Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Uric Acid and Clinical Outcomes in Young Patients with Ischemic Stroke
Authors Liu Y, Liu X , Jia J, Guo J, Li G, Zhao X
Received 11 May 2022
Accepted for publication 14 September 2022
Published 29 September 2022 Volume 2022:18 Pages 2219—2228
DOI https://doi.org/10.2147/NDT.S373493
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jun Chen
Yanfang Liu,1– 3,* Xinmin Liu,1,2,* Jiaokun Jia,1– 3 Jiahuan Guo,1,2 Guangshuo Li,1,2 Xingquan Zhao1– 4
1Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, People’s Republic of China; 2China National Clinical Research Center for Neurological Diseases, Beijing, People’s Republic of China; 3Research Unit of Artificial Intelligence in Cerebrovascular Disease, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 4Beijing Institute of Brain Disorders, Collaborative Innovation Center for Brain Disorders, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xingquan Zhao, Email [email protected]
Background and Purpose: There is limited available evidence for the relationship between uric acid (UA) levels and ischemic stroke in young adults. We aimed to explore the association between UA levels and acute ischemic stroke (AIS) in young patients.
Materials and Methods: This was a prospective and observational study. We recruited young patients aged 18– 45 years with AIS at our tertiary hospital. Patients were categorized into four groups according to quartiles of UA levels. The primary outcome was functional outcome at 3 months. The secondary outcomes included stroke severity, in-hospital complications, and functional outcome at discharge. Modified Rankin Scale (mRS) scores were used to assess functional outcome as poor (mRS=2-6) or favorable(mRS=0-1).
Results: A total of 636 patients were enrolled in the current analysis. The four groups were defined as follows: Q1≤ 289.8 μmol/L, 289.8 μmol/ L
Conclusion: Serum UA was a protective factor for stroke severity and in-hospital pneumonia after AIS in young patients. However, we were unable to identify the predictive significance of UA for functional outcome either at discharge or at 3 months after AIS.
Keywords: uric acid, young ischemic stroke, outcome, stroke severity, complication
Introduction
Stroke remains the principal leading cause of morbidity and mortality worldwide.1 In contrast to the decreasing incidence of stroke in older adults, the incidence of ischemic stroke in young adults aged 18–45 years is increasing.2,3 Acute ischemic stroke (AIS) in young adults can incur loss of the ability to work and high healthcare costs.2,4,5 It is noteworthy that traditional risk factors of ischemic stroke among young patients differ from that among the general or older population. Therefore, it is imperative that we seek to identify novel biomarkers and improve the clinical prognosis of young subjects with ischemic stroke.
Uric acid (UA) is the product of purine nucleotide catabolism. UA is the most abundant natural antioxidant in human plasma, accounting for 65% of the total antioxidant capacity of plasma.6 Severe oxidative stress and free radical production can be used to predict the poor prognosis of ischemic stroke. UA has been considered as a neuroprotective agent by virtue of the capacity of free radical scavenging in preclinical ischemic stroke models.7–9 Several observational human studies demonstrated that increased serum UA levels were associated with better clinical outcome after AIS.10–12 A randomized-controlled trial of intravenous UA administered during alteplase treatment for AIS also revealed that UA therapy had an excellent safety profile and ability to prevent early ischemic deterioration among thrombolysed patients.13
However, conflicting data have been reported regarding whether increased levels of serum UA are significantly associated with favorable outcomes in patients after ischemic stroke. Hyperuricemia was previously shown to be correlated with vascular risk factors such as hypertension,14 diabetes15 and other metabolic disease16 and could significantly increase the risk of ischemic stroke in younger and older populations.17–20 Another study demonstrated that increased UA levels were predictors of cytotoxic injury and infarct expansion after stroke.21,22 However, the relationship between serum UA and the prognosis of ischemic stroke remains controversial. Previous studies were limited to the general or older populations. There is a scarcity of studies exploring the effect of UA on prognosis after AIS in young patients. The aim of our study was to determine the association of UA levels with stroke severity, in-hospital complications, and functional outcome after AIS in young patients.
