received sliding scale insulin in co-administration with basal insulin. Despite its ease of use, insulin pen use was very low, at 14.6%. Significant differences were observed between the basal insulin groups for glycated hemoglobin level and dosing frequency. Hypoglycemia was uncommon -17.2% of patients experienced at least one event, and there was no significant difference in the prevalence of hypoglycemia between the groups.
Conclusion: These data suggest the underutilization of basal insulin in the LTC setting and worryingly high combinational use with sliding scale insulin. Differences in glycated hemoglobin and dosing frequencies between types of basal insulin warrant further comparative effectiveness studies.
Keywords: long-term care, nursing homes, type 2 diabetes mellitus, insulin detemir, insulin glargine, NPH insulin
Introduction
Diabetes is a common condition in the long-term care (LTC) setting, where approximately 25% of LTC facility residents in the US are affected.1 Diabetes management in this setting is complicated because, compared with individuals without diabetes, diabetes is often associated with increased rates of premature mortality, functional disability, cognitive dysfunction, and comorbidities (eg, hypertension, stroke, coronary heart disease, depression, dementia) in elderly patients.2,3 Factors such as renal insufficiency, coexisting illnesses, polypharmacy, irregular meal patterns, and cognitive and functional impairment can lead to an increased risk of hypoglycemia in the elderly;4 therefore, optimizing the treatment of diabetes is a vital component of the care required in LTC facilities.1,5
When considering diabetes management in the LTC setting, long-acting insulin analogs are a favorable treatment option because of their “peakless” profile, which reduces the risk of hypoglycemia compared with intermediate-acting insulins, such as neutral protamine Hagedorn (NPH) insulin.6 In patients with type 2 diabetes mellitus (T2DM), adding a long-acting insulin analog to an oral antidiabetic drug (OAD) is a simple, safe, and effective strategy for introducing insulin therapy when OADs are no longer adequate to control hyperglycemia.1
Despite recommendations on the use of insulin analogs in elderly patients with T2DM, the use of regular insulin and sliding scale insulin (SSI) regimens continue to be commonplace.7,8 To the authors’ knowledge, no investigation has been undertaken of basal insulin use and its associated outcomes among elderly nursing home residents with T2DM.
The objective of this retrospective study was to determine practice patterns and clinical outcomes associated with the use of basal insulin regimens in elderly T2DM patients residing in US LTC facilities.
Methods
Study design
This was a cross-sectional retrospective study conducted in elderly T2DM patients residing in US LTC facilities. The objective was to describe practice patterns and clinical outcomes associated with three insulin regimens: the long-acting insulin analog glargine (GLA); the long-acting insulin analog detemir (DET); and the intermediate-acting insulin NPH. Data were extracted from medical charts and merged with preexisting data from the Minimum Data Set (MDS). The MDS is a standardized and comprehensive assessment instrument that describes medical diagnoses, chronic health conditions, medication use and other interventions, cognitive function, psychosocial wellbeing, functional status, and other aspects of the health of patients residing in LTC facilities. Chart data provided information on patient characteristics, dosing regimens, and glycemic profiles as well as other pharmacological treatments and clinical outcomes not captured in the MDS. Data were collected from September 2010 through to September 2011. As the study used preexisting, de-identified data, it was exempt from approval by an Institutional Review Board committee.
Patients
Patients were eligible for inclusion in the study if they: had been newly admitted to the LTC facility after January 1, 2009 and resident in the LTC facility for ≥3 months; had at least one full MDS assessment; had at least two records of insulin dispensing (at least one of which was GLA, DET, or NPH); and had a diagnosis of diabetes (as recorded on the MDS form). Exclusion criteria included a diagnosis of type 1 diabetes, pump-administered insulin at any point after LTC admission, and a comatose state or receiving hospice care on or at any point after the date of LTC admission.
Study measures
To determine practice patterns and clinical outcomes associated with insulin use, the following data were collected: patient demographics and clinical characteristics (including age, sex, race, body mass index, activities of daily living/assistance, comorbidities, and use of other non-insulin antidiabetic agents), information on co-administration of prandial/bolus insulins or SSI, and information on dosing frequency and route of administration. Patients who changed insulin regimens during the course of the study were categorized as having received multiple regimens.
