Back to Journals » OncoTargets and Therapy » Volume 13
Upregulation of TRIAP1 by the lncRNA MFI2-AS1/miR-125a-5p Axis Promotes Thyroid Cancer Tumorigenesis
Authors Yu T, Tong L, Ao Y, Zhang G, Liu Y, Zhang H
Received 29 October 2019
Accepted for publication 16 May 2020
Published 17 July 2020 Volume 2020:13 Pages 6967—6974
DOI https://doi.org/10.2147/OTT.S236476
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianmin Xu
Tianyu Yu,1,* Lingling Tong,2,* Yu Ao,3 Genmao Zhang,4 Yunpeng Liu,5 Hejia Zhang4
1Department of Thyroid Surgery, Jilin University China-Japan Union Hospital, Changchun 130033, People’s Republic of China; 2Department of Gynaecology and Obstetrics, Jilin University China-Japan Union Hospital, Changchun 130033, People’s Republic of China; 3Department of Pediatrics, Jilin University First Hospital, Changchun 130031, People’s Republic of China; 4Department of Ultrasound, Jilin University China-Japan Union Hospital, Changchun 130033, People’s Republic of China; 5Department of Thoracic Surgery, Jilin University First Hospital, Changchun 130031, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hejia Zhang
Department of Ultrasound, Jilin University China-Japan Union Hospital, Changchun 130033, People’s Republic of China
Email [email protected]
Background: Thyroid cancer is a very common endocrine cancer worldwide. How long noncoding RNA (lncRNA) regulates thyroid cancer is elusive. LncRNA MFI2-AS1 has been demonstrated to initiate colorectal cancer. Nevertheless, the role of MFI2-AS1 in thyroid cancer remains unknown. This study aims to determine the roles of MFI2-AS1 in thyroid cancer.
Methods: qRT-PCR was used to determine the expression of MFI2-AS1 in thyroid cancer tissues and cells. Proliferation was determined by using CCK8 and colony formation assays. Transwell assay was utilized to analyze migration and invasion. Luciferase reporter assay was performed to confirm the interaction between MFI2-AS1 and miR-125a-5p.
Results: MFI2-AS1 was shown to be highly expressed in thyroid cancer tissues and predicted poor prognosis. Knockdown of MFI2-AS1 inhibited proliferation, colony formation, migration and invasion of thyroid cancer cells in vitro. Bioinformatics screening identified MFI2-AS1 as the sponge for miR-125a-5p. And miR-125a-5p was further confirmed to target TRIAP1 directly. Our data further demonstrated that MFI2-AS1 promoted TRIAP1 expression via repressing miR-125a-5p. Finally, TRIAP1 was found to be upregulated in thyroid cancer tissues and its restoration reversed the effects of MFI2-AS1 depletion.
Conclusion: Our results elucidated a novel mechanism that MFI2-AS1 promotes thyroid cancer progression via the miR-125a-5p/TRIAP1 pathway.
Keywords: MFI2-AS1, miR-125a-5p, TRIAP1, proliferation, thyroid cancer
Introduction
Thyroid cancer is a prevalent endocrine tumor worldwide with an increasing mobility.1 About 1% of cancer-induced deaths are generated by thyroid cancer.2 Compared to other cancers, thyroid cancer displays a good prognosis.3 However, thyroid cancer patients with aggressive metastasis show a quite unsatisfactory outcome.4 Thus, investigation to elucidate the molecular mechanism of thyroid cancer progression is crucial and urgently required. And developing novel therapeutic targets will be important for thyroid cancer treatment.5
Recent studies have defined noncoding RNAs with over 200 nucleotides in length as long noncoding RNAs (lncRNAs).6 More and more evidences show many lncRNAs are dysregulated in tumor tissues.7 Their aberrant expression is usually associated with dysregulation of proliferation, metastasis, survival and drug resistance in cancer cells.8 For example, lncRNA RP11 promotes colorectal cancer (CRC) metastasis via increasing Zeb1 expression.9 LncRNA EPB41L4A-AS2 represses liver cancer growth through regulating FOXL1 expression.10 LncRNA GAS5 controls the radiosensitivity of prostate tumor cell via inhibiting miR-18a activity.11 Additionally, Linc01194 overexpression promotes CRC proliferation and migration and predicts poor prognosis.12 Thus, exploring the important lncRNAs will be important to understand the mechanism of tumorigenesis.
