Back to Journals » OncoTargets and Therapy » Volume 12
Upregulation of valosin-containing protein (VCP) is associated with poor prognosis and promotes tumor progression of orbital B-cell lymphoma
Received 31 July 2018
Accepted for publication 29 November 2018
Published 27 December 2018 Volume 2019:12 Pages 243—253
DOI https://doi.org/10.2147/OTT.S182118
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Wenwen Zhu,1 Di Li,2,3 Lihua Xiao4
1Department of Ophthalmology, Affiliated Hospital of Weifang Medical College, Weifang, Shandong, China; 2Institute of Rehabilitation Medicine of China, China Rehabilitation Science Institute, China Rehabilitation Research Center, Beijing, China; 3Beijing Key Laboratory of Neural Injury and Rehabilitation, Beijing, China; 4Institute of Orbital Disease, General Hospital of Chinese People’s Armed Police Forces, Beijing, China
Objective: This study aimed to investigate the relationship between VCP expression and the prognosis of orbital B-cell lymphoma patients and the influence of downregulation of VCP on the apoptosis and invasion abilities of lymphoma cells.
Methods: We recruited 66 orbital B-cell lymphoma patients. VCP expression in 66 samples of orbital B-cell lymphoma was determined by immunohistochemistry using monoclonal VCP antibodies. Based on VCP-expression levels detected by immunohistochemistry, we chose ten cases of orbital tumor paraffin tissue from the patients. Total RNA was extracted and differences in VCP gene-expression levels compared among patients using quantitative reverse-transcription (qRT) PCR. We used siRNA to knock down VCP in the lymphoma cell lines Raji and SUDHL4. qRT-PCR and Western blot were applied to detect VCP mRNA and protein expression, respectively. SUDHL48 assays were applied to investigate cell proliferation. Hoechst 33258 staining and flow-cytometry analysis were applied to investigate cell apoptosis. Transwell assays were applied to investigate invasive ability. Survival analysis was used to evaluate prognostic values.
Results: Expression levels of VCP were correlated with the stage, tumor grade, and recurrence rate of patients. VCP mRNA-expression levels were consistent with VCP-expression levels in orbital B-cell lymphoma tissue. Moreover, survival analysis revealed that lower VCP-expression levels were correlated with longer overall survival of orbital B-cell lymphoma patients. Downregulation of VCP with siRNA did not inhibit cell proliferation. However, it dramatically increased apoptosis and suppressed the invasion of B-cell lymphoma cells.
Conclusion: VCP expression played an important role in the progression of orbital B-cell lymphoma. VCP could be a useful marker for predicting the prognosis of orbital B-cell lymphoma patients. VCP may be a potential therapeutic target for orbital B-cell lymphoma.
Keywords: VCP, orbital B-cell lymphoma, prognosis, invasion, apoptosis
Introduction
Non-Hodgkin lymphoma (NHL) of the orbit and ocular adnexa is the most common primary orbital malignancy.1,2 In recent years, the incidence of orbital and ocular adnexal NHL has been increasing.3 As we know, NHL constitutes a group of very heterogeneous neoplasms, deriving from a clonal proliferation of B or T lymphocytes. About 95%–100% of reported cases of orbital and ocular adnexa NHL are B-cell neoplasms,4 which are divided into two groups. Low-grade lymphoma includes extranodal marginal-zone B-cell lymphoma (BCL) of mucosa-associated lymphoid-tissue (MALT) lymphoma, follicular lymphoma (FL; grades 1 and 2), small lymphocytic lymphoma, and lymphoplasmacytoid lymphoma. High-grade lymphoma includes diffuse large BCL (DLBCL), mantle-cell lymphoma, FL grade 3, lymphoblastic lymphoma, and even Burkitt’s lymphoma. Moreover, it is remarkable that as a part of systemic disease, orbital BCL has the possibility of recurrence in the orbit and even distant organs after initial therapy. Besides that, as BCL is a malignant tumor, speculation on patient’ prognosis is very important for treatment and follow-up.