The primary clinical outcome of interest was glycemic control as measured by the mean levels across all observed records for glycated hemoglobin (HbA1c), fasting blood glucose (FBG) levels during all available follow-up periods available under the Medication Administration Record Sheet, and preprandial blood glucose (PBG) levels. In addition, the percentage of patients with at least one measurement of FBG ≤100 mg/dL, PBG ≤125 mg/dL, and HbA1c ≤7.0% or HbA1c <8.5% was obtained. HbA1c data were obtained from medical records and FBG and PBG levels were obtained from the Medication Administration Record Sheet. Although recent guidelines have recommended an HbA1c goal of ≤7.5% for otherwise healthy, elderly diabetes patients, the HbA1c goal of ≤7.0% was ubiquitously accepted at the time the study was conducted. The HbA1c<8.5% goal has been recommended for those elderly patients with complex needs, and cognitive and functional impairments who are likely to make up a large proportion of the LTC population.3
Data on the occurrence of specific adverse events associated with the insulin regimens (GLA, DET, and NPH) were collected, and included the incidence of hypoglycemia-related events (overall incidence and incidence of moderate events, defined as events with a confirmed blood glucose level of <50 mg/dL) and other adverse events, ie, falls, ketoacidosis, hospitalizations, infections, and emergency room visits.
Statistical analyses
Data were analyzed using mean values, medians, ranges, and standard deviations (SDs) of continuous variables, and frequency distributions for categorical variables. Outcomes were compared between treatment groups. P-values were derived from Mann–Whitney U-tests for dichotomous variables, and from Student’s t-tests for continuous variables.
Results
Patient demographics and baseline characteristics
A total of 2,096 elderly, insulin-treated patients with T2DM were identified from 117 facilities. Of these, 59.5% of patients (N=1,247) had at least one record of dispensation of basal insulin: 933 (74.8%) were treated with GLA, 199 (16.0%) with DET, and 115 (9.2%) with NPH during the observation period, and were included in the analysis.
The baseline demographic and clinical characteristics of the 1,247 patients, stratified by the type of insulin they received, are summarized in Tables 1 and 2. Baseline demographics were similar across the treatment groups. The mean age of patients was 73.6 years (SD ±12.0), and most patients were women (60.3%) and white (52.5%) (Table 1). Assessment of assisted daily living status indicated that 12.0% of patients were able to live independently with regards to their daily activities, 14.3% required supervision but without assistance, 34.0% required limited assistance, and 21.6% extensive assistance. Mean follow-up duration was 49.0 days (SD ±29.4) (range 6–424 days).
  | Table 1 Patient baseline characteristics |
The most common comorbidities reported in this patient population were Alzheimer’s disease/dementia (50.8%) and stroke (24.3%) (Table 2). Almost all patients (95.8%) used medications to treat chronic conditions, most commonly with antihypertensive drugs (81.4%), anticoagulant/antiplatelet drugs (58.5%), or antidepressant drugs (56.9%). Concomitant OADs – most commonly metformin (18.7%) or sulfonylureas (12.8%) – were used by 30.8% of patients. Although patients in the NPH insulin group tended to have less depression and lower associated concomitant medication use compared with patients in the GLA or DET groups, there were no significant between-group differences.
Insulin treatment regimen, administration, and dosage
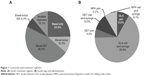
The most commonly-used insulin treatment regimen was basal insulin co-administered with SSI, followed by basal insulin only (Figure 1A). Approximately 17% of patients received an additional bolus insulin, either alone or with supplemental SSI.
Insulin pen use was generally low; 14.6% of patients received insulin via an injectable pen device (Figure 1B). A significantly lower proportion of patients in the GLA group (111 of 993 [11.9%]) compared with the DET group (68 of 199 [34.2%]) received their analog insulin via a pen (P<0.0001). Very few patients (2 of 115 [1.7%]) received NPH via a pen.
There were no significant differences between the groups with regards to mean daily insulin dose: 26.8 units/day (SD ±19.1) in the GLA group; 27.7 units/day (SD ±22.0) in the DET group; and 26.8 units/day (SD ±19.1) in the NPH group. However, significantly fewer patients in the GLA group required twice-daily insulin injections (5.6%) compared with either the DET (22.1%; P<0.0001) or NPH insulin (64.3%; P<0.0001) groups.
