Back to Journals » Research and Reports in Urology » Volume 12
Urinary Cytochrome C and Caspase-3 as Novel Biomarker of Renal Function Impairment in Unilateral Ureteropelvic Junction Obstruction Model of Wistar Rats
Authors Sibarani J , Tjahjodjati T , Atik N, Rachmadi D, Mustafa A
Received 24 April 2020
Accepted for publication 18 June 2020
Published 3 July 2020 Volume 2020:12 Pages 217—224
DOI https://doi.org/10.2147/RRU.S259237
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jan Colli
Jupiter Sibarani,1 Tjahjodjati Tjahjodjati,1 Nur Atik,2 Dedi Rachmadi,3 Akhmad Mustafa1
1Department of Urology, Faculty of Medicine Universitas Padjadjaran, Hasan Sadikin Hospital Bandung, Bandung, Indonesia; 2Department of Biomedical Sciences, Faculty of Medicine Universitas Padjadjaran, Hasan Sadikin Hospital Bandung, Bandung, Indonesia; 3Department of Pediatric, Faculty of Medicine Universitas Padjadjaran, Hasan Sadikin Hospital Bandung, Bandung, Indonesia
Correspondence: Akhmad Mustafa Jl. Eijkman no. 38, Bandung, Indonesia
Email [email protected]
Introduction: Prolonged obstruction in UPJO would lead to kidney destruction. It is important to find a non-invasive biomarker for early detection of renal impairment before definitive treatment for UPJO. In this study, we aim to evaluate the role of urinary cytochrome c and caspase-3 as a novel biomarker to predict renal function impairment in a UPJO model in Wistar rats.
Methods: Twenty-five male Wistar rats were separated into 3 groups. Group I consists of 5 rats without any treatment or model, group II consists of 5 rats (sham group), and group III consists of 15 rats with the unilateral partial ureteral obstruction model. After 4, 15, and 21 days of observation, the urine was collected and rats were sacrificed to collect the glomerular count in the kidney. Measurement of cytochrome c and caspase-3 was done using the ELISA method, while the glomerular count was done using a light microscope. Data were analyzed using factorial repeated measures ANOVA test and correlation test with Pearson and processed using SPSS version 20.0.
Results: The UPJO group has a significant increase in cytochrome c concentration and caspase-3 concentration compared to the control group and sham group (p< 0.05). There was a significant decrease in the normal glomerulus count of the UPJO group (p< 0.05). There was a significant relationship between the decrease in glomerulus count with the concentration of cytochrome c and caspase-3 in the UPJO group.
Conclusion: There was a significant relationship between the decrease in glomerulus count with the increase in the concentration of cytochrome c and caspase-3 in the UPJO group.
Keywords: ureteropelvic junction obstruction, cytochrome c, caspase-3
Introduction
Ureteropelvic junction obstruction (UPJO) is the most common cause of hydronephrosis in children.1 Despite extensive clinical and experimental studies over the past decades, fundamental issues regarding the evaluation and management of children with upper urinary tract obstruction remain unsolved.2 No reference standard is available to identify obstruction and degree of renal damage, and the diagnosis is usually achieved through repeating the various radiological investigations available.
The classic diagnostic tools for the investigation of children with upper tract renal obstruction include ultrasound scans, nuclear medicine assessments [(dimercaptosuccinic acid) scan and technetium-99m (Tc-99m DMSA) mercaptoacetyltriglycine (MAG3) renal studies], contrast studies such as voiding cystourethrogram (VCUG) and conventional markers of renal function (such as serum creatinine).3
However, these tests are not always adequate predictors for disease progression, particularly for borderline cases. Nevertheless, radiological investigations expose the child to radiation and may necessitate an injection of radiocontrast or radioisotope materials, which may be potentially harmful. Conservative treatment also conveys its own risk of prolonged obstruction, eventually leading to a progressive loss of nephrons with renal tubular atrophy and interstitial fibrosis.4 This process is mediated through various cytokines, leading to apoptosis of tubular cells. In the process, these cells release biological markers in the urine. Thus, it is possible to evaluate the progress of kidney destruction by the means of a non-invasive study of urinary biomarkers for assessment and prediction of functional and structural changes of the kidney. Hence, the use of urinary biomarkers has been advocated, which could discriminate patients whose renal function will deteriorate from those who will spontaneously improve at an early stage, preventing unnecessary surgery.