As we know, tumor recurrence is common, and sometimes existing treatment measures cannot effectively prevent local recurrence or central nervous system involvement.5 It is important to find effective predictors of tumor recurrence. It has been reported that age >60 years, elevated LDH level, advanced stage at presentation, high-grade histological subgroup, and presence of B symptoms have a negative impact on overall survival for the overall population.6 However, unlike visceral organ tumors, the position of orbital tumors is relatively shallow. We have found that the vast majority of orbital BCL patients were in stage I when patients visit for the first time and that International Prognostic Index values for most patients were 0–2. Histological subtyping of lymphoma is difficult for most pathology doctors. Studies have shown several clinicopathological factors to be prognosticators for orbital lymphoma. It has been reported that CD43,7 BCL10,8 and TKTL19 are involved in the progression of ocular adnexal lymphoma, however so far there is no generally accepted effective predictor for orbital BCL.
VCP is a member of the AAA superfamily of proteins, which plays an important role in various cellular activities, including the ubiquitin–proteasome system, endoplasmic reticulum-associated degradation of proteins, cell cycle, and DNA repair.10 Several studies have found that VCP was associated with the prognosis of gastric cancer,11 pancreatic cancer,12 liver cancer,13,14 esophageal carcinoma,15 non-small-cell lung cancer,16 prostate cancer,17 and breast carcinoma.18 Our previous studies found that expression levels of VCP were associated with prognosis of primary orbital MALT lymphoma. VCP could be a useful marker for predicting the prognosis of primary orbital MALT lymphoma.19
Here, we firstly detected VCP expression in 66 samples from orbital BCL. Combining these with the clinical data, we analyzed the correlation between VCP expression and the prognosis of orbital BCL patients. Then, we explored the effect of suppressing VCP expression on lymphoma-cell proliferation, apoptosis, and invasion.
Methods
Patients
A total of 71 patients underwent operations for orbital BCL at the Institute of Orbital Disease, General Hospital of Chinese People’s Armed Police Forces (Beijing, China) from December 2008 to December 2012, with follow-up until January 2018. We received a documented review and approval from the ethics committee for all studies involving relevant human material. In addition, written informed consent was provided by the patients or the patient’s parent or legal guardian if under the age of 18 years. Five of these patients were excluded from the present study for loss of follow-up. The remaining 66 patients were included. All patients had been diagnosed with orbital BCL by senior pathologists: 47 (71%) with low-grade lymphoma and 19 (29%) with high-grade lymphoma. There were 33 cases (50%) of MALT lymphoma, three (5%) of FL, 16 (24%) of DLBCL, eleven (17%) of lymphoplasmacytoid lymphoma, two (3%) of Burkitt’s lymphoma, and one (1%) of lymphoblastic lymphoma. There were 45 males and 21 females, with ages 3–85 years (median 59 years). The stage of the disease was classified according to the Ann Arbor staging system. There were 51 patients at stage I, seven at stage II, four at stage III, and four at stage IV. Before surgery, patients received comprehensive examinations: physical examination, laboratory examinations, chest X-ray, abdominal ultrasonography and/or thoracoabdominal computed tomography scan, and bone-marrow biopsy. All patients were classified according to the Ann Arbor system. During each operation, the surgeon removed the tumor as much as possible. After the incision had healed, stage I patients received the radiation therapy postoperatively. Stage II, III, and IV patients were treated by radiotherapy combined with chemotherapy. Regular follow-up was carried out for all postoperative patients, with face-to-face visits for at least 5 years. Through examination, recurrences in orbital or distant organs could be detected in timely fashion.
Immunohistochemistry and evaluation of staining intensity
Samples from the surgery resections were fixed in 10% formalin and conventionally paraffin-embedded. Each sample was cut at 4 μm for two continuous sections, on which immunohistochemical staining was performed for monoclonal VCP antibodies (Abcam, Cambridge, UK). Vascular endothelial staining was as the internal positive control. Unimmunized mouse IgG serum (Vector Laboratories, Burlingame, CA, USA) was used as the primary antibody for the negative control (NC). Stained sections were evaluated by a senior pathologist without prior knowledge of the design of this experiment. The percentage of positive cells and intensity of VCP immunochemical staining were evaluated in ten randomly selected areas (200×). The percentage of positive cells was categorized into four grades: 0 for <5% positive cells, 1 for 6%–25% positive cells, 2 for 26%–50% positive cells, 3 for 51%–75% positive cells, and 4 for >75% positive cells. Staining intensity of cytoplasm and nuclei was categorized into three grades: 1 for weakly positive, 2 for moderately positive, and 3 for strongly positive. The final score was the multiplication of the percentage and intensity scores. Final scores <6 were defined as low VCP expression. Final scores ≥6 were defined as high VCP expression.
