Back to Journals » Journal of Pain Research » Volume 13
Upregulation of μ-Opioid Receptor in the Rat Spinal Cord Contributes to the α2-Adrenoceptor Agonist Dexmedetomidine-Induced Attenuation of Chronic Morphine Tolerance in Cancer Pain
Authors Zhang P , Bu J, Wu X, Deng L, Chi M, Ma C, Shi X, Wang G
Received 27 July 2020
Accepted for publication 4 September 2020
Published 15 October 2020 Volume 2020:13 Pages 2617—2627
DOI https://doi.org/10.2147/JPR.S274225
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Robert B. Raffa
Pinyi Zhang,1 Jianlong Bu,2 Xiaohong Wu,1 Lin Deng,1 Meng Chi,1 Chao Ma,3 Xiaoding Shi,1 Guonian Wang3
1Department of Anesthesiology, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China; 2Department of Thoracic Surgery, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China; 3Department of Anesthesiology, The Fourth Hospital of Harbin Medical University, Harbin, People’s Republic of China
Correspondence: Guonian Wang
Department of Anesthesiology, The Fourth Hospital of Harbin Medical University, Nangang District, Harbin 150081, People’s Republic of China
Tel +86 15804616023
Email [email protected]
Background: Sustained morphine treatment for cancer pain has been limited due to analgesic tolerance. Opioid receptor internalization and desensitization mediated by downregulation of mu-opioid receptor (MOR) expression have been confirmed as one of the mechanisms of chronic morphine tolerance. In addition to the opiate system, the α 2-adrenergic system is involved in the development of morphine tolerance. Several studies reported that co-administration of α 2-adrenoceptor agonist dexmedetomidine inhibits morphine tolerance in normal or neuropathic pain animals. However, the effect of dexmedetomidine on morphine tolerance has not been studied in cancer pain. Therefore, we investigated the effect of intrathecal injection of dexmedetomidine on the development of morphine tolerance in cancer pain and on the expression of MOR in the spinal cord of morphine-tolerant cancer pain rats.
Methods: The model was established using a rat’s right hind paw injection of Walker 256 cancer cells. Subcutaneous morphine (10mg/kg) was administrated twice daily for 7 days; meanwhile, the rats received intrathecal α 2-adrenoceptor agonist dexmedetomidine (10μ/kg) or antagonist MK-467 (0.25mg/kg) in test groups. Rats receiving drug vehicle served as the control group. Antinociception was detected by von Frey filaments and hot-plate tests. The expression of MOR in the spinal cord was examined through real-time reverse transcription polymerase chain reaction and Western blotting. The data were analyzed via analysis of variance followed by Student t-test with Bonferroni correction.
Results: Seven-day chronic morphine administration elicited notable analgesic tolerance in the rats with cancer pain. Co-administration of α 2-adrenoceptor agonist dexmedetomidine enhanced morphine analgesia and attenuated morphine tolerance, which could be blocked by α 2-adrenoceptor antagonist MK-467. Furthermore, pre-treatment of dexmedetomidine significantly upregulated MOR protein expression without a notable change in MOR mRNA expression in the spinal cord.
Conclusion: Our findings suggest that intrathecal injection of dexmedetomidine enhanced morphine analgesia and attenuated morphine tolerance in cancer pain, potentially by upregulating MOR expression in the spinal cord. The α 2-adrenoceptor agonist may provide a more versatile analgesia option for morphine treatment for cancer pain.
Keywords: μ-opioid receptor, MOR, α 2-adrenoceptor, α 2-AR, dexmedetomidine, DEX, morphine tolerance, cancer pain
Introduction
Cancer pain remains a major medical issue that cannot be effectively controlled in the clinic.1 Morphine, a prototypical opiate analgesic, is one of the most commonly used drugs to treat moderate-to-severe pain. However, the development of chronic analgesic tolerance, requiring an increased dose for the same level of analgesia, has led to an underutilization of morphine in long-term treatment.2 On the basis of the definition of opioid tolerance, the prevalence of opioid tolerance in cancer patients ranges between 40% and 80%.3 High-dose opioids in cancer patients are associated with decreased survival.4 To improve cancer pain management, a new therapeutic approach is needed to better control morphine tolerance.