Nowadays, there are several markers to evaluate kidney function. These markers are able to evaluate glomerulus filtration and tubule function. Those markers are creatinine, urea, cystatin, β-trace protein (BTP), Inulin, Iohexol, radioactive marker, proteinuria, etc. Unfortunately, all of these markers are detected when there is already destruction of the kidney. Some research efforts have focused on the discovery and validation of novel serum and urine biomarkers to detect renal injury prior to extensive structural damage.5
Cytochrome c is best known as an indicator of cell death burden in any organ or tissue. It is released during mitochondrial damage that is associated with the processing of apoptosis, cell lysis during necrosis, and even reversible mitochondrial and cell injury.6 Cytochrome c is a protein with a size of 12.3 kDa. Cytochrome c contains a heme ring and is dissolved in the mitochondrial intermembrane fluid. Cytochrome c has various functions including a role in cell respiration, the process of apoptosis, ROS formation, destruction of ROS, and cardiolipin peroxidation. After leaving the mitochondria, cytochrome c will bind to Apaf-1, then form an apoptosome.7 This process is an initial part of apoptosis.
In the complicated molecular mechanism of apoptosis, cysteinyl aspartate-specific proteinases (caspases) have a key role, as they lead to the most of morphological and biochemical changes in cellular death. The mechanism of activation of caspases goes through two pathways; receptor-mediated death signaling (extrinsic signaling) or mitochondria regulated (intrinsic signaling). One of the key elements in renal apoptosis is caspase-3.8
Due to their roles in apoptosis, cytochrome c and caspase-3 were expected to be detected even before renal injury occurred. This study aims to evaluate the role of urinary cytochrome c and caspase-3 as a novel biomarker of initial kidney destruction events in a UPJO model of Wistar rats.
Materials and Methods
Research on experimental animals must take into account the advantages and disadvantages of the number of experimental animals used. Too many animals can cause losses in the form of money, time and energy, and is unethical. However, too few animals can weaken the strength of research, and lead to no scientific conclusions. To get the number of animals needed in this study, the number of samples was calculated using the Federer formula. The Federer formula for sample size in animal study is [(T-1)(N-1)>15, where N: number of samples, T: number of groups]; it was found that 25 male Wistar rats were sufficient to conduct this study. The study was done in June 2019. The rats aged 8 weeks and weighing 150–200 g were separated into 5 groups (Figure 1). All operations were performed under sterile conditions. The animals were anesthetized with 20mg/100g ketamine injection intramuscularly. The unilateral UPJO model was created in the left UPJ by using the method described by Boyarsky and Martinez.9 A median incision in the abdomen was made around 3 cm through the peritoneum to identify the UPJ. In the intervention group, a nylon 4.0 suture was inserted through the ureter (distally from the UPJ) into the kidney. The UPJ was then tied using silk 4.0, and then the nylon suture was pulled until it came off (Figure 2).
 |
Figure 1 Research flow. |
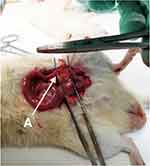 |
Figure 2 Unilateral UPJO model using Wistar rats. A indicates the left ureter. |
Samples of urine were collected on day 4, 15, and 21 after the procedure in a sterile container to be analyzed for the level of cytochrome c and caspase-3. The samples were yielded by complete random sampling. The cytochrome c and caspase-3 were measured by using ELISA (Rat Cyt-C (cytochrome c) ELISA Kit Catalog No. E-EL-R0006 and Rat CASP3 (Caspase-3) ELISA Kit). Histological preparations were made from the rats’ kidneys to assess the amount of normal glomerulus and tubulus from the intervention group.
All animal models used in this study were housed in a 100 cm2 cages, one for each rat. This study was conducted in Biochemical and Bioscience Laboratory, Brawijaya University Malang, and had been accepted for ethical clearance from the ethical committee. All study objects received treatments according to animal welfare guidelines according to William Russel and Rex Burch.10
For statistical analysis, the samples were processed using SPSS version 20.0 in Windows. Values of p≤0.05 were considered statistically significant. Two-way repeated ANOVA (analysis of variants) statistical test was used to assess the level of cytochrome c and simple linear regression was used to assess the comparison amount of glomerulus and caspase-3.