Cell-line cultures
The human Burkitt’s lymphoma cell line Raji was purchased from the China Infrastructure of Cell Line Resources (Beijing, China). The human DLBCL cell line SUDHL4 was purchased from Jennio Biotech (Guangzhou, China). Cell lines were cultured in RPMI 1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS, 100 U/mL penicillin, and 100 U/mL streptomycin. Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2.
siRNA transfection
Transfection of siRNA was done using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s protocol. VCP-homo-siRNA (sense 5′-GGGCACAUGUGAUUGUUAUTT-3′ and antisense 5′-AUAACAAUCACAUGUGCCCTT-3′) and NC siRNA (sense 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense 5′-ACGUGACACGUUCGGAGAATT-3′) were designed and synthesized by GenePharma (Shanghai, China). The cells were seeded into six- or 24-well plates and transfected with VCP siRNA (to a final concentration of 100 nM) using Lipofectamine 2000 for 48 hours. VCP silencing was confirmed by quantitative reverse-transcription (qRT) PCR for mRNA expression.
Quantitative reverse-transcription PCR
Total RNA of the cells or tissue was extracted using RNeasy Plus animal RNA-isolation kit with spin column (Beyotime, Haimen, China) according to the manufacturer’s protocol. Then, cDNA was synthesized with a PrimeScript RT reagent kit with gDNA eraser (Perfect Real Time; Takara, Kusatsu, Japan) at 42°C for 2 minutes to remove gDNA, followed by 37°C for 15 minutes and 85°C for 5 seconds. qRT-PCR was carried out on an Mx3000p system (Agilent, Santa Clara, CA, USA) at 95°C for 10 minutes, followed by 40 cycles at 95°C for 30 seconds, 58°C for 30 seconds, and 72°C for 20 seconds, according to SYBR Premix Ex Taq (TLI RNase H Plus; Takara) instructions. Data were analyzed with the 2−ΔΔCt method. Specific primers were synthesized by Taihe Biotechnology (Beijing, China): VCP, forward 5′-CCCTGTGCCTGCTTCTTT-3′ and reverse 3′-GCTGCTCCCTTTCCACCA-5′; GAPDH, forward 5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse 3′-AGGGGCCATCCACAGTCTTC-5′.
Western blot
Cells were collected and lysed with RIPA lysis buffer (Beyotime) supplemented with a protease-inhibitor cocktail (Hoffman-La Roche, Basel, Switzerland) and then centrifuged at 12,000 g, 4°C for 10 minutes to obtain the supernatant. Cell lysates containing 15 μg protein were heated for 5 minutes at 100°C, separated by 12% SDS-PAGE, and transferred onto polyvinylidene difluoride membranes (Immobilon-P; Millipore, Billerica, MA, USA). After incubation in 5% BSA–0.1% Tween 20 in TBS (TBST) at room temperature for 2 hours to block aspecific binding, the membranes were incubated with primary antibodies (1:1,000) in 0.1% TBST at 4°C overnight. VCP and β-actin antibodies were purchased from CST Signaling Technology. Next, these membranes were washed three times with TBST and incubated with the secondary antibody (1:5,000) for 1 hour at room temperature. The membranes were washed three times with TBST and visualized by enhanced chemiluminescence using a SuperSignal West Pico trial kit (Thermo Fisher Scientific).
SUDHL4–8 for proliferation assays
CCK8 was used to determine cell-proliferation ability according to the manufacturer’s instructions. Cell suspensions were seeded into each well of 96-well culture plates. SUDHL4–8 reagent (10 μL) was added to each well for 1 hour’s incubation at 37°C, 5% CO2. Finally, the OD450 values of the 96-well plates were measured with an EnSpire Multimode plate reader (PerkinElmer, Waltham, MA, USA).