Glycemic control
A total of 485 patients (38.9%) had at least one HbA1c measurement recorded in their medical chart; at least one HbA1c measurement was recorded for 40.0% of patients in the GLA group, 38.2% of patients in the DET group, and 31.3% of patients in the NPH group. Among those with at least HbA1c measurement, mean HbA1c in the GLA group was 7.31% (SD ±1.31), which was significantly lower than that in the DET group (7.72% [SD ±1.41]; P=0.0184) and numerically higher than the NPH group (6.93% [SD ±1.25]; P=0.1099) (Figure 2A). Similar percentages of patients in all three treatment groups had at least one measurement of HbA1c of ≤7.0% (ie, 47.5% of patients in the GLA group, 39.5% in the DET group, and 61.1% in the NPH group: GLA versus DET P=0.2034 and GLA versus NPH P=0.1147) (Figure 2B). Despite numerical differences, there were no significant differences in the proportions of patients with at least one measurement of HbA1c <8.5% during the observation period for the three treatment groups (ie, 83.7% of patients in the GLA group, 79.0% of patients in the DET group, and 86.1% of patients in the NPH group: GLA versus DET P=0.3219 and GLA versus NPH P=0.7010).
Similar percentages of patients in all three treatment groups had at least one measurement of FBG ≤100 mg/dL (ie, 51.7% of patients in the GLA group, 46.1% of patients in the DET group, and 50.9% of patients in the NPH group: GLA versus DET P=0.1681 and GLA versus NPH P=0.8732) (Figure 2B). There were no significant differences between GLA, DET, or NPH with regards to mean FBG levels (151.8 mg/dL, 154.3 mg/dL, and 145.9 mg/dL, respectively) (Figure 2C). Similar percentages of patients in each group had at least one measurement of PBG ≤125 mg/dL (Figure 2B).
Adverse events
The incidences of patients experiencing at least one adverse event while receiving the three insulin regimens are summarized in Table 3. Overall, the most common adverse event was infections (15.9%). A significantly lower proportion of patients in the GLA group (9.3%) than in the DET group (16.6%) had a urinary infection (P=0.0025); there were no significant differences between the three treatment groups in the incidence of other adverse events. Only a small proportion of patients had ketoacidosis during the observation period (overall incidence rate of 0.33 events per 100 person-months).
  | Table 3 Incidence of adverse events |
Of the 1,247 patients included in the analysis, 214 (17.2%) experienced at least one hypoglycemic event during follow-up, and 47 patients (3.8%) had at least one moderate hypoglycemic event – there were no significant between-group differences (Table 3). The incidence rate of hypoglycemic events per 100 person-months was 24.3 in the GLA group, 18.6 in the DET group, and 15.7 in the NPH group. The incidence rates of moderate hypoglycemic events were 3.2 per 100 person-months in the GLA group, 4.8 per 100 person-months in the DET group, and 1.8 per 100 person-months in the NPH group.
Discussion
Data from several studies suggest that a significant proportion of elderly LTC facility residents receive suboptimal diabetes care,1 despite the identification of numerous opportunities for improving care quality among nursing home residents with diabetes.9 For example, the development of treatment algorithms, together with the implementation of quality improvement tools that follow appropriate process and outcome indicators have been proposed as a means of improving the management of diabetes in this setting.9 Surprisingly little research has been carried out, however, to understand the pattern of insulin use in this elderly LTC population, given that the addition of a basal insulin to OADs is a recognized strategy for introducing insulin therapy.1,10 This study, one of the first of its kind, described basal insulin use and its associated outcomes among elderly LTC facility residents with T2DM.
One of the main treatment patterns identified in this study indicated that <60% of patients had received basal insulin; the remainder had been exclusively treated with SSI and more than half of these patients had used basal insulin in conjunction with SSI. This high prevalence of SSI is a cause for concern, given that its use is inconsistent with current recommendations5,8,11 and is associated with limited therapeutic success7 and high burden of unnecessary finger sticks.11 The continued use of SSI may be due to clinical inertia and its position as a “medical myth” passed on through generations of health care professionals.7 Staff or clinicians may not be aware of the ineffectiveness of SSI.12 SSI may also be perpetuated as it provides the opportunity to administer various doses of insulin in a reactive manner for a wide range of blood glucose levels without having to call the practitioner or analyze glucose patterns to make a logical change in the insulin regimen. The change in medical culture required to move away from SSI is not simple and requires the involvement of the multidisciplinary team;13 perhaps greater involvement from pharmacy staff, who can help promote the individualization of treatment, may aid the shift away from SSI.12
Concomitant OADs were used by 30.8% of patients, most commonly metformin (18.7%) or sulfonylureas (12.8%). Among patients who had received basal insulin, one in four received basal only with a majority requiring additional prandial insulin (basal insulin plus SSI comprised the predominant insulin regimen used).
