Back to Journals » Journal of Pain Research » Volume 13
Use of a Long-Acting Opioid Microsphere Formulation to Overcome Difficulties in Swallowing Pain Medication
Authors Anderson N, Gillman AG, Wasan AD
Received 14 February 2020
Accepted for publication 17 April 2020
Published 6 May 2020 Volume 2020:13 Pages 955—960
DOI https://doi.org/10.2147/JPR.S249592
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Nathan Anderson,1 Andrea G Gillman,1 Ajay D Wasan1,2
1Department of Anesthesiology and Perioperative Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; 2UPMC Pain Medicine, Pittsburgh, PA, USA
Correspondence: Ajay D Wasan Tel +1 412 665 8048
Fax +1 412 665 8033
Email [email protected]
Purpose: Xtampza ER® (XER) is a long-acting oxycodone formulation which was designed to be abuse-deterrent and to overcome capsule-swallowing issues. This pilot study evaluated the effectiveness of XER at reducing swallowing difficulty while providing effective analgesia in the setting of chronic pain.
Subjects and Methods: Eleven subjects with chronic pain who reported pill-swallowing difficulty were enrolled in a 6-week uncontrolled open-label pilot study in which their prescribed daily opioid medication was converted to XER. Swallowing difficulty, pain intensity, opioid satisfaction, and secondary indicators of pain response were recorded for subjects throughout the study.
Results: Both swallowing difficulty and opioid satisfaction (XER vs baseline opioid) improved significantly over the 6-week study period (p < 0.05), while pain intensity ratings demonstrated no significant change. No significant change was noted in any of the secondary pain, mental health, or physical function measures after conversion to XER compared to baseline.
Conclusion: Subjects experienced improvement in both swallowing and opioid medication satisfaction after conversion to XER with no significant change in pain intensity and related measures.
Keywords: opioid medication, swallowing difficulty, chronic pain, abuse deterrent formulation
Introduction
To achieve optimal care for pain patients, pain medication should provide satisfactory analgesia and minimize patient burdens such as the effort required to swallow pills. Although there are risks of abuse, overdose, and side effects,1 long-term low- or moderate-dose opioid medication can provide effective and safe analgesia in well-selected patients with chronic pain. However, long-acting opioid pill capsules are often large and difficult for patients to swallow.2 Studies have shown that 20% of adult patients have difficulty swallowing their medications, and up to 10% refuse specific pharmaceutical therapies because they cannot swallow the pills.3,4 Pill swallowing difficulty can be due to several factors, including capsule size, outer coating texture, shape, and taste,5,6 and it is likely that swallowing difficulties compromise the quantity, quality, and satisfaction with pain relief from oral opioids. This issue disproportionately affects patients with cancer pain, as chemotherapy, radiation, and/or surgical treatments may result in increased swallowing difficulties.
Xtampza ER® (XER) is a long-acting oxycodone formulation that was designed to be abuse-deterrent and to help patients overcome capsule-swallowing issues. The XER capsule contains extended-release microspheres that can be opened by the patient and mixed into soft food.7 It is also an abuse-deterrent formulation (ADF), as the microspheres are not crushable or dissolvable in liquid,8–10 and stable extended release formulations like XER provide a disincentive to overtake or manipulate the medication in an attempt to abuse it. The package insert for XER recommends that the microspheres be added to soft food such as applesauce before oral consumption,7 which makes them much easier to swallow than a standard opioid pill. As it is specifically designed to overcome capsule-swallowing issues, XER may, therefore, be an important tool for personalized pain care for patients who have difficulty swallowing large pills.
The present study investigated swallowing satisfaction, pain relief, and pain-related outcomes in patients who transitioned from “traditional” opioid pills to XER over a 6-week period. We hypothesized that the subjects would report comparable pain relief and improved ability to swallow opioid medication through the dispersal of the microspheres in soft food. The primary hypothesis was that after being converted to XER, the subjects would report no meaningful differences in the degree of daily and weekly average pain intensity. The secondary hypothesis was that after being converted to XER, the subjects would report significant improvement in ratings of difficulty swallowing their pain medication.
Subjects and Methods
All study procedures were approved by the University of Pittsburgh Institutional Review Board, and written informed consent was obtained from each participant. This study was conducted in accordance with the Declaration of Helsinki.