Hoechst 33258 staining and flow cytometry for cell-apoptosis assays
Cells were stained with Hoechst 33258 for apoptotic cells. Cells were washed by PBS and stained with Hoechst 33258 (5 μg/mL) for 30 minutes at room temperature. Apoptotic cells were counted under fluorescence microscopy. To analyze cell apoptosis, cells were collected and incubated with FITC–annexin V and PI for 15 minutes. Data acquisition and analysis were performed in flow cytometer (Becton Dickinson, San Jose, CA, USA). All experiments were repeated three times independently.
Transwell assays for invasion ability
Transwell assays were performed in 24-well transwell inserts with a Corning Costar 8 μm-pore polycarbonate membrane (Corning, NY, USA). Cells (100 μL) were plated in RPMI 1640 medium without FBS in the upper Matrigel-coated chamber and incubated for 10 minutes at 37°C and 5% CO2 to allow the cells to settle down. Then, RPMI 1640 medium containing 20% FBS was added to the lower chamber for culture. After incubation for 24 hours at 37°C in 5% CO2, invasive cells in the lower chamber were stained with Hoechst 33258 and counted under fluorescence microscopy. All experiments were repeated three times independently.
Statistical analysis
Statistical analysis was performed with SPSS 17.0. Statistical differences among values were analyzed with independent t-tests. The relationship between VCP expression and clinicopathological features was analyzed with χ2 tests. Univariate survival analysis was analyzed with the Kaplan–Meier method. Multivariate analysis was analyzed with the Cox regression model. P<0.05 was considered statistically significant.
Results
Patient outcomes
After follow-up for at least 5 years, we found that 24 of 66 (36.4%) patients had suffered tumor recurrence and 20 of 66 (30.3%) had died due to tumors. Median disease-free survival was 63.5 (2–105) months. Median overall survival time was 68 (2–109) months. Disease-free and overall survival rates were 63.6% and 69.7%, respectively.
VCP-expression level correlated with clinical and pathological characteristics of orbital BCL
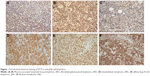
A total of 66 cases of orbital BCL specimens were investigated. Immunohistochemistry assays found that VCP staining was located mainly in cytoplasm and nuclei (Figure 1). We investigated the correlation between VCP expression and clinical and pathological features (Table 1). Interestingly, we found VCP expression was higher in tumors of Ann Arbor stage II–IV than stage I (P=0.005). Notably, VCP expression of orbital high-grade lymphoma was higher than low-grade lymphoma (P=0.039). The recurrence rate of patients with low VCP expression was significantly lower than those with high VCP expression (P=0.000). In orbital tumor tissue of ten orbital BCL patients, VCP mRNA expression was consistent with VCP expression. According to the immunohistochemistry results, VCP mRNA expression in tumor tissue of the high-VCP-expression group was significantly higher than the low-VCP-expression group (P<0.05, Figure 2).
  | Table 1 VCP expression and its relationship with clinical pathological characteristics of 66 orbital B-cell lymphoma patients |
  | Figure 2 VCP mRNA expression in the tumor tissue. |
Survival analysis
Surprisingly, univariate analysis found that disease-free and overall survival rates of patients were significantly correlated with VCP expression and Ann Arbor stage. Histological subtype was significant for overall survival, but not for disease-free survival. Compared with patients with high VCP expression, those with low VCP expression had better overall survival and disease-free survival (disease-free survival, 85.3% vs 40.6%, P=0.000; overall survival, 88.2% vs 50%, P=0.001; Table 2 and Figure 3). Multivariate analysis showed that VCP expression and Ann Arbor stage were independent prognostic factors for disease-free and overall survival (Table 3).