Results
The result of cytochrome c examination using the ELISA method showed that there were concentration differences between control, sham, and UPJO groups. Cytochrome c concentration was higher in the UPJO group compared to the control and sham group, as seen in Table 1. There was also an increasing tendency in cytochrome c concentration in the UPJO group from day 0 to day 4; then, the concentration went relatively stable as seen in Figure 3.
 |
Table 1 Comparison of Cytochrome c Concentration |
 |
Figure 3 Cytochrome c concentration pattern. |
The result from the statistical analysis showed that there is a significant effect in the UPJO group on the concentration of cytochrome c in urine. The UPJO group has a significant increase in cytochrome c levels compared to the control group and the sham group (p<0.05) (Table 1). Table 2 shows the mean value of urine caspase-3 concentrations in the sham group, the control group, and the UPJO group on the 4th, 15th, and 21st days. The results showed that the concentration of caspase-3 in the UPJO group was higher than the control and sham group. In Figure 4 the concentration of caspase-3 in the UPJO group has an upward trend between day 0 to day 4 then went relatively stable. The result from the statistical analysis showed that the UPJO group has a significant effect on the caspase-3 level. There was a significant increase in caspase-3 levels in the UPJO group compared to the control group and sham group (p<0.05).
 |
Table 2 Comparison of Caspase-3 Concentration |
 |
Figure 4 Caspase-3 concentration pattern. |
The result of the count of the normal glomerulus and tubulus per low power field using a light microscope showed a decreasing trend in the UPJO group, as seen in Figure 5. The trend showed a gradual decrease from day 0 to days 4, 15, until 21. Table 3 shows that the glomerulus count in the UPJO group was lower than the control and sham group. Results from statistical analysis showed that the UPJO group has a significant effect on the normal glomerular count. The UPJO group has a significant decrease in normal glomerulus count compared to the control group and sham group (p<0.05).
 |
Table 3 Comparison of Glomerulus Count |
 |
Figure 5 Glomerulus count pattern. |
The relationships between normal glomerular count with caspase-3 concentration and cytochrome c in urine were analyzed using parametric linear regression analysis. Glomerulus count has a strong reversal correlation with cytochrome c concentration (r=−0.845; p=0.000) and caspase-3 (r=−0.730; p=0.000), while caspase-3 correlated strongly in the same direction with cytochrome c (r=0.740; p=0.000). The result showed there was a significant relationship between the decrease in glomerulus count with the concentration of cytochrome c and caspase-3 in the UPJO group (Table 4).
 |
Table 4 Pearson Correlation Between Residual Linear Regression Equation with Caspase-3 Levels and Urine Cytochrome Levels and Normal Glomerular Amounts |
Discussion
Kidney damage that occurs in UPJO varies greatly: changes in tubular size, chronic tubulointerstitial damage, glomerulosclerosis, fibrosis, and in severe circumstances, renal dysplasia occurs. The decrease in the number of glomeruli also occurs as a result of nephrogenesis disruption and nephron damage that has formed through the process of apoptosis.11,13 Blockage in the kidney also causes tubular atrophy and tubular cell death, in which the main mechanism of cell death is apoptosis.1,11,13
Cytochrome c is a small spherical protein involved in the electron transport chain of mitochondria.14,16 Its function is to facilitate energy production at the cellular level. It is now known that cytochrome c has a role in the intrinsic pathway of the process of cell apoptosis.15,16 Cytochrome c is rapidly released after damage from a particular type of cell.8 Increased levels indicated mitochondrial damage, as one of the earliest signs of injury and cell death.17 Thus, before the kidney damage process occurs, it is likely that cytochrome c can already be detected in urine. In other words, an increase in cytochrome c in urine can be used as a reference for taking action in UPJO patients.