Materials and Methods
Study Design and Participants
This study was a single-center, prospective, and observational cohort study conducted in Beijing Tiantan Hospital, Capital Medical University. A total of 691 young patients diagnosed with ischemic stroke were enrolled between January 2019 and December 2021. All patients underwent cranial computed tomography (CT) or/and magnetic resonance imaging (MRI) scans to avoid misdiagnosis. The inclusion criteria were as follows: (1) 18–45 years-of-age; (2) patient met the diagnostic criteria for ischemic stroke according to World Health Organization criteria;23 3) patient was admitted to hospital within 3 days of onset. (4) informed consent was provided by the patient or legally authorized representative. The exclusion criteria were as follows: (1) venous infarction or acute intracerebral hemorrhage (ICH); (2) head trauma and other ischemic stroke mimics; (3) diagnosed with major comorbidities or late-stage diseases; (4) pre-stroke modified Rankin Scale (mRS) ≥2; Major comorbidities or late-stage diseases included heart failure defined as left ventricular ejection fraction ≤40%, end-stage kidney disease defined as estimated glomerular filtration rate (eGFR) <15 mL/min per 1.73 m2, liver failure and malignant tumors with a life expectancy of <3 months.
Demographic and Clinical Information
Demographic information, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), smoking, drinking, medical history, medication history, and National Institutes of Health Stroke Scale (NIHSS) were collected on admission. Fast blood samples were collected within 24 hours of admission. UA levels were estimated by photometry methods at the time of admission. Other laboratory examinations were also detected within 24 hours of admission, including triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), lipoprotein cholesterol (LDL-C), glycated hemoglobin (GHb), fasting blood glucose (FBG), homocysteine (Hcy), estimated glomerular filtration rate (eGFR), hypersensitive-C reactive-protein (hs-CRP), erythrocyte sedimentation rate (ESR) and platelets (PLT). Stroke subtypes were classified by the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification criteria into four categories as large-artery atherosclerosis, small-vessel occlusion, cardioembolism, undetermined etiology, and other other determined etiology.24
Follow-Up and Outcomes
Face-to-face interviews were performed when each patient was discharged, and telephone interviews were performed by well-trained research personnel at 90 days after the onset of stroke. The primary outcome was the 90-day functional outcome appraised by mRS scores. Due to the significant demand to return work by the young people, an mRS of 0-1 was regarded as a favorable outcome, while an mRS score of 2–6 was considered as a poor outcome in this study. The secondary outcomes were stroke severity, in-hospital complications, and functional outcome at discharge. Stroke severity was assessed by NIHSS score at admission. Moderate-severe stroke was defined as an NIHSS score ≥5.25–27 The in-hospital complications included pneumonia, urinary tract infection, deep vein thrombosis (DVT), and infectious diarrhea. Pneumonia was defined as respiratory crackles, new purulent sputum, or positive sputum culture, and supported by typical chest X-ray findings. Urinary tract infection was defined as clinical symptoms of urinary tract infection combined with a positive urine examination or culture. Deep vein thrombosis was defined as clinical diagnosis of deep vein thrombosis with radiographic evidence. Infectious diarrhea was defined as diarrhea occurring more than three times a day, accompanied by a positive stool test.
Statistical Analysis
The enrolled patients were divided into four groups (Q1-Q4) according to quartile levels of UA. Categorical variables were presented as frequencies with percentages. The Chi-squared test or Fisher’s exact test was used to analyze the categorical variables. The continuous variables were expressed as the mean ± standard deviation (SD) or the median and inter-quartile range (IQR) depending on whether the distribution of continuous variables was normal. If the distribution was normal, the comparisons were performed by one-way analysis of variance (ANOVA), otherwise the Mann–Whitney U-test was used.
Multivariate logistic regression analysis was adopted to determine the association of UA levels and stroke severity, in-hospital pneumonia, and functional outcome at discharge and at 3 months. In the first model, we adjusted only gender and age. In the second model, we added all potential co-variates with a p-value <0.05. A further linear trend test was then used to calculate P for trend of Q2-Q4 with reference to Q1.