  | Table 2 Univariate analysis of clinicopathological factors for disease-free and overall survival of 66 orbital B-cell lymphoma patients |
  | Table 3 Multivariate analysis of clinicopathological factors for disease-free and overall survival of 66 orbital B-cell lymphoma patients |
Downregulation of VCP expression induced apoptosis and suppressed invasion of BCL cells
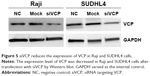
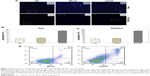
To investigate the effect of VCP on the proliferation, apoptosis and invasion of BCL cells, siRNA targeting VCP (siVCP) was transfected in Raji cells and SUDHL4 cells. qRT-PCR results showed that the mRNA level of VCP in siVCP cells was knocked down significantly compared with the NC group (Figure 4). VCP expression was also significantly decreased after cells had been treated with siVCP (Figure 5). We checked if VCP could influence the proliferation, apoptosis, and invasion of BCL cells at 72 hours. Cell proliferation was measured by SUDHL4–8 assay. Cell apoptosis was evaluated by Hoechst 33258 staining and flow cytometry. Cell-invasion ability was measured by transwell assay. There was no significant difference in proliferative capacity after cells had been treated with siVCP. Silencing of the VCP gene by siVCP did not inhibit cell proliferation (Figure 6); however, interestingly, downregulation of VCP by siVCP produced a higher percentage of apoptotic cells compared with the NC group (Figure 7). Hoechst 33258 staining showed the apoptosis rate of the siVCP group was nearly three times that of the NC group (P<0.05). Flow cytometry showed that cell apoptosis was enhanced obviously after the VCP gene had been silenced by siVCP in Raji cells (P<0.05). Downregulation of VCP promoted the apoptosis of cells. It was also found that VCP knockdown by siVCP decreased the invasion ability of BCL cells compared with the NC group (P<0.05). Therefore, downregulation of VCP was able to suppress the invasion of BCL cells (Figure 8).
Discussion
In this study, we found that VCP-expression levels in orbital BCL were different. VCP expression was associated with Ann Arbor stage, histological subtype, and the recurrence rate in patients. Furthermore, survival analysis revealed that higher expression VCP was associated with unfavorable overall and disease-free survival of orbital BCL patients. As such, VCP could be a potential marker for prognosis of orbital BCL patients. Then, we found that VCP had no significant effect on lymphoma-cell proliferation, but downregulation of VCP expression could promote lymphoma-cell apoptosis and suppress invasion ability. Therefore, VCP may play an important role in promoting lymphoma-cell progression and invasion. VCP may be a potential target for new drugs in the future.
It has been reported that CB5083 is a potent, selective, and orally bioavailable inhibitor of VCP.20 Gugliotta et al found that CB5083 induced a significant reduction in viability in human B-ALL cell lines. Early and strong induction of apoptosis was also demonstrated in human B-ALL cell lines treated with CB5083.21 We will explore the effect of CB5083 on human BCL cells and its therapeutic effect on BCL in an animal model.
The Burkitt lymphoma cell line Raji and DLBCL cell line SUDHL4 cell were high-grade lymphoma cells, the VCP expression of which was high. Here, we used Raji and SUDHL4 cells for VCP gene silencing. Whether VCP gene silencing can affect apoptosis and invasion abilities in primary orbital lymphoma cells needs to be determined.
Univariate analysis found that histological subtype was associated with overall survival, but not disease-free survival. Multivariate analysis found that histological subtype was not associated with overall or disease-free survival. As such, in this study the histological subtype of orbital BCL did not influence survival as much as we thought. Perhaps it is important to employ standardized and effective treatment for improving the survival of high-grade orbital BCL patients.
We investigate only correlations between VCP expression and prognosis of orbital BCL. Whether VCP is associated with other types of lymphoma in orbit and BCL in other organs still needs to be researched.
Conclusion
Our results identified that VCP could be a potential marker for prognosis of orbital BCL patients. VCP may be a potential target for new drugs in the future.
Acknowledgment
This study was supported by Shandong Provincial Natural Science Foundation, China (ZR2016HB10) and Shandong Province Medical and Health Science Technology Development Project (2017WS731).
Disclosure
The authors report no conflicts of interest in this work.