Research by Zager found an acute increase in cytochrome c levels in rats that were given ioversol contrast agents that triggered damage to the proximal tubules.6 This acute increase was related to a dysfunction in the mitochondria; thereby cytochrome c could be a biomarker of the “stress” conditions in the mitochondria.18 In addition, this study also found that the condition of acute kidney failure will be marked by an increase in cytochrome c level as an initial marker of the process of cell apoptosis.6
Another study conducted by Goldstein et al found that cytochrome c level increased within 5 minutes of cell damage. The study also found that in the condition of acute renal failure, damage from glomerulus and tubules would occur and increased the level of cytochrome c due to damage from mitochondria.19
Caspase is a cysteine protease.17 One of its derivatives is caspase-3 which is considered as one of the markers of the process of cell death or tubular cell apoptosis. Both the intrinsic and extrinsic pathways of the apoptotic process can trigger the activation of caspase-3.20 Cytochrome c that comes out of mitochondrial damage is released through the inflammatory process, which induces activation of caspase-9, which in turn activates the enzyme caspase-3.20 Caspase-3 will be activated in every process of renal cell apoptosis caused by obstructive nephropathy conditions.18 Inhibition of the activity of the caspase will decrease the apoptotic process in tubular cells and glomerular cells in ischemic kidney condition or reperfusion injury to the kidney, thereby preventing inflammation and fibrosis.20,21
These results are consistent with a study conducted by Vaara (2016) which found that caspase-3 was one of the markers that increased in patients with acute renal impairment, where caspase-3 was increased in the apoptosis process in tubular and glomerular cells.21,22 Increased caspase-3 in damaged glomerulus could be detected through urine and blood tests; both results showed a significant increase in the presence of ischemic kidney.21,22
Research conducted by Shirazi (2017) found that cytochrome c and caspase-3 could be markers of renal glomerular damage. They found that in infants with UPJO who underwent pyeloplasty surgery, at 6 months after surgery cytochrome c and caspase-3 levels were found to be decreased significantly. The decrease in both indicated that each has a significant correlation as a marker of kidney damage.23
In this study, we tried to investigate the association between the increase in cytochrome c concentration in urine and renal glomerular damage in rats. We found that there was a decrease in the number of glomeruli accompanied by an increase in cytochrome c on day 4 and day 15, but on day 21 cytochrome c levels began to decrease slightly. UPJO will cause urine obstruction from the kidney which will cause urinary stasis in the kidney. The complications of this condition are damage to the kidney tubules and glomerulus. The existence of cell damage will cause disruption to the mitochondria which will produce cytochrome c as a marker of the process of apoptosis in the glomerular cells. In this study, there was a decrease in the number of glomeruli and an increase in cytochrome c in the UPJO group on day 4 and day 15. On the 21st day the decrease of cytochrome c may have been caused by the small amount of the remaining glomerulus.
In this study, there was a difference in the concentration of caspase-3 between the UPJO group and the control group on days 4, 15, and 21. The caspase-3 concentration was significantly higher since the increase in the concentration of caspase-3 starting from day 4 of examination. Caspase-3 will increase in the apoptosis process, which will certainly occur in the UPJO group. We found that caspase-3 concentration tended to persist after day 15 and day 21, but there were still significant differences compared to the control group.
There was a decrease in the number of glomeruli accompanied by an increase in cytochrome c on days 4 and 15, but on day 21 cytochrome c levels began to decrease slightly. UPJO will cause obstructive urine from the kidney which will cause the condition of urinary stasis in the kidney. Complications of this condition are damage to the kidney tubules and glomerulus. The existence of cell damage will cause interference with the mitochondria which will produce cytochrome c as a marker of the process of apoptosis in glomerular cells. In this study, there was a decrease in the number of glomeruli and an increase in cytochrome c in the artificial UPJO group on days 4 and 15 of the examination. On the 21st day cytochrome c decreased, which may be due to the small amount of glomerulus remaining.
Conclusion
Cytochrome c and caspase-3 concentrations were seen increased significantly in the UPJO group, while there was a decrease in the normal glomerular count. The normal glomerular count decreased as the cytochrome c concentration and caspase-3 concentration increased. However, further research should be done in order to determine the cytochrome c and caspase-3 concentration and the cut-off point concentration in humans with UPJO. Increased caspase-3 and cytochrome c in UPJO condition can be proposed as an early biomarker for establishing the diagnosis and early treatment selection in UPJO.