The mechanism underlying morphine tolerance is complex. As a primary mediator of morphine analgesia, μ-opioid receptor (MOR) contributes to the formation and development of chronic morphine tolerance through downregulating the expression of MOR and uncoupling from G-proteins5.6 Accumulating evidence provides support for the involvement of the noradrenergic system in morphine actions7.8 The α2 adrenoceptors (α2-ARs), co-localized with MOR in the spinal cord,9 has been reported to have a conformational cross-talk to MOR controlling cell signaling in morphine dependence and withdrawal10.11 Moreover, synergistic interactions have also been observed between α2-AR and opioid analgesics.12 Dexmedetomidine (DEX) is a highly selective α2-AR agonist, which has been promoted for its antinociceptive potency with opioid-sparing effects in pain treatment, including acute inflammatory pain,13 post-operative pain14 and chronic neuropathic pain unresponsive to opioid analgesics.15 Some investigations also have indicated that combination use of DEX and morphine inhibited the analgesic tolerance to sustained morphine exposure in normal rats16,17 or long-term treatment for neuropathic pain.18 However, the effect of α2-AR agonist DEX on chronic morphine tolerance has not been studied in cancer-induced pain.
As we know, the underlying mechanisms of cancer pain appear very complex, having inflammatory, neuropathic and tumorigenic components. In this study, we investigated the effect of intrathecal DEX administration on the development of morphine tolerance in cancer pain treatment. Based on a rat model of cancer pain, α2-AR agonist DEX and antagonist MK-467 were intrathecally injected before subcutaneous administration of morphine to evaluated the effect of DEX on morphine analgesia. Furthermore, considering the co-localization and interaction between α2-AR and MOR, we also examined the DEX-induced effect on the level of MOR expression in the lumbar spinal cord of morphine-tolerant rats.
Materials and Methods
Animals
Adult male Wistar rats weighing 200 ± 20 g were provided by the Laboratory Animal Center of Harbin Medical University, Harbin, China. Rats were housed in a room maintained at 22 ± 1 °C, with a 12–12 h light-dark cycle, water and food ad libitum. This raising and treatment of the rats followed the policies and recommendations of the International Association for the Study of Pain (IASP), and the National Institutes of Health (NIH) guidelines for laboratory animals. The study was approved by the Animal Care and Use Committee of Harbin Medical University, Harbin, China.
Intrathecal Catheter Implantation
The rats were initially anesthetized with 5% sevoflurane in 30% O2 (remainder N2), then keeping autonomous respiration and inhalation anesthetized with 2–3% sevoflurane. The catheterization was performed as described by previous studies with modifications.19 Briefly, the animal was placed in a prone position, and a midline skin incision was made to expose the intervertebral membrane between L3 and L4. The tip of a 12 cm long catheter (Polyethylene PE 10; Becton Dickinson, Franklin Lake, NJ) was inserted into the subarachnoid space about 2.0 cm. The catheter was fixed to the muscle, and the other end was tunneled rostrally underneath the skin to exit 1.5 cm in the occipital region. The incisions were then closed in two layers using 4–0 silk. After 5 days of recovery in a separate cage, the lidocaine test was performed on the rats to evaluate whether i.t. catheterization was successful. Rats showing hind limbs paralysis within 30 s after i.t. administration of 2% lidocaine (10 μL) suggested successful i.t. catheterization. Rats that displayed any abnormal neurological signs were excluded from experiments.
Cell Preparation and Cancer Pain Model Establishment
The Walker 256 carcinoma cells were prepared from breast cancer cells of Wistar rats at the Cancer research institute of Heilongjiang (Harbin, China). Briefly, 2×107 cells (in 0.5 mL) were injected into the abdominal cavity of homologous Wistar rats. Three-milliliter ascites were collected sterilely after 7 days. Cells in ascites were washed twice and resuspended in phosphate buffered saline (PBS, pH 7.4) to a final concentration of 106 per 100 μL. The tumor cells were then kept on ice before injection.