References
Cani AK, Soliman M, Hovelson DH, et al. Comprehensive genomic profiling of orbital and ocular adnexal lymphomas identifies frequent alterations in MYD88 and chromatin modifiers: new routes to targeted therapies. Modern Pathology. 2016;29(7):685–697. | ||
Pinnix CC, Dabaja BS, Milgrom SA, et al. Ultra-low-dose radiotherapy for definitive management of ocular adnexal B-cell lymphoma. Head Neck. 2018;40(6):1335. | ||
Özkan MC, Palamar M, Tombuloğlu M, et al. Ocular adnexal lymphomas: single-center experience. Clin Lymphoma Myeloma Leuk. 2015;15 Suppl:S158–S160. | ||
Mckelvie PA. Ocular adnexal lymphomas: a review. Adv Anat Pathol. 2010;17(4):251–261. | ||
Tang LJ, Gu CL, Zhang P. Intraocular lymphoma. Int J Ophthalmol. 2017;10(8):1301–1307. | ||
Mulay K, Honavar SG. An update on ocular adnexal lymphoma. Semin Diagn Pathol. 2016;33(3):164–172. | ||
Nola M, Lukenda A, Bollmann M, Kalauz M, Petrovecki M, Bollmann R. Outcome and prognostic factors in ocular adnexal lymphoma. Croat Med J. 2004;45(3):328–332. | ||
Franco R, Camacho FI, Caleo A, et al. Nuclear BCL10 expression characterizes a group of ocular adnexa MALT lymphomas with shorter failure-free survival. Mod Pathol. 2006;19(8):1055–1067. | ||
Lange CA, Tisch-Rottensteiner J, Böhringer D, Martin G, Schwartzkopff J, Auw-Haedrich C. Enhanced TKTL1 expression in malignant tumors of the ocular adnexa predicts clinical outcome. Ophthalmology. 2012;119(9):1924–1929. | ||
Meyer H, Bug M, Bremer S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat Cell Biol. 2012;14(2):117–123. | ||
Yamamoto S, Tomita Y, Hoshida Y, et al. Expression level of valosin-containing protein is strongly associated with progression and prognosis of gastric carcinoma. J Clin Oncol. 2003;21(13):2537–2544. | ||
Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–1806. | ||
Yamamoto S, Tomita Y, Nakamori S, et al. Elevated expression of valosin-containing protein (p97) in hepatocellular carcinoma is correlated with increased incidence of tumor recurrence. J Clin Oncol. 2003;21(3):447–452. | ||
Yi P, Higa A, Taouji S, et al. Sorafenib-mediated targeting of the AAA+ ATPase p97/VCP leads to disruption of the secretory pathway, endoplasmic reticulum stress, and hepatocellular cancer cell death. Mol Cancer Ther. 2012;11(12):2610–2620. | ||
Yamamoto S, Tomita Y, Hoshida Y, et al. Expression level of valosin-containing protein (p97) is associated with prognosis of esophageal carcinoma. Clin Cancer Res. 2004;10(16):5558–5565. | ||
Peng J, Yang LX, Zhao XY, et al. VCP gene variation predicts outcome of advanced non-small-cell lung cancer platinum-based chemotherapy. Tumour Biol. 2013;34(2):953–961. | ||
Tsujimoto Y, Tomita Y, Hoshida Y, et al. Elevated expression of valosin-containing protein (p97) is associated with poor prognosis of prostate cancer. Clin Cancer Res. 2004;10(9):3007–3012. | ||
Cui Y, Niu M, Zhang X, Zhong Z, Wang J, Pang D. High expression of valosin-containing protein predicts poor prognosis in patients with breast carcinoma. Tumour Biol. 2015;36(12):9919–9927. | ||
Zhu WW, Kang L, Gao YP, et al. Expression level of valosin containing protein is associated with prognosis of primary orbital MALT lymphoma. Asian Pac J Cancer Prev. 2013;14(11):6439–6443. | ||
Anderson DJ, Le Moigne R, Djakovic S, et al. Targeting the AAA ATPase p97 as an approach to treat cancer through disruption of protein homeostasis. Cancer Cell. 2015;28(5):653–665. | ||
Gugliotta G, Sudo M, Cao Q, et al. Valosin-containing protein/p97 as a novel therapeutic target in acute lymphoblastic leukemia. Neoplasia. 2017;19(10):750–761. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.