A two-sided P value < 0.05 was regarded as statistically significant. All analyses were performed with SPSS version 24.0 for Windows (IBM, Chicago, IL, USA).
Results
Baseline Characteristics
After excluding 51 patients diagnosed with TIA and 4 patients without UA data, 636 young patients were finally enrolled in the current analysis (Figure 1). Of the total patients, 537 (84.4%) patients were male; the mean age (median (IQR)) of the whole patients was 37 (33,42) years. Patients were divided into four groups (Q1-Q4) according to the quartiles of UA levels, defined as follows: Q1≤289.8 µmol/L, 289.8 µmol/ L<Q2≤349.0 µmol/L, 349.0 µmol/L<Q3≤421 µmol/L, and Q4>421 µmol/L.
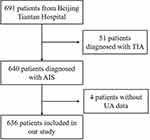 |
Figure 1 Flowchart of study population. |
Table 1 shows the differences in clinical features of patients among the four groups. As the UA levels increased, the proportion of males increased (P<0.001). Compared with the lowest UA levels, patients with higher UA levels were more likely to have a younger age; have a higher BMI and SBP; have higher proportion of OSAS, smoking, and drinking; and have higher levels of TG, TC, LDL-C, Hcy, eGFR, and ESR (all P <0.05). On the other hand, the levels of DBP and HDL-C were more likely to be lower in the group with higher UA levels (all P <0.05).
 |
Table 1 Characteristics and Clinical Information in Subjects |
Association Between Serum UA and Stroke Severity
As shown in Table 2, there was a higher proportion of moderate-severe stroke in the group with lower UA levels. In the multivariate logistic regression analysis, after adjustment for age and gender, compared to the Q1, higher UA levels were significantly associated with a lower proportion of moderate-severe stroke (odds ratio (OR) 0.792, 95% confidence interval (CI) 0.669–0.939, P for trend=0.007) (Table 3). After adjustment for age, gender, BMI, SBP, DBP, OSAS, smoking, drinking, TG, TC, HDL-C, LDL-C, Hcy, eGFR, and ESR, the statistical significance still remained (OR 0.786, 95% CI 0.647–0.956, P for trend=0.016) (Table 3).
 |
Table 2 Clinical Outcmes in Subjects |
 |
Table 3 Logistic Regression of the UA Levels on Stroke Severity |
Association Between Serum UA and in-Hospital Complications
Compared to the lowest UA levels, the incidence of in-hospital pneumonia was lower in groups with higher UA levels (P=0.001, Table 2). However, there was no significant difference in terms of other in-hospital complications, including urinary tract infection, DVT, and infection diarrhea between Q1-Q4 groups. As shown in Table 4, after adjusting for potential confounders (age, gender, BMI, SBP, DBP, OSAS, smoking, drinking, TG, TC, HDL-C, LDL-C, Hcy, eGFR, ESR, and NIHSS on admission), serum UA was inversely associated with in-hospital pneumonia and patients in the Q3 and Q4 showed a significant reduction in risk for in-hospital pneumonia when compared with those in the Q1 group (OR 0.222, 95% CI 0.061–0.807, OR 0.192, 95% CI 0.042–0.877, respectively, P for trend=0.012).
 |
Table 4 Logistic Regression of the UA Levels on in-Hospital Pneumonia |
Association Between Serum UA and Functional Outcome
As shown in Table 2, univariate analysis identified a significant difference in poor outcome at discharge or at 3 months among the Q1-Q4 groups. Compared to patients in the Q1, those with higher UA levels were at a lower risk of poor outcome both at discharge and at 3 months. After adjustment for age, gender, BMI, SBP, DBP, OSAS, smoking, drinking, TG, TC, HDL-C, LDL-C, Hcy, eGFR, ESR, serum UA was inversely associated with poor outcome at discharge and at 3 months (OR 0.724, 95% CI 0.598–0.876, P for trend=0.001, OR 0.760, 95% CI 0.618–0.935, P for trend=0.009, respectively) (Table 5). However, after further introducing NIHSS scores on admission into the regression model, the statistically significant association disappeared (Table 5).
 |
Table 5 Logistic Regression of the UA Levels on Functional Outcome |
Discussion
In the current study, we found that higher serum UA levels were independently associated with a lower proportion of moderate-severe stroke and a decreased risk of in-hospital pneumonia after AIS among young patients. However, UA levels were not significantly associated with functional outcome either at discharge or at 3 months after AIS.
