Back to Journals » OncoTargets and Therapy » Volume 12
Use of anlotinib in intra-abdominal desmoplastic small round cell tumors: a case report and literature review
Received 10 October 2018
Accepted for publication 3 December 2018
Published 18 December 2018 Volume 2019:12 Pages 57—61
DOI https://doi.org/10.2147/OTT.S190333
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Hui-Min Chen, Ge Feng
Nanjing Jiangbei People’s Hospital, Nantong University, Nanjing 220000, People’s Republic of China
Background: Intra-abdominal desmoplastic small round cell tumor (IADSRCT) is a highly invasive malignant tumor that is rare in clinical practice. Anlotinib is a multitarget receptor tyrosine kinase inhibitor which inhibits vascular endothelial growth factor receptor (VEGFR) 1–3, fibroblast growth factor receptor (FGFR) 1–4, platelet-derived growth factor receptors (PDGFR) α/β, c-Kit, and Met. In our study, we present a record of IADSRCT which was validly treated by anlotinib.
Case presentation: A 38-year-old man was admitted due to anterior abdominal wall nodule for 1 month. The nodule and intraperitoneal mass were resected and diagnosed IADSRCT. The patient received six cycles of adjuvant chemotherapy and his CT scan revealed metastasis in the right inguinal lymph node and omental lymph node. Anlotinib was then recommended. Anlotinib significantly reduced the lymph nodes after four cycles. The patient continued to use anlotinib as maintenance therapy, and the patient was in good condition. The side effects of anlotinib were high triglycerides and fatigue. However, its toxicity was controllable and tolerable.
Conclusion: This is the first report about anlotinib being effective in the treatment of IADSRCT. This report may provide a new option for the treatment of metastatic IADSRCT.
Keywords: intra-abdominal desmoplastic small round cell tumor, anlotinib, target therapy
Introduction
Intra-abdominal desmoplastic small round cell tumor (IADSRCT) is a very recently described tumor, characterized in 1989 by Gerald and Rosai,1,2 who identified the EWS-WT1 translocation and fusion protein as pathognomonic. If this fusion protein cannot be identified in the tissue, the diagnosis of IADSRCT cannot be made. It has been reported in the literature, with a higher incidence in children and young adults, a male predominance, and an average age of onset of 21 years. Prognosis for this disease is quite poor, with 5-year overall survival estimated at only 15%–30%.3 No standardized treatment guideline is presently available. Current therapy consists of surgical resection combined with chemotherapy and radiotherapy.4
Anlotinib is a novel oral multi-targeted receptor tyrosine kinase inhibitor, which has a broad spectrum of inhibitory action on tumor angiogenesis and growth.5 It inhibits vascular endothelial growth factor receptor (VEGFR) 1–3, fibroblast growth factor receptor (FGFR) 1–4, platelet-derived growth factor receptors (FDGFR) α/β, c-Kit, and Met.6,7 In phase I study, anlotinib showed promising anti-tumor potential against many types of tumor such as colon adenocarcinoma, non-small-cell lung cancer, renal clear cell cancer and medullary thyroid carcinoma.8,9 However, there are so far no reports of the treatment of IADSRCT with anlotinib. This study aims to present the case of a male patient with IADSRCT treated with anlotinib.
Ethics statement
This study was approved by the Institutional Ethics Review Board of Nanjing Jiangbei People’s Hospital, Nanjing 220000, People’s Republic of China. The patient agreed to participate in this study and submitted written informed consent, including publication of the case details, at the Nanjing Jiangbei People’s Hospital.
Case presentation
On October 26, 2017, a 38-year-old man visited the Nanjing Jiangbei People’s Hospital presenting with anterior abdominal wall nodule for 1 month. Physical examination showed that the nodule had a hard texture, unclear boundary, and a low degree of mobility. The laboratory assessment results were normal. Abdominal computed tomography (CT) showed a soft tissue nodule measuring 1.3×1.5 cm in the subcutaneous layer of the right abdominal wall and a cystic density mass measuring 9.9×8.7 cm in the peritoneal cavity (Figure 1). On November 2, 2017, the patient received laparoscopic tumor resection + anterior abdominal wall nodule resection at the Jiangsu Cancer Hospital. All neoplasms were resected and sent for pathological evaluation. The pathologic findings were supportive for the diagnosis of IADSRCT (Figure 2A and B). Immunohistochemistry findings showed that the tumor cells were positive for AE1/AE3, desmin, NSE, Ck7, CD34, CD99 and partially positive for CD56, GATA3, Syn (Figure 2C–E). The tumor stained negatively with P63, CK5/6, P40, S100, CD31, Ki-67 and the proliferation index was 40%.