According to our previous studies,20 the cancer pain model was established by inoculating Walker 256 carcinoma cells to the plantar region of the right hind paw of each rat with i.t. catheterization. After 5 days following tumor cell inoculation, a marked proliferation of tumor cells was detected, and the hind paw thermal hyperalgesia was also detected by behavior tests, suggesting a successful setup of the cancer pain model.
Induction of Morphine Tolerance and Drug Delivery
A total of 36 rats were randomly allocated to 6 groups (n=6). All drugs were administered twice daily (9:00 am and 6:00 pm) for 7 days. To induce morphine analgesic tolerance, a 7-day cumulative dosing regimen was used. The rats were injected intrathecal (i.t.) with normal saline 10 μL and subcutaneous (s.c.) with morphine sulfate (Northeast Pharmaceutical Group Co., Ltd., Harbin, China) 10mg/kg/mL (vehicle+morphine group; n=6). The analgesic effects of morphine were measured by the tail-flick test. Drugs were delivered as follows: in separate groups, rats received i.t. injection of dexmedetomidine (10 μg/kg, 20 μL; Heng Rui Pharmaceutical Co., Ltd. Jiangsu, China) and s.c. injection of morphine (DEX+morphine group; n=6), or i.t. injection of α2-AR antagonist MK-467 (0.25 mg/kg, 10 μL; USA) 30min prior to Dex and s.c. injection of morphine (MK-467+DEX+morphine group; n=6). DEX was injected 30 min prior to morphine. The rats received i.t. 10μL and s.c. 1mL/kg injection of normal saline served as the control group (vehicle+saline group; n=6). Furthermore, to test the effects of DEX on cancer pain, the rats received i.t. injections of DEX (DEX+saline group; n=6) or MK-467 30min prior to DEX (MK-467+DEX+saline group; n=6).
Behavioral Tests
All rats were acclimated to the testing environment for at least 3 days prior to baseline measurements. Behavioral assays were performed before drug administration on day 0 and 30 min after drug delivery in the morning from day 1 to day 7. The withdrawal threshold to mechanical stimuli was assessed by von Frey filaments (Stoelting Company, Wood Dale, IL, USA) using a staircase method. Three measurements were performed on the right paw of each rat, and the mean value of mechanical withdrawal thresholds (MWT) from both hind paws was used. Thermal withdrawal latencies (TWL) were determined for each rat using a hot-plate apparatus in a plastic cylinder (Technology & Market CORP, Chengdu, China). Rats were individually placed on the hot plate (52°C), and latency was defined as the time that elapsed before the rat licked a hind paw or jumped. The maximal cut-off time was 30 s to avoid damage to the paws. Three measurements at 10 min interval were averaged to generate the final values. All behavioral tests were performed by an experimenter blinded to the experiment.
Real-Time Quantitative PCR
The animals were sacrificed on day 7 of morphine treatment after the behavioral tests. RT-PCR was performed using lumbar L3-L5 segments of spinal cord ipsilateral to the tumor cell injections. Total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA), and a 2 μg aliquot was used for complementary deoxyribonucleic acid (cDNA) synthesis using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland). The cDNA was used as a template for RT-PCR amplification using Fast Start Universal SYBR Green Master Mix (Roche). Primers for MOR and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) were designed by PrimerExpress software (Applied Biosystems, Inc., Carlsbad, CA) and were as follows: MOR (forward: 5ʹ- GCTATCGGGCTCCAAAGAAA-3ʹ, reverse: 5ʹ- GGGTCCAGCAGACGATAAATAC-3ʹ); GAPDH (forward: 5ʹ- ATGCCATCACTGCCACTCA-3ʹ, reverse: 5ʹ- CCTGCTTCACCACCTTCTTG-3ʹ). Quantitative PCR amplification was performed according to the manufacturer’s instructions (30 seconds at 95°C, 40 cycles of 5 seconds at 95°C and 30 seconds at 55°C) using an ABI 7500 fast real-time PCR system (Applied Biosystems, Inc., Carlsbad, CA).