UA is a natural antioxidant in human plasma, with an excellent ability to remove hydroxyl radicals, peroxynitrite, and hydrogen peroxide and suppress lipid peroxidation.28 Numerous studies have investigated the correlation between UA levels and both stroke risk and clinical outcomes after stroke. In previous studies, hyperuricemia was proven to be associated with comorbidities of hypertension, diabetes, and other metabolic disease; these are known independent vascular risk factors for ischemic stroke.14–16 Elevated UA levels showed independent predictive ability for a high risk of ischemic stroke.17,29 A previous meta-analysis involving 37,386 males and 31,163 females indicated that increased UA was a significant risk factor for adult stroke, both for ischemic stroke and hemorrhagic stroke. However, the impact of serum UA on clinical outcomes after AIS remains controversial. Several studies indicated that higher UA levels were associated with favorable outcome in ischemic stroke. Sun et al and Wu et al performed studies on Chinese populations and concluded that higher UA levels predicted good outcome after AIS.11,30 It also demonstrated that lower UA levels predicted a higher risk of hemorrhagic transformation after ischemic stroke.12,31 Chamorro et al demonstrated a 12% increase in the odds of a good clinical outcome for each milligram per deciliter increase of serum UA among patients with AIS.32 Furthermore, analysis of the URICO-ICTUS trial further reported that UA combined with alteplase may prevent early stroke progression.33 However, other studies came to the contrary conclusion that high serum UA levels were related to poor outcomes after AIS. Weir et al reported that higher serum UA levels were independently associated with a poor outcome (dead or in care) and higher vascular event rates.34 Wang et al reported that higher serum UA levels independently predicted poor outcomes in diabetic patients with AIS, especially in patients aged ≤ 75 years.35 Notably, a U-shaped relationship between serum UA levels and outcomes after AIS has been reported in several studies.36,37
However, previous studies only focused on the general or older populations. To the best of our knowledge, there is a significant lack of studies investigating the relationship between UA levels and ischemic stroke in young patients. However, we were unable to identify a significant association between serum UA levels and functional outcome after AIS in young patients. These conflicting results could be due to the differences in age, gender, stroke etiology and treatment. It was noteworthy that serum UA levels were demonstrated to improve functional outcome in homogeneous populations, such as female patients, or patients with pretreatment hyperglycemia, early recanalization, or subtypes of large artery atherosclerosis.10,11,38–41 A previous study of a Chinese population found that the rate of intravenous thrombolysis and endovascular thrombectomy for AIS were 9.5% and 4.4%.42 However, within the enrolled population, the proportion of intravenous thrombolysis was 7.2% (46/636), which was lower than that of general population. Our current analysis included young patients aged 18–45 years and mild stroke (NIHSS<5) accounted for 70.9%(145/636) of these patients; these patients would be less likely to undergo emergency intravenous thrombolysis; this may explain the difference between previous studies and the present results. Females accounted for only 15.6% (99/636) of our study population and the rate of intravenous thrombolysis and subtypes of large artery atherosclerosis were relatively lower; this may explain the differences between our findings and those of previous studies.