Recently, a study suggested that lncRNA MFI2-AS1 (951 nucleotides in length without protein-coding ability) promotes CRC progression via upregulating MYCBP level.13 However, the role of MFI2-AS1 in thyroid cancer development has not been reported. In our study, we found that MFI2-AS1 was upregulated in thyroid cancer tissues and its upregulation indicated poor prognosis in patients. Then, we showed MFI2-AS1 knockdown suppressed proliferation, migration and invasion but inducing apoptosis in thyroid cancer cells. Furthermore, we found that MFI2-AS1 promoted TRIAP1 expression via restricting miR-125a-5p activity. Notably, overexpression of TRIAP1 abrogated the roles of MFI2-AS1 knockdown. Collectively, our findings illustrated the oncogenic functions of MFI2-AS1 and demonstrated its molecular mechanism in thyroid cancer.
Materials and Methods
Patients’ Samples
Forty-three human papillary thyroid cancer tissues and adjacent normal controls were obtained from Jilin University China–Japan Union Hospital. Patients who received radiotherapy or chemotherapy prior to surgery were excluded and other patient’s tissues were included. All patients provided written informed consents. This study was approved by the Ethics Committee of Jilin University China–Japan Union Hospital. This study was conducted in accordance with the Declaration of Helsinki.
Cell Cultures
Human thyroid cancer cell lines (SW579, K1 and TPC-1) and normal thyroid follicular epithelial cell line Nthy-ori 3–1 were from Cell Center of Shanghai Institutes for Biological Sciences (Shanghai, China) and maintained following previous study.7 In brief, all cells were cultured using DMEM medium completed with 10% FBS maintained under the following conditions: 95% humidity, 37°C and 5% CO2.
qRT-PCR Analysis
RNA was isolated from thyroid cancer cells using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. qRT-PCR was performed as previously described.14 In brief, RNA was reverse transcribed into cDNA using Tetro Reverse Transcriptase (Bioline). Then, qPCR experiments were conducted using SYBR Premix Ex Taq (Qiagen, Hilden, Germany). GAPDH was the normalized control. And relative expression was calculated according to the 2−ΔΔCT method.
Cell Transfection
shRNAs were designed and cloned into PLKO.1-Puro carrier (Sigma-Aldrich Chemical Company, St Louis, MO). Then, above plasmid was transfected into 293T cells with psPAX2 and pMD2.G (Addgen) for lentivirus production. After infection, the thyroid cancer cells were screened for stable cell lines.
CCK8 Assay
CCK8 assay was performed to test cell proliferation as described before.7 In brief, cells were seeded into the 96-well plates and cultured for 3 days. Then, CCK8 solution (10 μL) was added into each well and cultured for 2 h. After that 10 μL DMSO was added into each well and OD values were measured at 450 nm.
Caspase-3/7 Activity
Apoptosis was determined by measuring Caspase-3/7 activity using the Apo-ONE Homogeneous Caspase-3/7 Assay kit (Promega, Madison, WI, USA).
Transwell Assay
Transwell assay was used for migration and invasion analysis as described previously.7 Briefly, cells in serum-free medium were seeded into the upper transwell chambers (8 μm pores) which was precoated with Matrigel matrix (BD Bioscience, Franklin Lakes, NJ, USA) for the invasion analysis. The lower chamber was filled with medium containing 10% FBS as a chemoattractant. After cultured for 24 h, the migrated and invasive cells on the lower chamber were fixed and stained with 0.1% crystal violet (Sigma, St Louis, MO, USA). Cell numbers were counted using a light microscope.
RNA Immunoprecipitation (RIP) Assay
Imprint RNA immunoprecipitation kit (Sigma-Aldrich) was used for RIP assay. TPC-1 and K1 cells were lysed and cell lysates were incubated with anti-Ago2 or IgG control for 4 h at °C, followed by incubation with Protein A/G beads for another 2 h. Then the precipitated RNAs were isolated, and expression of MFI2-AS1 and miR-125a-5p was analyzed by qRT-PCR method.