Western Blotting
Western blotting was performed as our previous procedures.20 In brief, samples (L3–L5 segments of the spinal cord) were collected and washed with ice-cold phosphate buffered saline (PBS) before lysed in RIPA (radioimmunoprecipitation assay) lysis buffer (Solarbio, Beijing, China). Then, the whole sample lysates were collected for homogenization and centrifugation, and the protein concentration of the supernatant was measured by the Bradford method. The proteins were electrophoretically transferred onto polyvinylidene difluoride (PVDF) membranes, which were blocked with 3% non-fat dry milk for 1 h and incubated overnight at 4°C with primary antibodies for MOR (Abcam, 1:2000) and β-actin (ABclonal, 1:10,000, performed as a loading control). Then the filters were developed using ECL reagents (Meilunbio, Dalian, China) with secondary antibodies [goat anti-mouse or goat anti-rabbit horseradish peroxidase conjugated (HRP), 1:3000; Millipore, USA]. Densitometric scanning was used for quantitative analysis.
Statistical Analysis
Data were analyzed by the Statistical Product for Social Sciences (SPSS version 23.0). Data were presented as mean ± SD. For behavioral data, the difference between groups was determined by two-way repeated measures ANOVA followed by Student t-test with Bonferroni correction. MOR expression on mRNA and protein level were analyzed via one-way ANOVA followed by Student t-test with Bonferroni correction for multiple comparisons. We performed 7 comparisons at each period: vehicle + saline versus the other 5 groups, DEX+morphine versus vehicle+morphine, and MK-467+DEX+morphine versus vehicle+morphine. A P value less than the Bonferroni-corrected threshold of 0.0071 (0.05/7) was defined as statistically significant.
Results
Induction of Morphine Tolerance in Rats with Cancer Pain
Mechanical and thermal hyperalgesia were observed within 5 days after injection of Walker 256 tumor cells. Cancer induced pain behaviors in rats were indicated by significantly increased mechanical and thermal sensitivity. As presented in Figures 1 and 2, no significant difference was found in mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) in right hind paw among each group prior to drug administration (day 0). In the first three days, morphine administration produced a significant analgesic effect compared with the control group based on mechanical and thermal hyperalgesia (P<0.007). In the following days from day 4, in rats with morphine treatment, the level of morphine analgesia gradually decreased compared with day 1 (P<0.007). Furthermore, from day 4 to day 7, the MWT and TWL in the morphine group had no significant alternations compared with the control group, indicating that 7-day chronic morphine administration elicited notable analgesic tolerance in the rats with cancer pain.
The Effect of Dexmedetomidine on Mechanical Hyperalgesia in Development of Morphine Tolerance in Rats with Cancer Pain
As presented in Figure 1, compared with morphine group, MWT in DEX+morphine group significantly increased from day 2 to day 7, which suggested that pre-administration of DEX can strengthen the analgesic effect of morphine (P<0.007). Although MWT values of DEX-pretreated rats (DEX+morphine group) reduced substantially from day 3 to day 7 compared with day 1 (days 3–7: all P<0.007), the analgesic effect remained significantly higher than the control group (days 1–7: all P<0.007) on each day. Thus, pre-administration of DEX could prevent the analgesic tolerance formation of morphine (P<0.007). No significant difference was shown in MWT between the MK-467+DEX+morphine group and vehicle+morphine group. Thereby, the α2-AR antagonist MK-467 could reverse the effects of DEX on morphine analgesia and tolerance. The mechanical hyperalgesia of repeated administration of DEX alone (DEX+saline group) did not differ from the control group in the von Frey filaments test.
The Effect of Dexmedetomidine on Thermal Nociception in Development of Morphine Tolerance in Rats with Cancer Pain
As presented in Figure 2, compared with morphine group, TWL in DEX+morphine group significantly increased from day 4 to day 7, which suggested that pre-administration of DEX can strengthen the analgesic effect of morphine (P<0.007). Although TWL values of DEX-pretreated rats (DEX+morphine group) reduced on day 7 compared with day 1 (P<0.007), the analgesic effect remained significantly higher than the control group (days 1–7: all P<0.007) on each day. Thus, the pre-administration of DEX could prevent the analgesic tolerance formation of morphine (P<0.007). No significant difference was shown in TWL between the MK-467+DEX+morphine group and the vehicle+morphine group. Thereby, the α2-AR antagonist MK-467 could reverse the effects of DEX on morphine analgesia and tolerance. The thermal hyperalgesia of repeated administration of DEX alone (DEX+saline group) did not differ from the control group in the hot plate test.