Very fewer clinical studies are available relating to the relationship between UA levels and stroke severity after AIS. We found that lower serum UA levels on admission were an independent indicator of initial moderate-severe ischemic stroke. The generation of free radicals leads to oxidative stress and neuronal damage after ischemic stroke.43 Several studies have shown that exogenous UA might alleviate oxidative stress, neuroinflammation, and apoptotic cell death in murine models of acute ischemic stroke.7,8 Nevertheless, Cherubini et al reported that the levels of the majority of antioxidants, including serum UA, were reduced immediately after an acute ischemic stroke.44 Leinonen et al demonstrated that low plasma antioxidant activity was associated with high neurological impairment, as assessed by NIHSS scores in stroke;45 this was consistent with the results of our present study. Another study found that a reduction in UA levels from admission to day 7 was associated with an initially more severe ischemic stroke (NIHSS score >7).46 A reduction in UA levels in acute stroke may reflect the load of oxidative stress influenced by stroke severity. The sample time in our study fluctuated within 3–4 days after the onset of stroke. Therefore, serum UA might play a role of neuroprotection and anti-inflammation in the earlier acute stage of AIS. Furthermore, paying attention to the initial levels and dynamic changes of UA in the earlier stage of AIS (within 3 days after stroke onset) was of great significance to evaluate the oxidative stress and stroke severity.
A limited body of literature has explored the relationship between serum UA levels and in-hospital pneumonia after AIS in young patients. Previous studies reported that serum or salivary UA levels were lower in a group of patients with pneumonia.47,48 Another study demonstrated serum UA exerted anti-inflammation and protective effects on the mycoplasma pneumoniae pneumonia in children.49 The ability of UA to reduce oxidative stress and inflammation is well established in pneumonia. In an earlier study, we found that serum UA was a protective factor for in-hospital pneumonia after ICH in a large population.50 In the current study, we also found a significant association between higher UA levels and reduction of in-hospital pneumonia after AIS. Furthermore, patients with severe stroke often stay bedridden and suffer from poor swallowing function; these are known risk factors of hypostatic pneumonia or aspiration pneumonia. A low rate of moderate-severe stroke with higher UA levels could also explain the results. Therefore, it is of great value to monitor UA levels when predicting the risk of pneumonia after AIS. It is also important to conduct more clinical randomized controlled trials or animal experiments to investigate the influence of UA on pneumonia.
There were several limitations to our study that need to be considered. First, this was a single-center, hospital-based study and the study population was small and mostly involved males. Larger multi-center studies are now needed to support our findings. Moreover, we only collected the UA levels at a single time. The dynamic change of UA levels were not acquired. The serum UA levels at admission might not accurately represent the overall condition of UA. Finally, we were unable to collect medical treatment data associated with the use of UA lowering drugs; this might have had an impact on UA levels and further influenced clinical outcomes after AIS.
Conclusion
We found that higher serum levels of UA were independently associated with lower proportion of moderate-severe stroke and a decreased risk of in-hospital pneumonia after AIS in young patients. It was important that we pay attention to UA levels when evaluating stroke severity and predicting the risk for in-hospital pneumonia. However, we failed to identify a significant association between UA levels and functional outcome either at discharge or at 3 months after AIS.
Abbreviations
UA, uric acid; AIS, acute ischemic stroke; ICH, intracerebral hemorrhage; TIA, transient ischemic attack; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, Triglyceride; TC, Total Cholesterol; HDL-C, High-density lipoprotein cholesterol; LDL-C, Lipoprotein cholesterol; GHb, glycated hemoglobin; FBG, fasting blood glucose; Hcy, homocysteine; eGFR, estimated glomerular filtration rate; hs-CRP, hypersensitive-C reactive-protein; ESR, Erythrocyte Sedimentation Rate; PLT, platelets; NIHSS, National Institutes of Health Stroke Scale; DM, Diabetes mellitus; OSAS, Obstructive sleep apnea-hypopnea syndrome; DVT, deep vein thrombosis.
Ethics Statement
The study was performed according to the Declaration of Helsinki guidelines and was approved by the Ethics Committees of Beijing Tiantan Hospital (approval number: KY2022-142-02). The patients/participants provided their written informed consent to participate in this study.