Luciferase Reporter Assay
MFI2-AS1 and miR-125a-5p interaction were analyzed using miRcode software (http://www.mircode.org/). miR-125a-5p and TRIAP1 interaction was predicted using TaregetScan7. The sequences containing wild-type (WT) or mutant (MUT) miR-497 binding were constructed into psiCHECK2 vector (Promega Corporation, Madison, WI, USA). For luciferase reporter assay, reporters and miR-125a-5p mimics were transfected into thyroid cancer cells using Lipofectamine 3000; 48 h later, Firefly and Renilla luciferase activities were measured using the Dual Luciferase Reporter Assay system (Promega Corporation) according to the manufacturer’s instruction.
Statistical Analysis
All results were analyzed using SPSS 22.0 (IBM Corporation, Armonk, NY, USA) and expressed as mean ± SD. A Student’s t-test was performed for comparison between two groups. A one-way ANOVA followed by Tukey’s post-hoc test was performed for comparisons between multiple groups. Spearman’s rank correlation analysis was used to determine the correlation between MFI2-AS1 and miR-125a-5p expression. A p < 0.05 indicates that the difference is meaningful.
Results
MFI2-AS1 is Upregulated in Thyroid Cancer
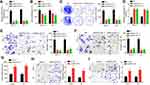
The expression of MFI2-AS1 in thyroid cancer was firstly analyzed using bioinformatics method. According to the TCGA data using TANRIC tool (http://ibl.mdanderson.org/tanric/_design/basic/query.html), TANRIC expression was significantly upregulated in tumor tissues (Figure 1A). Similarly, qRT-PCR showed that MFI2-AS1 level was increased in 43 thyroid cancer tissues (Figure 1B). Consistently, MFI2-AS1 expression was higher in thyroid cancer cell lines than that in Nthy-ori 3–1 cells (Figure 1C). We then analyzed the subcellular location of MFI2-AS1 and found that it was mainly located in the cytoplasm of TPC-1 and K1 cells (Figure 1D).
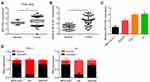 |
Figure 1 MFI2-AS1 is upregulated in thyroid cancer. (A) MFI2-AS1 expression was analyzed in TCGA data using the TANRIC online tool (https://ibl.mdanderson.org/tanric/_design/basic/query.html). TCGA: The Cancer Genome Atlas. (B) MFI2-AS1 expression was analyzed in 43 thyroid cancer tissues and their adjacent normal tissues. (C) MFI2-AS1 expression in thyroid cancer cell lines was measured. (D) The subcellular distribution of MFI2-AS1 in thyroid cancer cells were analyzed by qRT-PCR. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was the cytoplasmic control. U6 (RNA, U6 small nuclear 1) was the nuclear control. *p<0.05. |
MFI2-AS1 Promotes Thyroid Cancer Progression
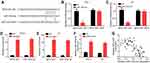
Then, MFI2-AS1 function in thyroid cancer was investigated. MFI2-AS1 expression was decreased by transfection with two independent shRNAs in TPC-1 and K1 cells (Figure 2A). CCK8 assay and colony formation assay indicated MFI2-AS1 knockdown inhibited tumor cell proliferation (Figure 2B and C). And the activity of Caspase-3/7 was increased after MFI2-AS1 knockdown (Figure 2D), indicating MFI2-AS1 regulates apoptosis. Furthermore, Transwell assay showed that MFI2-AS1 knockdown reduced the numbers of cell migration and invasion (Figure 2E and F). To further validate the role of MFI2-AS1, we overexpressed MFI2-AS1. Through CCK8 and transwell assays, we found that MFI2-AS1 overexpression promoted the proliferation, migration and invasion of TPC-1 and K1 cells (Figure 2G–I). Thus, MFI2-AS1 regulates the malignant behaviors of thyroid cancer cells.