Of the different basal insulins investigated in this study, GLA had been used by three in four patients, a finding in line with previous studies.14 Another pattern identified in this study was that NPH was mostly used twice daily, and patients receiving DET were significantly more likely to receive insulin twice daily than those receiving GLA. Injectable pen use was low despite the availability, for all three insulins, of insulin pens. A possible explanation for this is the relatively high acquisition cost of insulin pens and the high use of SSI, which is married to the syringe vial paradigm. Insulin pens are easier to use and offer improved treatment adherence and persistence, and a lower incidence of hypoglycemia compared with vial and syringe administration in real-world ambulatory settings.15 Diabetes management in elderly LTC facility residents might, therefore, be improved by switching to insulin pens.
With regard to glycemic control, differences were found between the three treatment groups. A significantly lower HbA1c level was reported for GLA-treated patients than for those treated with DET. This finding is consistent with a previous real-world study that reported better glycemic control in patients initiating insulin therapy with GLA, than those initiating with DET.16 However, it should be noted that tight HbA1c control may not be appropriate for all patients and assessment of comparative effectiveness was not the goal of this study.
An HbA1c target level of <7.0% is a reasonable goal for most patients and provides the potential benefit of reducing microvascular and macrovascular disease. However, this goal carries an increased risk of hypoglycemia that must be weighed against its potential benefits in elderly patients with T2DM.4 A low percentage of patients achieved at least one measurement of HbA1c ≤7.0% in the current study, supporting the suggestion that achievement of this target HbA1c level is not necessarily an appropriate goal for elderly T2DM patients residing in LTC facilities.3,17 Due to the unique presentations and challenges of diabetes and its chronic complications in the elderly, treatment goals in this population should be tailored to the individual patient.2,18 The majority of patients in all three treatment groups reached the comparatively less stringent HbA1c target of <8.5%, which may be a more appropriate HbA1c level for functionally and cognitively impaired elderly patients in an LTC setting.
With regards to safety and tolerability, the prevalence of hypoglycemia did not significantly differ between treatment groups. The proportion of LTC residents with at least one hypoglycemic event was approximately 17%, which is in accordance with previous studies reporting hypoglycemia prevalence rates in elderly patients with diabetes (range 15%–27%).19,20 There were no significant differences between the three treatment groups in the incidence of most adverse events, although a significantly lower rate of urinary tract infections was detected in the GLA group compared with the DET group.
One of the strengths of this study was that chart data were used to provide information on patient characteristics, dosing regimens, and glycemic profiles, as well as other pharmacological treatments and clinical outcomes not captured in the MDS. Furthermore, this was a large-scale study that included >1,200 elderly LTC facility residents with T2DM receiving insulin therapy. Nevertheless, data collection relied on convenience sampling so these findings cannot be generalized to the overall US elderly LTC facility population. Other limitations were that the study’s cross-sectional design did not permit assessment of the comparative effectiveness of the three insulin treatments; the data collection was retrospective and relied on medical chart abstraction and the MDS (including that for hypoglycemia events) and therefore was unable to capture information such as patient education, dietary control, and lifestyle, which are important for understanding comprehensive management of elderly diabetes patients; and the information collected could also have been subject to measurement errors. There could also be an element of interindividual variability of OAD treatment due to genetic mechanisms,21 which may influence these results. Furthermore, because the timing of the HbA1c measurement could not be determined, the relationship between HbA1c and insulin treatment cannot be inferred and the levels might have been related to other treatments. The findings of this study should, therefore, be interpreted conservatively and require confirmation in randomized trials.
Conclusion
The data from this retrospective study conducted in elderly T2DM patients reveal that, among US LTC facility residents, basal insulin may still be underutilized and that when used it is often in conjunction with SSI. Vial and syringe use was still the dominant mode of administration despite the potential benefits of insulin pens. Pragmatic randomized trials should be conducted to investigate if specific basal insulins have a therapeutic advantage in this patient group.
Acknowledgments
This study was funded by Sanofi US, Inc. The authors had access to all data and were responsible for preparing the manuscript for publication. Editorial and writing support was provided by Ewen Legg, PhD, of Excerpta Medica, funded by Sanofi US, Inc.
The manuscript has been read and approved by all authors and the authorship requirements stated by the ICMJE have been met. All authors participated in the development of the concept and study design, they all interpreted the data, reviewed the draft manuscript, provided comments, and approved the final document. KLD, JLM, and BSK collected the data and conducted the analyses.