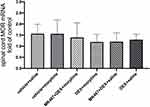
The Effect of Dexmedetomidine on MOR mRNA Expression in Morphine-Tolerant Rats with Cancer Pain
RT-PCR was used to determine the mRNA expression of MOR in the L3-L5 spinal cord. The vehicle + saline group served as the control group. As shown in Figure 3, there was no significant difference in MOR mRNA expression among the six groups.
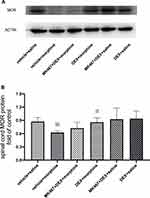
The Effect of Dexmedetomidine on MOR Protein Expression in Morphine-Tolerant Rats with Cancer Pain
Furthermore, Western blotting was performed to determine the protein level of MOR in the L3-L5 spinal cord. As presented in Figure 4, compared with the control group, chronic morphine administration (vehicle+morphine group) significantly decreased the protein expression of MOR in cancer pain rats (※P=0.003<0.007). Pre-administration of DEX (DEX+morpnine group) significantly increased MOR protein expression in the spinal cord compared with morphine administration alone (vehicle+morphine group) (#P=0.004<0.007). No significant difference was found between MK-467+DEX+morphine group and vehicle+morphine group, indicating that the α2-AR antagonist MK-467, 30 min prior to DEX administration, could partially block the effect of DEX on morphine-induced downregulation of MOR protein expression.
Discussion
In this study, we found that intrathecal injection of α2-AR agonist DEX significantly decreased the mechanical and thermal hyperalgesia in morphine-treated rats with cancer pain, which could be reversed by the α2-AR antagonist MK-467. This indicated that DEX enhanced the analgesic effect of morphine and attenuated the development of analgesic tolerance to morphine in cancer pain via an α2-AR pathway. Furthermore, we found that co-injection of DEX with morphine increased MOR protein expression in the spinal cord, while no evident influence in mRNA expression has been found. Our findings suggested that the α2-AR agonist DEX may play a positive role of attenuating morphine tolerance in cancer pain treatment potentially via upregulating MOR expression at a post-translational level.
Studies have shown that MORs are crucial for both positive and negative effects of opioids. After knocking out the MOR gene, morphine not only loses its analgesic effect but also no longer causes side effects such as analgesic tolerance, hyperalgesia, and drug addiction.21 Thus, the MOR is one of the core components when investigating morphine tolerance. One proposed mechanism of antinociceptive tolerance after sustained opioid exposure is through agonist induced receptor desensitization and downregulation of functional receptors present in target neurons.22 A decrease in functional, cell surface MOR as a result of prolonged morphine exposure is a characteristic common to many GPCR that have been studied to date and as such, receptor desensitization and downregulation as a cellular mechanism of drug tolerance is widely accepted.22 Studies of heroin users found that high opioid use correlates with the downregulation of the MOR on circulating leukocytes.23 Studies have shown that cancer pain generally required significantly higher doses of morphine as compared to inflammatory pain.24 Moreover, it has been demonstrated that chronic pain and opioid administration are two main mechanisms inducing MOR downregulation.25 Thus, from a molecular perspective, we speculate that chronic morphine tolerance is more likely to induce downregulation of MOR in cancer pain model as compared to that in non-cancer model, which appears little or no decrease in MOR expression and receptor binding.26 In this study, sustained morphine treatment significantly decreased the protein expression of MOR in the spinal cord of cancer pain rats in the vehicle+morphine group as compared with the control group. Our findings strongly support that morphine-induced downregulation of MOR expression in cancer pain promotes the development and maintenance of morphine tolerance.21
The role of α2-AR agonists in the development of morphine tolerance has been studied in several researches. Based on a normal rat or non-cancer pain rat model,17 co-administration of DEX has been shown to inhibit the development of chronic analgesic tolerance to morphine. Whereas the underlying molecular mechanism still remains unclear, and the effect of α2-AR agonist DEX on morphine tolerance in cancer pain has not been reported. In this study, we used a morphine tolerance model induced on cancer pain rats to find that intrathecal injection of DEX increased the analgesic effects of morphine while attenuating morphine tolerance during long-term treatment for cancer pain. Opioid and adrenergic receptors both belong to the family of Gi/Go-protein–coupled receptors (GPCR). A co-expression of the two receptors has been reported in the spinal cord superficial dorsal horn layers I and II, an