MFI2-AS1 is the Sponge for miR-125a-5p
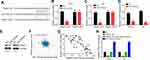
LncRNAs are potential sponges for miRNAs.10 Thus, the possible target miRNAs of MFI2-AS1 were analyzed and we identified miR-125a-5p. We constructed luciferase reporter vectors (Figure 3A). Luciferase reporter assay showed that miR-125a-5p inhibited the activity of WT reporter in TPC-1 and K1 cells (Figure 3B and C). And RIP assay showed that miR-125a-5p interacted with MFI2-AS1 in TPC-1 and K1 cells (Figure 3D and E). Moreover, MFI2-AS1 knockdown increased miR-125a-5p expression (Figure 3F). And miR-125a-5p was negatively correlated with MFI2-AS1 in thyroid cancer tissues (Figure 3G). Hence, MFI2-AS1 interacted with miR-125a-5p to restrict its activity.
miR-125a-5p Targets TRIAP1 Directly
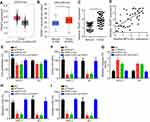
The potential targets of miR-125a-5p were further analyzed and we identified TRIAP1. To demonstrate their interaction, we constructed luciferase reporter vectors (Figure 4A). Luciferase reporter assay showed that TRIAP1-WT activity was decreased by miR-125a-5p mimics (Figure 4B and C). And miR-125a-5p also reduced the expression of TRIAP1 in thyroid cancer cells (Figure 4D and E). TCGA data using StarBase v3.0 tool indicated miR-125a-5p was negatively correlated with TRIAP1 in thyroid cancer tissues (Figure 4F), which was further validated by qRT-PCR in 43 tumor tissues (Figure 4G). Moreover, we found that TRIAP1 expression was suppressed after MFI2-AS1 knockdown (Figure 4H). And inhibiting miR-125a-5p could rescue the expression of TRIAP1 (Figure 4H). Therefore, TRIAP1 was targeted by miR-125a-5p and upregulated by MFI2-AS1.
MFI2-AS1 Promotes Thyroid Cancer Progression via Upregulating TRIAP1
Through bioinformatics examination, TRIAP1 was upregulated in thyroid cancer tissues (Figure 5A and B). And qRT-PCR also indicated TRIAP1 was upregulated in tumor tissues (Figure 5C), implying TRIAP1 may be an oncogene. We found TRIAP1 was positively correlated with MFI2-AS1 in thyroid cancer tissues (Figure 5D). Thus, we sought to determine whether MFI2-AS1 promotes thyroid cancer progression via TRIAP1. CCK8 and colony formation assay showed that knockdown of MFI2-AS1 or TRIAP1 inhibited cell proliferation (Figure 5E and F). And restoration of TRIAP1 promoted proliferation of MFI2-AS1-depleted cells (Figure 5E and F). Similarly, the apoptosis, migration and invasion of tumor cells were affected by MFI2-AS1 and TRIAP1 in a similar manner (Figure 5G–I). Thus, MFI2-AS1 promotes thyroid cancer development via TRIAP1.
Discussion
In this study, we showed that lncRNA MFI2-AS1 was upregulated in thyroid cancer tissues. And its expression was correlated with patients’ prognosis. Moreover, loss-of-function assays indicated that MFI2-AS1 knockdown led to decreased proliferation, migration and invasion while causing more apoptosis in thyroid cancer cells in vitro. Mechanistically, we demonstrated that MFI2-AS1 sponged miR-125a-5p to facilitate TRIAP1 expression and thyroid cancer development. Therefore, our study convincingly supports MFI2-AS1 as a novel oncogenic lncRNA in thyroid cancer.
In the past decade, lncRNAs have been found to play significant roles in various tumors and be potential therapeutic targets, including in thyroid cancer.15,16 LncRNAs possess the ability to regulate cancer cell proliferation, apoptosis and angiogenesis.15 Several lncRNAs are shown to regulate tumorigenesis of thyroid cancer. For instance, LINC00313 promotes migration, invasion and EMT of thyroid cancer via affecting ALX4 expression.17 LncRNA H19 is overexpressed in thyroid cancer and enhances EMT of thyroid cancer.18 LncRNA SNHG16 is a driver of thyroid cancer growth and metastasis.7 Additionally, LINC00514 is found to be upregulated in thyroid cancer tissues and regulates proliferation, migration and invasion via the miR-204-3p/CDC23 axis.19 Although lncRNA MFI2-AS1 was reported to promote CRC progression, its role in thyroid cancer is unclear.13 Our data showed MFI2-AS1 expression was elevated in thyroid cancer and its knockdown led to inhibition of tumor cell proliferation, migration and invasion. Besides, MFI2-AS1 knockdown promoted apoptosis of thyroid cancer cells.