Disclosure
KLD and JLM are employees of RTI Health Solutions, which received funding from Sanofi US, Inc., to conduct this study. WW is an employee of Sanofi US, Inc. BSK is an employee of AnalytiCare, LLC, which received funding from Sanofi US, Inc., to conduct this study. NP previously received speaking honoraria and served as a consultant for Sanofi US, Inc. The authors have no further conflicts of interest in this work.
References
Haas LB. Optimizing insulin use in type 2 diabetes: role of basal and prandial insulin in long-term care facilities. J Am Med Dir Assoc. 2007; 8(8):502–510. | ||
American Diabetes Association. Standards of medical care in diabetes – 2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. | ||
Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35(12):2650–2664. | ||
Fravel MA, McDanel DL, Ross MB, Moores KG, Starry MJ. Special considerations for treatment of type 2 diabetes mellitus in the elderly. Am J Health Syst Pharm. 2011;68(6):500–509. | ||
American Medical Directors Association [webpage on the Internet]. Columbia, MD: Clinical practice guidelines in the long term care setting: diabetes management; 2010. Available from: http://www.amda.com/tools/guidelines.cfm#diabetes. Accessed March 20, 2014. | ||
Rosenstock J. Management of type 2 diabetes mellitus in the elderly: special considerations. Drugs Aging. 2001;18(1):31–44. | ||
Umpierrez GE, Palacio A, Smiley D. Sliding scale insulin use: myth or insanity? Am J Med. 2007;120(7):563–567. | ||
Pandya N, Thompson S, Sambamoorthi U. The prevalence and persistence of sliding scale insulin use among newly admitted elderly nursing home residents with diabetes mellitus. J Am Med Dir Assoc. 2008;9(9): 663–669. | ||
Feldman SM, Rosen R, DeStasio J. Status of diabetes management in the nursing home setting in 2008: a retrospective chart review and epidemiology study of diabetic nursing home residents and nursing home initiatives in diabetes management. J Am Med Dir Assoc. 2009;10(5): 354–360. | ||
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–1379. | ||
Pandya N, Wei W, Meyers JL, Kilpatrick BS, Davis KL. Burden of sliding scale insulin use in elderly long-term care residents with type 2 diabetes mellitus. J Am Geriatr Soc. 2013;61(12):2103–2110. | ||
Browning LA, Dumo P. Sliding-scale insulin: an antiquated approach to glycemic control in hospitalized patients. Am J Health Syst Pharm. 2004;61(15):1611–1614. | ||
Nau KC, Lorenzetti RC, Cucuzzella M, Devine T, Kline J. Glycemic control in hospitalized patients not in intensive care: beyond sliding-scale insulin. Am Fam Physician. 2010;81(9):1130–1135. | ||
Goykhman S, Drincic A, Desmangles JC, Rendell M. Insulin glargine: a review 8 years after its introduction. Expert Opin Pharmacother. 2009; 10(4):705–718. | ||
Davis SN, Wei W, Garg S. Clinical impact of initiating insulin glargine therapy with disposable pen versus vial in patients with type 2 diabetes mellitus in a managed care setting. Endocr Pract. 2011;17(6): 845–852. | ||
Xie L, Wei W, Pan C, Du J, Baser O. A real-world study of patients with type 2 diabetes initiating basal insulins via disposable pens. Adv Ther. 2011;28(11):1000–1011. | ||
Brown AF, Mangione CM, Saliba D, Sarkisian CA; California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51(5 Suppl):S265–S280. | ||
Rizvi AA. Management of diabetes in older adults. Am J Med Sci. 2007; 333(1):35–47. | ||
Feil DG, Rajan M, Soroka O, Tseng CL, Miller DR, Pogach LM. Risk of hypoglycemia in older veterans with dementia and cognitive impairment: implications for practice and policy. J Am Geriatr Soc. 2011; 59(12):2263–2272. | ||
Bellissimo JL, Holt RM, Maus SM, Marx TL, Schwartz FL, Shubrook JH. Impact of activity participation and depression on glycemic control in older adults with diabetes: glycemic control in nursing homes. Clin Diabetes. 2011;29(4):139–144. | ||
Semiz S, Dujic T, Causevic A. Pharmacogenetics and personalized treatment of type 2 diabetes. Biochem Med (Zagreb). 2013;23(2): 154–171. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.