Acknowledgments
The authors thank all the participating patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This research was supported by CAMS Innovation Fund for Medical Sciences (2019-I2M-5-029), Beijing Municipal Committee of Science and Technology (Z201100005620010), Beijing Hospitals Authority Innovation Studio of Young Staff Funding Support (202112), and National Key Research and Development Program of China (2018YFC1312200 /2018YFC1312204).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Lanas F, Seron P. Facing the stroke burden worldwide. Lancet Glob Health. 2021;9(3):e235–e236. doi:10.1016/s2214-109x(20)30520-9
2. Ekker MS, Boot EM, Singhal AB, et al. Epidemiology, aetiology, and management of ischaemic stroke in young adults. Lancet Neurol. 2018;17(9):790–801. doi:10.1016/s1474-4422(18)30233-3
3. George MG, Tong X, Bowman BA. Prevalence of cardiovascular risk factors and strokes in younger adults. JAMA Neurol. 2017;74(6):695–703. doi:10.1001/jamaneurol.2017.0020
4. Bhatt N, Malik AM, Chaturvedi S. Stroke in young adults: five new things. Neurol Clin Pract. 2018;8(6):501–506. doi:10.1212/cpj.0000000000000522
5. Khan SU, Khan MZ, Khan MU, et al. Clinical and economic burden of stroke among young, midlife, and older adults in the United States, 2002–2017. Mayo Clin Proc Innov Qual Outcomes. 2021;5(2):431–441. doi:10.1016/j.mayocpiqo.2021.01.015
6. Mármol F, Sanchez J, Martínez-Pinteño A. Effects of uric acid on oxidative and nitrosative stress and other related parameters in SH-SY5Y human neuroblastoma cells. Prostaglandins Leukot Essent Fatty Acids. 2021;165:102237. doi:10.1016/j.plefa.2020.102237
7. Aliena-Valero A, Rius-Pérez S, Baixauli-Martín J, et al. Uric acid neuroprotection associated to IL-6/STAT3 signaling pathway activation in rat ischemic stroke. Mol Neurobiol. 2021;58(1):408–423. doi:10.1007/s12035-020-02115-w
8. Wang Q, Zhao H, Gao Y, et al. Uric acid inhibits HMGB1-TLR4-NF-κB signaling to alleviate oxygen-glucose deprivation/reoxygenation injury of microglia. Biochem Biophys Res Commun. 2021;540:22–28. doi:10.1016/j.bbrc.2020.12.097
9. Jiménez-Xarrié E, Pérez B, Dantas AP, et al. Uric acid treatment after stroke prevents long-term middle cerebral artery remodelling and attenuates brain damage in spontaneously hypertensive rats. Transl Stroke Res. 2020;11(6):1332–1347. doi:10.1007/s12975-018-0661-8
10. Wang C, Cui T, Wang L, et al. Prognostic significance of uric acid change in acute ischemic stroke patients with reperfusion therapy. Eur J Neurol. 2021;28(4):1218–1224. doi:10.1111/ene.14643
11. Sun Z, Feng J, He M, et al. Higher uric acid is associated with better discharge recovery and short-term outcome in stroke patients treated with thrombolysis. Neurol Sci. 2021;42(8):3225–3231. doi:10.1007/s10072-020-04919-z
12. Song Q, Wang Y, Cheng Y, Liu J, Wei C, Liu M. Serum uric acid and risk of hemorrhagic transformation in patients with acute ischemic stroke. J Mol Neurosci. 2020;70(1):94–101. doi:10.1007/s12031-019-01404-x
13. Chamorro A, Amaro S, Castellanos M, et al. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): a randomised, double-blind phase 2b/3 trial. Lancet Neurol. 2014;13(5):453–460. doi:10.1016/s1474-4422(14)70054-7
14. Viazzi F, Antolini L, Giussani M, et al. Serum uric acid and blood pressure in children at cardiovascular risk. Pediatrics. 2013;132(1):e93–99. doi:10.1542/peds.2013-0047
15. De Cosmo S, Viazzi F, Pacilli A, et al. Serum uric acid and risk of CKD in type 2 diabetes. Clin J Am Soc Nephrol. 2015;10(11):1921–1929. doi:10.2215/cjn.03140315
16. Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–1821. doi:10.1056/NEJMra0800885
17. Shi J, Yan G, Cao L, et al. The contribution of plasma uric acid to the risk of stroke in hypertensive populations. Cardiovasc J Afr. 2020;31(6):298–303. doi:10.5830/cvja-2020-023
18. Zhang S, Liu L, Huang YQ, Lo K, Tang S, Feng YQ. The association between serum uric acid levels and ischemic stroke in essential hypertension patients. Postgrad Med. 2020;132(6):551–558. doi:10.1080/00325481.2020.1757924
19. Norvik JV, Schirmer H, Ytrehus K, et al. Uric acid predicts mortality and ischaemic stroke in subjects with diastolic dysfunction: the Tromsø Study 1994–2013. ESC Heart Fail. 2017;4(2):154–161. doi:10.1002/ehf2.12134
20. Seki H, Kaneko H, Morita H, et al. Relation of serum uric acid and cardiovascular events in young adults aged 20–49 years. Am J Cardiol. 2021;152:150–157. doi:10.1016/j.amjcard.2021.05.007
21. Sato T, Sakai K, Komatsu T, et al. Risk factors for infarct expansion are different between lacunar and giant lacunar infarction. Atherosclerosis. 2020;292:17–22. doi:10.1016/j.atherosclerosis.2019.10.018
22. Ament Z, Bevers MB, Wolcott Z, Kimberly WT, Acharjee A. Uric acid and gluconic acid as predictors of hyperglycemia and cytotoxic injury after stroke. Transl Stroke Res. 2021;12(2):293–302. doi:10.1007/s12975-020-00862-5
23. Stroke WH. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO task force on stroke and other cerebrovascular disorders. Stroke. 1989;20(10):1407–1431. doi:10.1161/01.str.20.10.1407.
24. Adams HP
25. Zhao M, Guan L, Collet JP, Wang Y. Relationship between ischemic stroke locations, etiology subtypes, neurological outcomes, and autonomic cardiac function. Neurol Res. 2020;42(8):630–639. doi:10.1080/01616412.2020.1782103
26. Kang K, Lee WW, Lee JJ, Park JM, Kwon O, Kim BK. Association of higher waist circumference with milder stroke severity in acute ischaemic stroke. Neurol Res. 2018;40(9):785–794. doi:10.1080/01616412.2018.1479346
27. Liao CH, Liao NC, Chen WH, et al. Penumbra volume predicts unfavorable outcome in patients with acute minor stroke or transient ischemic attack. J Chin Med Assoc. 2020;83(6):551–556. doi:10.1097/jcma.0000000000000342
28. Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res. 1998;53(5):613–625. doi:10.1002/(SICI)1097-4547(19980901)53:5<613::AID-JNR11>3.0.CO;2-1
29. Tariq MA, Shamim SA, Rana KF, Saeed A, Malik BH. Serum uric acid - Risk factor for acute ischemic stroke and poor outcomes. Cureus. 2019;11(10):e6007. doi:10.7759/cureus.6007
30. Wu S, Pan Y, Zhang N, Jun WY, Wang C. Lower serum uric acid level strongly predict short-term poor functional outcome in acute stroke with normoglycaemia: a cohort study in China. BMC Neurol. 2017;17(1):21. doi:10.1186/s12883-017-0793-6
31. Tian Y, Xie Q, You J, Yang S, Zhao H, Song Y. Lower uric acid level may be associated with hemorrhagic transformation after intravenous thrombolysis. Neurol Sci. 2021. doi:10.1007/s10072-021-05760-8
32. Chamorro A, Obach V, Cervera A, Revilla M, Deulofeu R, Aponte JH. Prognostic significance of uric acid serum concentration in patients with acute ischemic stroke. Stroke. 2002;33(4):1048–1052. doi:10.1161/hs0402.105927
33. Amaro S, Laredo C, Renú A, et al. Uric acid therapy prevents early ischemic stroke progression: a tertiary analysis of the URICO-ICTUS trial (Efficacy study of combined treatment with uric acid and r-tPA in acute ischemic stroke). Stroke. 2016;47(11):2874–2876. doi:10.1161/strokeaha.116.014672