Increasing researches denote that lncRNA is potential sponges for miRNAs.20 LncRNAs could bind to the response elements of target miRNAs and restrict their activity, leading to upregulation of mRNAs.21 For example, LncRNA SNHG3 sponges miR-151-3p to upregulate RAB22A and promote osteosarcoma progression.22 LncRNA SNHG16 contributes to osteosarcoma via inhibiting miR-340.23 MFI2-AS1 was previously shown to sponge miR-574-5p in CRC.13 In our study, we demonstrated that MFI2-AS1 is the sponge for miR-125a-5p in thyroid cancer. We demonstrate their direct interaction in thyroid cancer cells. Our results also imply miR-125a-5p suppressed the progression of thyroid cancer, which is consistent with a previous study.24 However, how miR-125a-5p regulates thyroid cancer development is poorly studied. Our work further demonstrated that miR-125a-5p directly targets TRIAP1, whose expression was upregulated by MFI2-AS1 in thyroid cancer. TRIAP1 was found to regulate nasopharyngeal carcinoma, ovarian cancer and prostate cancer.25–27 Its effect on thyroid cancer remains unclear. Our data suggested that TRIAP1 was upregulated in thyroid cancer tissues. Importantly, inhibition of TRIAP1 led to suppression on proliferation, migration and invasion of thyroid cancer cells. Thus, we demonstrated that TRIAP1 is a new oncogene in thyroid cancer. Moreover, rescue assays showed that TRIAP1 restoration reversed the roles of MFI2-AS1 knockdown on thyroid cancer cells. Therefore, TRIAP1 is the downstream oncogenic signaling of MFI2-AS1 in thyroid cancer.
In summary, our study demonstrated MFI2-AS1/miR-125a-5p/TRIAP1 axis is a new signaling pathway in regulating thyroid cancer progression and may be a possible therapeutic target for tumor treatment. However, there is a limitation in our study. In vivo animal experiment needs to be performed to validate the role of MFI2-AS1/miR-125a-5p/TRIAP1 axis in the future.
Data Sharing Statement
All generated data are included in this manuscript.
Disclosure
The authors report no funding and no conflicts of interest for this work.
References
1. Wang X, Zhang Q, Cai Z, Dai Y, Mou L. Identification of novel diagnostic biomarkers for thyroid carcinoma. Oncotarget. 2017;8(67):111551–111566. doi:10.18632/oncotarget.22873
2. Sharifi A, Shojaeifard A, Soroush A, Jafari M, Abdehgah AG, Mahmoudzade H. Predictors of regional lymph node recurrence after initial thyroidectomy in patients with thyroid cancer. J Thyroid Res. 2016;2016:4127278. doi:10.1155/2016/4127278
3. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13(3):184–199. doi:10.1038/nrc3431
4. Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. 2006;106(3):524–531. doi:10.1002/cncr.21653
5. Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013;381(9871):1058–1069. doi:10.1016/S0140-6736(13)60109-9
6. Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157(1):77–94. doi:10.1016/j.cell.2014.03.008
7. Wen Q, Zhao L, Wang T, et al. LncRNA SNHG16 drives proliferation and invasion of papillary thyroid cancer through modulation of miR-497. Onco Targets Ther. 2019;12:699–708. doi:10.2147/OTT.S186923
8. Zhu SP, Wang JY, Wang XG, Zhao JP. Long intergenic non-protein coding RNA 00858 functions as a competing endogenous RNA for miR-422a to facilitate the cell growth in non-small cell lung cancer. Aging (Albany NY). 2017;9(2):475–486. doi:10.18632/aging.101171
9. Wu Y, Yang X, Chen Z, et al. m(6)A-induced lncRNA RP11 triggers the dissemination of colorectal cancer cells via upregulation of Zeb1. Mol Cancer. 2019;18(1):87. doi:10.1186/s12943-019-1014-2
10. Wang YG, Wang T, Shi M, Zhai B. Long noncoding RNA EPB41L4A-AS2 inhibits hepatocellular carcinoma development by sponging miR-301a-5p and targeting FOXL1. J Exp Clin Cancer Res. 2019;38(1):153.