Subjects
This was a pilot, uncontrolled open-label study. Subjects were 11 individuals ages 31–60 experiencing chronic noncancer pain on a daily basis for a minimum of six months. Subjects had primary pain diagnoses of low back pain (18%), panniculitis (18%), osteoarthritis (18%), ankylosing spondylitis (9%), Ehlers-Danlos syndrome (9%), complex regional pain syndrome (9%), cervical spinal stenosis (9%), and abdominal pain (9%), and were not receiving treatment for cancer or cancer-related pain at the time of the study. Prior to enrollment in the study, all subjects had been prescribed opioids for daily use with a maximum daily dosage of 200 morphine milligram equivalents (MME). All subjects reported difficulty swallowing their prescribed opioid medication of at least 3 on a 0–10 numeric rating scale, where 0 = “No trouble at all” and 10 = “The greatest possible difficulty swallowing pills”. In addition to pill swallowing difficulties, 82% of the subjects reported difficulty swallowing solids, and 27% reported difficulty swallowing liquids.
Study Timeline and Measures
Subjects were followed for six weeks. Clinic visits occurred in weeks 1, 2, 3, and 6, with shorter surveys sent to the subjects via text message in weeks 4 and 5. The baseline visit included confirmation of study eligibility and a medical history review by the study physician. Urine drug toxicology screening was obtained if there was not one available in the electronic medical record within three months. Subjects with toxicology results showing illegal drugs or non-prescribed opioids in the urine were excluded. The Pennsylvania State Prescription Drug Monitoring Program database (Harrisburg, PA) was reviewed to confirm that each subject’s opioid medications were prescribed by a single practice at the dosage reported by the subject.
During the week 2 visit, all subjects had their previous long-term opioid prescription converted to XER by the study physician using a standard conversion table. Only 75% of the calculated dose was initially converted to account for incomplete cross tolerance. Subjects then visited the clinic again in week 3 for evaluation and adjustment of XER dose for the final weeks of the study.
All study measures were collected using REDCap (Nashville, TN). Subjects completed the PROMIS-29 Adult Profile v2.0,11 a shortened version of the Baylor Dysphagia Questionnaire,12 and a Pill Swallowing Difficulty Question at all clinic visits and in weeks 4 and 5 via a text message link to the REDCap survey. The Pill Swallowing Difficulty Question stated: “How would you rate the difficulty swallowing your current opioid medications (weeks 1–2)/the study pain medication (weeks 3–6)?” with answers presented on a numeric scale ranging from “0 - No trouble at all” to “10 - The greatest possible difficulty.” The Opioid Medication Satisfaction Question was collected in weeks 1, 3, 5, and 6 and stated: “How satisfied are you with your current opioid medication (week 1)/the study medication, XER (weeks 3, 5, and 6)?” with answers presented on a numeric scale ranging from “0 - Not satisfied at all” to “10 - Completely satisfied.” The Patient Global Impression of Change13 was also collected in weeks 5 and 6.
Subjects were asked to complete daily Pain and Medication Diaries to monitor their pain levels and medication compliance. These diary questions were sent and captured via Short Message Services (SMS - “text”) messages relayed via the Mosio application (Seattle, WA). These text messages stated: (1) “What is your level of pain now? Text 0–10. 0 = no pain, 10 = pain as bad as you can imagine,” (2) “What was your average level of pain over the last 24 hours? Text 0–10. 0 = no pain, 10 = pain as bad as you can imagine,” and (3) “Did you remember to take your study medication yesterday? Text Y or N.”
At the conclusion of the study, subjects were given the option of returning to their baseline opioid regimen or continuing to use XER in coordination with their prescribing physician.
Statistical Analysis
Baseline characteristics were analyzed using basic descriptive statistics (Table 1). Univariate and multivariate repeated measures analysis of variance (ANOVA) were performed to analyze changes from baseline (week 1) to the end of the study (week 6). The primary outcome analyzed was change in pain intensity using the daily average pain intensity measure from the text message Pain Diary and the weekly average pain intensity measure from the PROMIS-29 profile. The secondary outcomes analyzed were changes in pill swallowing difficulty, opioid satisfaction, impression of change, and PROMIS T-Scores for pain interference, anxiety, depression, satisfaction with social roles, sleep disturbance, and physical function. All analyses were performed using SPSS v.25.