area of projection for most ascending nociceptive A and C fibers.27 Moreover, their post-receptor signal transduction pathways may be similarly shared by the two receptors on the same cell.28 Considering the mechanisms of opioid tolerance involves changes at the receptor level as well as at downstream sites,29 we therefore hypothesize that the effect of α2-AR agonists on morphine tolerance may occur in two ways: (1) the interaction with MOR and (2) the involvement in post-receptor signaling pathways. Accumulating evidence has shown that chronic morphine treatment would not alter the transcriptional ability of the MOR gene but may regulate the synthesis of MORs at the post-transcriptional level,30.31 Many laboratories examined the effects of morphine treatment on MOR mRNA levels and failed to find a significant effect,32.33,34 Similarly, rather than MOR mRNA expression, we observed that intrathecal injection of DEX evidently increased the protein expression of MOR, which was downregulated by chronic morphine exposure, and further attenuated the mechanical and thermal hyperalgesia of rats due to morphine analgesic tolerance. These findings suggested that DEX attenuated morphine tolerance in cancer pain rats via modulating the expression MOR on a post-transcriptional level. The post-transcriptional processing of MOR proteins essentially relies on specific interactions between cis-acting elements mainly localized in the untranslated region (UTR) of the transcript and the trans-acting factors [RNA-binding proteins (RBPs) and non-coding regulatory RNAs] that bind to these sequences,35.36 In the cancer pain morphine tolerance model, the specific post-transcriptional process of DEX-induced upregulation on MOR expression remains to be further investigation.
As previously mentioned, opioids and α2-AR agonists may interact at multiple levels in association with analgesic, neuroinflammatory, and immunomodulatory effects. It has been confirmed that sustained application of morphine enhances the neuroimmune reactivity mediated by the innate immune receptor Toll-like receptor 4 (TLR4).37 TLR4, expressed in a wide variety of cells, can recognize the invariant molecular structures of pathogens and participate in innate immunity.38 Through binding to the glycoprotein myeloid differentiation factor-2 (MD-2) on TLR4,39,40 morphine initiates an inflammatory response via activating nuclear factor kappa B (NF-κB) and p38 mitogen activated protein kinase (MAPK) phosphorylation,37 further resulting in the release of proinflammatory cytokines including tumor necrosis factor (TNF), Interleukin-1 beta (IL-1β), and Interleukin 6 (IL-6),39,40. These series of spinal TLR4 activities oppose the antinociceptive effect of morphine, and further contributes to analgesic tolerance. α2-ARs have been detected in microglial cells of the spinal cord and DRG, as an agonist of α2-AR, DEX has been reported a potential neuroprotective effect through modulating central immune activities. In rat models of inflammatory pain and neuropathic pain, co-administration of DEX and morphine downregulates the expression of TLR4 mediated NF-κB activation, and then effectively inhibits the morphine-induced inflammatory responses,41.42 Collectively, we speculate that other than modulating MOR expression, the attenuation of DEX in morphine tolerance in cancer pain may also exert via spinal glial signaling pathways.
Numerous studies have shown that the central noradrenergic system is involved in many actions of morphine,8 wherein the analgesic synergism between α2-AR agonists and opioid agonists exists in the spinal cord.12 On one hand, α2-AR agonists potentiate morphine analgesia not only in acute pain models but also in rodent models of chronic pain,43.44 Similarly, in our research on a cancer pain model, the behavioral tests showed that intrathecal injection of DEX significantly increased the nociceptive efficacy of morphine in long-term pain treatment. This opioid–adrenoceptor synergy has been further confirmed by the clinical studies concerning intrathecal DEX and morphine reduced the morphine consumption in patients with refractory cancer pain without serious side effects.45 On the other hand, the analgesic effect of α2-AR agonists is enhanced in animals with morphine tolerance, since chronic systemic morphine administration upregulated the expression of α2A, α2B and α2C-AR in lumbar DRG and the dorsal horn.46 In this study, we observed that intrathecal delivery of DEX relieved the cancer-evoked pain in the state of morphine tolerance via upregulating MOR expression in spinal cord. Although we did not test the expression of α2-AR in DRG and spinal cord, it could be speculated that the upregulation of α2-AR expression might contribute to the attenuated development of morphine tolerance in the cancer pain rats receiving combination of DEX and morphine.