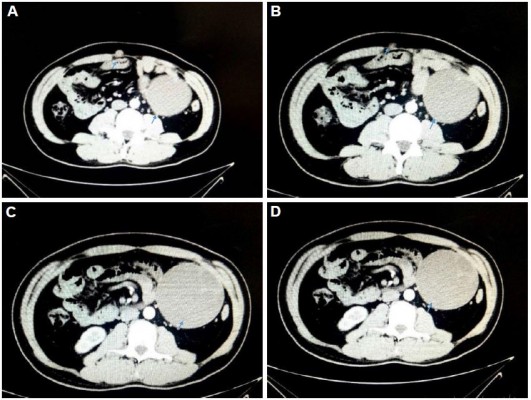
Postoperatively, from December 2017 to April 2018, the patient received six cycles of chemotherapy (ifosfamide 3.6×5 days, liposomal doxorubicin 60 mg×1 day) as adjuvant therapy. On May 20, 2018, the patient’s CT scan revealed metastasis in the right inguinal lymph node and omental lymph node. The size of the enlarged lymph nodes was 29.3×19.8 mm and 9.5×8.6 mm, respectively (Figure 3A and C). On June 1, 2018, anlotinib was administered at a dose of 12 mg, once daily, 2-weeks on/1-week off. The size of the right inguinal lymph node and omental lymph node were reduced to 17.8×14.9 mm and 8.9×7.9 mm, respectively after two cycles of treatment (Figure 3B and D). The efficacy was evaluated as stable disease (SD). After four cycles of anlotinib treatment, the size of lymph nodes was further reduced. A CT scan revealed SD. The patient continued to use anlotinib as maintenance therapy. Until now, anlotinib was continued for an additional cycle, the side effects of anlotinib were high triglyceride and fatigue. However, its toxicity was controllable and tolerable.
Discussion
IADSRCT is an aggressive malignancy that occurs predominantly in young adults and nearly 80% of cases have been found in young males.10 Pathologically, it is characterized by abdominopelvic sarcomatosis exhibiting multi-lineage cellular nests of epithelial, muscular, mesenchymal, and neural differentiation admixed with desmoplastic stroma. Clinically, the patients commonly present with an abdominal mass and distension, ascites and hydronephrosis, with or without weight loss. Radiologically, IADSRCT shows a huge solid mass within the peritoneal cavity and is frequently accompanied by intra-peritoneal seeding.11 Also, it may invade the surrounding intraperitoneal and retroperitoneal abdominal organs directly, but hematogenous metastases are rare.
Microscopically, IADSRCT characteristically shows well-defined nests of small cells in a distinctive desmoplastic stroma. Tumor cells have scanty cytoplasm and indistinct cellular borders. It formed a pathognomonic EWSR1-WT1 t(11;22)(p13:q12) chromosomal translocation that pairs the Ewing sarcoma (ES) gene (EWSR1) with the Wilm’s tumor suppressor gene (WT1). The functional loss of the WT1 tumor suppressor protein and the oncogenic effects caused by the aberrant 59 kDa fusion protein results in hundreds or thousands of nodules coating the intraabdominal serosal and subdiaphragmatic surfaces.12–14
Chemotherapy, resection, and radiation therapy form the basis of IADSRCT treatment. No single therapy has been accepted as the standard strategy. The chemotherapy is based on combinations of vincristine, doxorubicin, cyclophosphamide, ifosfamide, etoposide and dactinomycin. P6 chemotherapy regimen proposed by Kushner et al15 (1, 2, 3, and 6 cycles: cyclophosphamide + doxorubicin + vincristine; 4, 5, 7 cycles: ifosfamide + etoposide) is a representative adjuvant chemotherapy regimen for the treatment of DSRCT.
Cyclophosphamide/topotecan, temozolomide/irinotecan, and high-dose ifosfamide are common second-line regimens. Less common salvage regimens include gemcitabine/docetaxel, cyclophosphamide/vinorelbine, and dacarbazine.16 Owing to peritoneal implantation, tumor tissue residue is often present even after resection. Therefore, hyperthermic intraperitoneal chemotherapy (HIPEC) with cisplatin was considered as an effective adjunctive therapy. Radiation was commonly used for palliation at the time of tumor recurrence. In addition, a substantial number of DSRCTs overexpress VEGFR-2, and a handful of patients have had clinical responses to sunitinib, sorafenib, or pazopanib. Other biological therapies, mammalian target of rapamycin (mTOR) inhibitors, including anti-ganglioside GD2 antibodies, imatinib, and a combination of insulin-like growth factor 1 and mTOR inhibitors, have shown limited success.17–19
Anlotinib (AL3818) hydrochloride is a novel multitarget tyrosine kinase inhibitor (TKI) for tumor angiogenesis and proliferative signaling. The prime targets of anlotinib include receptor tyrosine kinases vascular endothelial growth factor receptor 1–3, endothelial growth factor receptor (EGFR), fibroblast growth factor receptor 1–4, platelet-derived growth factor receptor α and β, and stem cell factor receptor. Response to anlotinib was noted in a wide range of tumor types, including renal carcinoma, medullary thyroid carcinoma, non-small-cell lung cancer, colorectal cancer, melanoma, thymic carcinoma, and adenoid cystic carcinoma.20,21 The most common adverse events were triglyceride elevation, hand-foot skin reaction, hypertension, fatigue, proteinuria pharyngalgia, and pneumothorax. However, it was not reported whether anlotinib exerts effects in the treatment of IADSRCT.