Acknowledgments
The study was supported partly by the Academic Leadership Grant (ALG) Universitas Padjadjaran (contract no. 2476/UN6.C/LT/2018).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tekgul S, Riedmiller H, Hoebeke P, et al. Dilatation of the upper urinary tract (UPJ and UVJ obstruction). EAU Guidelines on Paediatric Urology. 2016:49–51
2. Tabari AK, Atqiaee K, Mohajerzadeh L, et al. Early pyeloplasty versus conservative management of severe ureteropelvic junction obstruction in asymptomatic infants. J Pediatr Surg. 2019. doi:10.1016/j.jpedsurg.2019.08.006
3. Corbett HJ, McCarthy L. Hydronephrosis in children: pelviureteric junction dysfunction. Surgery (Oxford). 2013;31(3):135–139. doi:10.1016/j.mpsur.2013.01.003
4. Chertin B, Pollack A, Koulikov D, et al. Conservative treatment of ureteropelvic junction obstruction in children with antenatal diagnosis of hydronephrosis: lessons learned after 16 years of follow-up. Eur Urol. 2006;49(4):734–739. doi:10.1016/j.eururo.2006.01.046
5. Shirazi M, Eslahi A, Shari V, Rahimi F, Safarpour A. Evaluation of caspase 3 enzyme and TNF-alpha as biomarkers in ureteropelvic junction obstruction in children- a preliminary report. Pak J Med Sci. 2017;33(2):315–319. doi:10.12669/pjms.332.11934
6. Zager RA, Johnson AC, Hanson SY. Proximal tubular cytochrome c efflux: determinant, and potential marker, of mitochondrial injury. Kidney Int. 2004;65(6):2123–2134. doi:10.1111/j.1523-1755.2004.00638.x
7. Basnakian A, Kaushal G, Shah S. Apoptotic pathways of oxidative damage to renal tubular epithelial cells. Antioxid Redox Signal. 2002;4(6):915–924. doi:10.1089/152308602762197452
8. Jeruc J, Vizjak A, Rozman B, Ferluga D. Immunohistochemical expression of activated caspase-3 as a marker of apoptosis in glomeruli of human lupus nephritis. Am J Kidney Dis. 2006;48(3):410–418. doi:10.1053/j.ajkd.2006.05.019
9. Wen JG. Partial unilateral ureteral obstruction in rats. Neurourol Urodyn. 2002;21(3):231–250. doi:10.1002/nau.10006
10. Russell WMS, Burch RL. The Principles of Humane Experimental Technique. London, UK: Methuen; 1959:238.
11. Flechner S, Finke J, Dan Fairchild R. Basic principles of immunology in urology. In: Dalam Wein A, Kavoussi J, editors. Campbell-Walsh Urology, 10th Edition, International Edition. Saunders, Elsevier; 2012:495–529.
12. Gowda S, Desai P, Kulkarni S, Hull V, Math A, Dan Vernekar S. Markers of renal function tests. N Am J Med Sci. 2010;2(4):170–173.
13. McIlwain D, Berger T, Mak T. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5(4):a008656. doi:10.1101/cshperspect.a008656
14. Yeagle PL. The Membranes of Cells. Academic Press; 2016 Feb 17.
15. Garrido C, Galluzzi L, Brunet M, et al. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13(9):1423–1433. doi:10.1038/sj.cdd.4401950
16. George BP, Abrahamse H. Increased oxidative stress induced by rubus bioactive compounds induce apoptotic cell death in human breast cancer cells. Oxid Med Cell Longev. 2019;2019:1–18. doi:10.1155/2019/6797921
17. Eleftheriadis T, Pissas G, Liakopoulos V, Stefanidis I. Sitokrom c as a potentially clinical useful marker of mitochondrial and cellular damage. Front Immunol. 2016;7:279. doi:10.3389/fimmu.2016.00279
18. Zager RA, Johnson ACM, Hanson S. Radiocontrast media induced tubular injury: evaluation of oxidant stress and plasma membrane integrity. Kidney Int. 2003;2003(64):128–139. doi:10.1046/j.1523-1755.2003.00059.x
19. Goldstein JC, Waterhouse NJ, Juin P, et al. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol. 2000;2(3):156–6212. doi:10.1038/35004029
20. Madsen MG, Norregaard R, Frokijaer J, Jorgensen TM. Urinary biomarkers in prenatally diagnosed unilateral hydronephrosis. J Pediatr Urol. 2011;7(2):105–112. doi:10.1016/j.jpurol.2010.12.004
21. Vaara ST, Lakkisto P, Immonen K, et al. Urinary biomarkers indicative of apoptosis and acute kidney injury in the critically Ill. PLoS One. 2016;11(2):e0149956. doi:10.1371/journal.pone.0149956
22. Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17(6):1503–1520. doi:10.1681/ASN.2006010017
23. Delanaye P, White CA, Ebert N, Rule AD. Assessing kidney function. Chronic Renal Dis. 2020:37–54. Academic Press.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