11. Yang J, Hao T, Sun J, Wei P, Zhang H. Long noncoding RNA GAS5 modulates alpha-Solanine-induced radiosensitivity by negatively regulating miR-18a in human prostate cancer cells. Biomed Pharmacother. 2019;112:108656. doi:10.1016/j.biopha.2019.108656
12. Wang XX, Liu ZM, Tong H, et al. Linc01194 acts as an oncogene in colorectal carcinoma and is associated with poor survival outcome. Cancer Manag Res. 2019;11:2349–2362. doi:10.2147/CMAR.S189189
13. Li C, Tan F, Pei Q, et al. Non-coding RNA MFI2-AS1 promotes colorectal cancer cell proliferation, migration and invasion through miR-574-5p/MYCBP axis. Cell Prolif. 2019;52:e12632.
14. Wang Y, Cheng Q, Liu J, Dong M. Leukemia stem cell-released microvesicles promote the survival and migration of myeloid leukemia cells and these effects can be inhibited by MicroRNA34a overexpression. Stem Cells Int. 2016;2016:9313425. doi:10.1155/2016/9313425
15. de Oliveira JC, Oliveira LC, Mathias C, et al. Long non-coding RNAs in cancer: another layer of complexity. J Gene Med. 2019;21(1):e3065.
16. Renganathan A, Felley-Bosco E. Long noncoding RNAs in cancer and therapeutic potential. Adv. Exp. Med. Biol. 2017;1008:199–222.
17. Zhao X, Hu X. Downregulated long noncoding RNA LINC00313 inhibits the epithelial-mesenchymal transition, invasion, and migration of thyroid cancer cells through inhibiting the methylation of ALX4. J Cell Physiol. 2019;234(11):20992–21004. doi:10.1002/jcp.28703
18. Liang WQ, Zeng CCF, Sun SM, Lu XF, Peng CY, Lin HY. Long noncoding RNA H19 is a critical oncogenic driver and contributes to epithelial-mesenchymal transition in papillary thyroid carcinoma. Cancer Manag Res. 2019;11:2059–2072. doi:10.2147/CMAR.S195906
19. Li X, Zhong W, Xu Y, Yu B, Liu H. Silencing of lncRNA LINC00514 inhibits the malignant behaviors of papillary thyroid cancer through miR-204-3p/CDC23 axis. Biochem Biophys Res Commun. 2019;508(4):1145–1148. doi:10.1016/j.bbrc.2018.12.051
20. Zhao Y, Wang H, Wu C, et al. Construction and investigation of lncRNA-associated ceRNA regulatory network in papillary thyroid cancer. Oncol Rep. 2018;39(3):1197–1206. doi:10.3892/or.2018.6207
21. Su X, Xing J, Wang Z, Chen L, Cui M, Jiang B. microRNAs and ceRNAs: RNA networks in pathogenesis of cancer. Chin J Cancer Res. 2013;25(2):235–239. doi:10.3978/j.issn.1000-9604.2013.03.08
22. Zheng S, Jiang F, Ge D, et al. LncRNA SNHG3/miRNA-151a-3p/RAB22A axis regulates invasion and migration of osteosarcoma. Biomed Pharmacother. 2019;112:108695.
23. Su P, Mu S, Wang Z. Long noncoding RNA SNHG16 promotes osteosarcoma cells migration and invasion via sponging miRNA-340. DNA Cell Biol. 2019;38(2):170–175. doi:10.1089/dna.2018.4424
24. Huang P, Mao LF, Zhang ZP, et al. Down-regulated miR-125a-5p promotes the reprogramming of glucose metabolism and cell malignancy by increasing levels of CD147 in thyroid cancer. Thyroid. 2018;28(5):613–623. doi:10.1089/thy.2017.0401
25. Li YQ, Tang XR, He QM, et al. Overexpression of mitochondria mediator gene TRIAP1 by miR-320b loss is associated with progression in nasopharyngeal carcinoma. PLoS Genet. 2016;12(7). doi:10.1371/journal.pgen.1006183
26. Liu P, Qi XR, Bian C, et al. MicroRNA-18a inhibits ovarian cancer growth via directly targeting TRIAP1 and IPMK. Oncol Lett. 2017;13(6):4039–4046. doi:10.3892/ol.2017.5961
27. Ketteler J, Panic A, Reis H, et al. Progression-related loss of stromal caveolin 1 levels mediates radiation resistance in prostate carcinoma via the apoptosis inhibitor TRIAP1. J Clin Med. 2019;8(3):348. doi:10.3390/jcm8030348
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.