In our present study, the patient developed right inguinal lymph node and omental lymph node metastasis after abdominal surgery and six cycles of chemotherapy. Anlotinib significantly reduced swollen lymph nodes and progress free survival had reached nearly 4 months. Meanwhile, the patient suffered no severe toxicity except for high triglyceride and fatigue, which were controllable and well tolerated. At the present time, the best therapy for patients with IADSRCT has yet to be determined. Because of the rarity of the disease and the small number of patients, multi-institutional clinical trials for IADSRCT are not available. Reports of individual responses and the clinical courses of patients with IADSRCT treated using various regimens may thus assist physicians seeking the best therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
Gerald WL, Rosai J. Case 2. Desmoplastic small cell tumor with divergent differentiation. Pediatr Pathol. 1989;9(2):177–183. | ||
Gerald WL, Miller HK, Battifora H, Miettinen M, Silva EG, Rosai J. Intra-abdominal desmoplastic small round-cell tumor. Report of 19 cases of a distinctive type of high-grade polyphenotypic malignancy affecting young individuals. Am J Surg Pathol. 1991;15(6):499–513. | ||
Dufresne A, Cassier P, Couraud L, et al. Desmoplastic small round cell tumor: current management and recent findings. Sarcoma. 2012;714986. | ||
Hayes-Jordan A, Laquaglia MP, Modak S. Management of desmoplastic small round cell tumor. Semin Pediatr Surg. 2016;25(5):299–304. | ||
Sun Y, Niu W, du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. 2016;9(1):105. | ||
Lin B, Song X, Yang D, Bai D, Yao Y, Lu N. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene. 2018;654:77–86. | ||
Taurin S, Yang CH, Reyes M, et al. Endometrial cancers harboring mutated fibroblast growth factor receptor2 protein are successfully treated with a new small tyrosine kinase inhibitor in an orthotopic mouse model. Int J Gynecol Cancer. 2018;28(1):152–160. | ||
Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(4):504–535. | ||
Han BH, Li K, Wang QM, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2018;V4N11:1569–1575. | ||
Reisner D, Brahee D, Patel S, Hartman M. A case of desmoplastic small round cell tumor. J Radiol Case Rep. 2015;9(8):1–7. | ||
Hayes-Jordan A, Laquaglia MP, Modak S. Management of desmoplastic small round cell tumor. Semin Pediatr Surg. 2016;25(5):299–304. | ||
Gerald WL, Ladanyi M, De Alava E, et al. Clinical, pathologic, and molecular spectrum of tumors associated with t(11;22)(p13;q12): desmoplastic small round-cell tumor and its variants. J Clin Oncol. 1998;16(9):3028–3036. | ||
Hill DA, Pfeifer JD, Marley EF, et al. WT1 staining reliably differentiates desmoplastic small round cell tumor from Ewing sarcoma/primitive neuroectodermal tumor. An immunohistochemical and molecular diagnostic study. Am J Clin Pathol. 2000;114(3):345–353. | ||
Huang J, Sha L, Zhang H, Tang X, Zhang X. Desmoplastic small round cell tumor in transverse colon: report of a rare case. Int Surg. 2015;100(5):809–813. | ||
Kushner BH, LaQuaglia MP, Wollner N, et al. Desmoplastic small round-cell tumor: prolonged progression-free survival with aggressive multimodality therapy. J Clin Oncol. 1996;14:1526–1531. | ||
Mora J, Cruz CO, Parareda A, De Torres C. Treatment of relapsed/refractory pediatric sarcomas with gemcitabine and docetaxel. J Pediatr Hematol Oncol. 2009;31:723–729. | ||
Subbiah V, Brown RE, Jiang Y, et al. Morphoproteomic profiling of the mammalian target of rapamycin (mTOR) signaling pathway in desmoplastic small round cell tumor (EWS/WT1), Ewing’s sarcoma (EWS/FLI1) and Wilms’ tumor (WT1). PLoS One. 2013;8(7):e68985. | ||
Thijs AM, van der Graaf WT, van Herpen CM. Temsirolimus for metastatic desmoplastic small round cell tumor. Pediatr Blood Cancer. 2010;55(7):1431–1432. | ||
Naing A, Lorusso P, Fu S, et al. Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing’s sarcoma family tumors. Clin Cancer Res. 2012;18(9):2625–2631. | ||
Beedie SL, Mahony C, Walker HM, Chau CH, Figg WD, Vargesson N. Shared mechanism of teratogenicity of anti-angiogenic drugs identified in the chicken embryo model. Sci Rep. 2016;6(1):30038. | ||
Syed YY. Anlotinib: first global approval. Drugs. 2018;78(10):1057–1062. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.