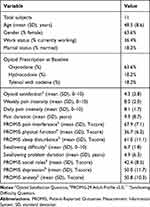 |
Table 1 Baseline Characteristics of Enrolled Subjects |
Results
Baseline Characteristics
Twelve subjects were enrolled, and 11 subjects completed the 6-week study. The twelfth subject was not able to complete the required urine drug screening in a timely manner and was removed from the study by the principle investigator. Demographic information and baseline reported measures of the 11 subjects are found in Table 1. Of the eleven subjects, 7 (63.6%) were prescribed oxycodone, 2 (18.2%) were prescribed hydrocodone, and 2 (18.2%) were prescribed Tylenol with codeine. Seven of the eleven subjects (63.6%) were female with a mean (standard deviation - SD) age of 48.5 (8.6) years. Four subjects (36.4%) were currently employed, and two (18.2%) were married. The mean (SD) pain duration was 9.9 (8.7) years, and the mean swallowing problem duration was 4.9 (6.3) years. Mean (SD) pain intensity at baseline was 8.1 (1.7) for daily pain ratings from text message links averaged across the first 7 days of the study and 8.0 (2.0) for weekly pain ratings from the PROMIS-29 profile.
Results of the daily Medication Diary text message questions showed that 9 of the 11 subjects (81.8%) reported 100% medication compliance for the entire 5-week XER dosing period. The remaining 2 subjects were unable to receive text messages for approximately 2 weeks of the XER dosing period due to technical difficulties. These subjects reported 100% medication compliance in the received text messages and verbally reported that they took their study medication every day during the period when text messages were not received.
Primary Outcomes
The primary outcome of change in pain intensity is displayed in Figure 1A. Repeated measures univariate ANOVA showed no significant difference in average pain intensity values on both the daily and weekly surveys. The daily pain intensity scores showed 12.5% (3.1%) improvement, and the weekly pain intensity scores showed 9.3% (29.8%) improvement from baseline to the end of the study, but this was not a significant change for either measure, F(1, 10) = 1.379–1.729, p = 0.218–0.268.
Secondary Outcomes
Secondary outcome data for swallowing, opioid satisfaction, and global impression of change are found in Table 2, and swallowing difficulty is shown in Figure 1A. Subjects reported a decrease in swallowing difficulty of −5.7 (2.5) points, and this was a significant change, F(1, 10) = 55.074, p < 0.001. From baseline when the subjects were taking their previously prescribed opioid medication to the end of the study when subjects were taking XER, opioid medication satisfaction increased by an average of 2.8 (4.0), which was also a significant change, F(1, 10) = 5.388, p < 0.05. At the end of the study, mean (SD) global impression of change was 1.3 (1.1), which falls between 1 = “A little bit improved” and 2 = “Somewhat improved” on the answer scale.
 |
Table 2 Secondary Subject-Reported Outcomes Related to Satisfaction |
Additional secondary outcome measures of PROMIS pain, mental health, and physical health domain T-Scores are shown in Figure 1B. The subjects reported mild improvement across all domains on average: physical function and social roles T-Scores showed mean (SD) increased from baseline of 0.7 (5.0) and 1.1 (4.9) respectively. Mean (SD) changes in depression, anxiety, pain interference, and sleep disturbance T-Scores from baseline to the end of the study ranged from −0.4 (7.3) to −3.7 (6.2). None of these PROMIS measures showed significant changes from baseline to the end of the study, F(1,10) = 0.027–3.858, p = 0.078–0.874.
Discussion
This pilot study demonstrates that patients who report difficulty swallowing opioid pain medication can be converted to XER with little difficulty and show both decreased swallowing difficulty and improved overall satisfaction with their pain medication. None of the subjects in the study had issues opening the capsules or spreading the microspheres in soft food. There were also no appreciable negative differences in pain, function, or sleep scores after the conversion to XER.
A recent systematic review found that self-reported pill swallowing difficulties affect 10–40% of patients in this country.14 This review also found that 70% of patients who report swallowing difficulties also report skipping medications, and 10–59% tamper with their medications (such as crushing or dissolving tablets) in order to take them. Both skipping and tampering with medications are problematic adherence issues for opioid prescribing, as these behaviors are often indicative of opioid misuse. Tampering with opioid medication can be especially dangerous because patients may rapidly absorb all of the drug in a short or long-acting formulation, and this places the patient at an increased risk of accidental overdose. The abuse-deterrent formulation of XER prevents the patient from tampering with the medication and therefore alleviates these issues. In addition to its abuse-deterrent formulation, the openable capsule of XER makes it an especially attractive opioid pain medication for patients afflicted with cancer pain who are more likely to have pill swallowing difficulties as an effect of cancer-related treatments. Of note, variations in diet (such as liquid vs. solid, or vegetarian vs. meat-eating) have not been shown to affect absorption of the long-acting oxycodone microspheres.