34. Weir CJ, Muir SW, Walters MR, Lees KR. Serum urate as an independent predictor of poor outcome and future vascular events after acute stroke. Stroke. 2003;34(8):1951–1956. doi:10.1161/01.str.0000081983.34771.d2
35. Wang P, Li X, He C, et al. Hyperuricemia and prognosis of acute ischemic stroke in diabetic patients. Neurol Res. 2019;41(3):250–256. doi:10.1080/01616412.2018.1553347
36. Kuo CF, See LC, Yu KH, Chou IJ, Chiou MJ, Luo SF. Significance of serum uric acid levels on the risk of all-cause and cardiovascular mortality. Rheumatology. 2013;52(1):127–134. doi:10.1093/rheumatology/kes223
37. Yang Y, Zhang Y, Li Y, et al. U-shaped relationship between functional outcome and serum uric acid in ischemic stroke. Cell Physiol Biochem. 2018;47(6):2369–2379. doi:10.1159/000491609
38. Amaro S, Llull L, Renú A, et al. Uric acid improves glucose-driven oxidative stress in human ischemic stroke. Ann Neurol. 2015;77(5):775–783. doi:10.1002/ana.24378
39. Llull L, Laredo C, Renú A, et al. Uric acid therapy improves clinical outcome in women with acute ischemic stroke. Stroke. 2015;46(8):2162–2167. doi:10.1161/strokeaha.115.009960
40. Chamorro Á, Amaro S, Castellanos M, et al. Uric acid therapy improves the outcomes of stroke patients treated with intravenous tissue plasminogen activator and mechanical thrombectomy. Int J Stroke. 2017;12(4):377–382. doi:10.1177/1747493016684354
41. Wang YF, Li JX, Sun XS, Lai R, Sheng WL. High serum uric acid levels are a protective factor against unfavourable neurological functional outcome in patients with ischaemic stroke. J Int Med Res. 2018;46(5):1826–1838. doi:10.1177/0300060517752996
42. Tu WJ, Chao BH, Ma L, et al. Case-fatality, disability and recurrence rates after first-ever stroke: a study from bigdata observatory platform for stroke of China. Brain Res Bull. 2021;175:130–135. doi:10.1016/j.brainresbull.2021.07.020
43. Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999;9(1):119–131. doi:10.1111/j.1750-3639.1999.tb00214.x
44. Cherubini A, Polidori MC, Bregnocchi M, et al. Antioxidant profile and early outcome in stroke patients. Stroke. 2000;31(10):2295–2300. doi:10.1161/01.str.31.10.2295
45. Leinonen JS, Ahonen JP, Lönnrot K, et al. Low plasma antioxidant activity is associated with high lesion volume and neurological impairment in stroke. Stroke. 2000;31(1):33–39. doi:10.1161/01.str.31.1.33
46. Brouns R, Wauters A, Van De Vijver G, De Surgeloose D, Sheorajpanday R, De Deyn PP. Decrease in uric acid in acute ischemic stroke correlates with stroke severity, evolution and outcome. Clin Chem Lab Med. 2010;48(3):383–390. doi:10.1515/cclm.2010.065
47. Klein Kremer A, Kuzminsky E, Bentur L, Nagler RM. Salivary and serum analysis in children diagnosed with pneumonia. Pediatr Pulmonol. 2014;49(6):569–573. doi:10.1002/ppul.22794
48. Matsusaka K, Kawakami G, Kamekawa H, et al. Pneumonia risks in bedridden patients receiving oral care and their screening tool: malnutrition and urinary tract infection-induced inflammation. Geriatr Gerontol Int. 2018;18(5):714–722. doi:10.1111/ggi.13236
49. Pan C, Chen Y, Wang S, Li M, Qu S. The study of routine laboratory factors in children with mycoplasma pneumoniae pneumonia: serum uric acid may have anti-inflammatory effect. J Clin Lab Anal. 2021;35(11):e24026. doi:10.1002/jcla.24026
50. Liu X, Cao Z, Gu H, et al. Uric acid and clinical outcomes among intracerebral hemorrhage patients: results from the china stroke center alliance. Front Neurol. 2020;11:609938. doi:10.3389/fneur.2020.609938
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