Using an open label, noninferiority design, the present study showed that patients can reliably take opioid medication as prescribed without having to tamper with the pills. It should be noted that open-label study designs can lead to expectation bias from both the subjects and the study physicians.15 As the sample size is small (N=11), it is best to consider this a proof-of-concept study. However, small proof-of-concept studies are not without merit, as they provide critical evidence to support medical interventions. Although it may seem obvious that conversion to XER would improve patients’ swallowing difficulties and decrease tampering, results from small proof-of-concept studies such as this one are necessary to show that this “common sense” is actually accurate. We hope that the present study’s findings will raise awareness among providers of the issue of pill swallowing difficulties with opioid pain medication and provide a straightforward solution should they encounter this issue among their patients.
Conclusion
It is important for clinicians to inquire about pill swallowing difficulties in their patients who are prescribed opioids. The present study shows that conversion to the openable, abuse-deterrent oxycodone formulation XER improves pill swallowing difficulties, increases overall satisfaction with the pain medication regimen, and is unlikely to negatively affect self-reported pain, mental health, or physical function.
Data Sharing Statement
Individual de-identified participant data for all study measures will be available for qualified academic, government, and industry researchers for seven years after the publication of this paper. All requests for study data should be directed to the corresponding author.
Acknowledgments
This investigator-initiated study was funded by Collegium Pharmaceutical. This study was registered on ClinicalTrials.gov as clinical trial NCT03588806.
Disclosure
Dr Ajay D Wasan reports grants from Collegium Pharmaceutical, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Ballantyne JC. Opioids for the treatment of chronic pain: mistakes made, lessons learned, and future directions. Anesth Analg. 2017;125(5):1769–1778. doi:10.1213/ANE.0000000000002500
2. Schiele JT, Quinzler R, Klimm HD, Pruszydlo MG, Haefeli WE. Difficulties swallowing solid oral dosage forms in a general practice population: prevalence, causes, and relationship to dosage forms. Eur J Clin Pharmacol. 2013;69(4):937–948. doi:10.1007/s00228-012-1417-0
3. Pergolizzi JV
4. Llorca PM. Discussion of prevalence and management of discomfort when swallowing pills: orodispersible tablets expand treatment options in patients with depression. Ther Deliv. 2011;2(5):611–622. doi:10.4155/tde.11.32
5. Fields J, Go JT, Schulze KS. Pill properties that cause dysphagia and treatment failure. Curr Ther Res Clin Exp. 2015;77:79–82. doi:10.1016/j.curtheres.2015.08.002
6. Nativ-Zeltzer N, Bayoumi A, Mandin VP, et al. Validation of the PILL-5: a 5-item patient reported outcome measure for pill dysphagia. Front Surg. 2019;6:43. doi:10.3389/fsurg.2019.00043
7. Xtampza ER Label. FDA. 2016. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208090s000lbl.pdf.
8. Lee YH, Brown DL, Chen HY. Current impact and application of abuse-deterrent opioid formulations in clinical practice. Pain Physician. 2017;20(7):E1003–E1023.
9. Stanos SP, Bruckenthal P, Barkin RL. Strategies to reduce the tampering and subsequent abuse of long-acting opioids: potential risks and benefits of formulations with physical or pharmacologic deterrents to tampering. Mayo Clin Proc. 2012;87(7):683–694. doi:10.1016/j.mayocp.2012.02.022
10. Maincent J, Zhang F. Recent advances in abuse-deterrent technologies for the delivery of opioids. Int J Pharm. 2016;510(1):57–72. doi:10.1016/j.ijpharm.2016.06.012
11. Hays RD, Spritzer KL, Schalet BD, Cella D. PROMIS((R))-29 v2.0 profile physical and mental health summary scores. Qual Life Res. 2018;27(7):1885–1891. doi:10.1007/s11136-018-1842-3
12. Dysphagia Questionnaire. Centers BASM, ed
13. Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther. 2004;27(1):26–35. doi:10.1016/j.jmpt.2003.11.003
14. Forough AS, Lau ET, Steadman KJ, et al. A spoonful of sugar helps the medicine go down? A review of strategies for making pills easier to swallow. Patient Prefer Adherence. 2018;12:1337–1346. doi:10.2147/PPA.S164406
15. Roydhouse JK, Fiero MH, Kluetz PG. Investigating potential bias in patient-reported outcomes in open-label cancer trials. JAMA Oncol. 2019;5(4):457–458. doi:10.1001/jamaoncol.2018.6205
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