Further researches are planned to be carried out from the following aspects. Firstly, based on a cancer pain model, the effect of DEX in chronic morphine tolerance will be examined using different dosages. Secondly, the expression of α2-AR will be tested to verify our speculation concerning the upregulation of α2-AR by sustained morphine stimulation in cancer pain. Thirdly, the spinal glia pathways involved in DEX attenuating morphine tolerance will be evaluated in cancer pain. As such, the mechanism underlying the effect of DEX on morphine tolerance in cancer pain can be better clarified.
In conclusion, this study demonstrates that α2-AR agonist DEX enhances morphine analgesia and attenuates the development of morphine tolerance in cancer pain. This effect is accompanied by an upregulation of the morphine-induced MOR expression in the spinal cord. Our findings provide a further understanding of the interaction between α2-AR and opioid system, showing the therapeutic benefit of DEX combined with morphine for cancer pain treatment.
Acknowledgments
We thank professor Xuan Li (Harbin Medical University Cancer Hospital) for her help with the English language copy editing.
Author Contributions
Wang Guonian and Zhang Pinyi designed experiments, Zhang Pinyi prepared the manuscript. Wang Guonian generated the idea and provided valuable advice on the research. Bu Jianlong, Wu Xiaohong, Deng Lin and Shi Xiaoding did Western blotting and RT-PCR experiments. Bu Jianlong, Chi Meng and Ma Chao did behavioral tests. Bu Jianlong helped with data collection and statistical analyses. All authors read and edited the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 8177051154) and the Fundamental Research Funds for the Provincial Universities.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Mercadante S, Portenoy RK. Opioid poorly-responsive cancer pain. Part1: clinical considerations. J Pain Symptom Manage. 2001;21:144–150.
2. Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112(3):372–380. doi:10.1016/j.pain.2004.09.019
3. Mercadante S. Breakthrough pain in cancer patients: prevalence, mechanisms and treatment options. Curr Opin Anaesthesiol. 2015;28:559–564. doi:10.1097/ACO.0000000000000224
4. Portenoy RK, Sibirceva U, Smout R, et al. Opioid use and survival at the end of life: a survey of a hospice population. J Pain Symptom Manage. 2006;32:532–540. doi:10.1016/j.jpainsymman.2006.08.003
5. Wu Q, Zhang L, Law PY, Wei LN, Loh HH. Long-term morphine treatment decreases the association of mu-opioid receptor (MOR) mRNA with polysomes through miRNA23b. Mol Pharmacol. 2009;75:744–750. doi:10.1124/mol.108.053462
6. Starowicz K, Obara I, Przewłocki R, Przewlocka B. Inhibition of morphine tolerance by spinal melanocortin receptor blockade. Pain. 2005;117:401–411. doi:10.1016/j.pain.2005.07.003
7. Mansikka H, Zhou L, Donovan DM, Pertovaara A, Raja SN. The role of mu-opioid receptors in inflammatory hyperalgesia and alpha 2-adrenoceptor-mediated anti-hyperalgesia. Neuroscience. 2002;113(2):339–349. doi:10.1016/S0306-4522(02)00189-6
8. Sierralta F, Naquira D, Pinardi G, Miranda HF. Alpha-adrenoceptor and opioid receptor modulation of clonidine-induced antinociception. Br J Pharmacol. 1996;119:551–554. doi:10.1111/j.1476-5381.1996.tb15707.x
9. Jordan BA, Gomes I, Rios C, Filipovska J, Devi LA. Functional interactions between mu opioid and alpha 2A-adrenergic receptors. Mol Pharmacol. 2003;64:1317–1324. doi:10.1124/mol.64.6.1317
10. Vilardaga JP, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ. Conformational cross-talk between α2A-adrenergic and m-opioid receptors controls cell signaling. Nat Chem Biol. 2008;4:126–131. doi:10.1038/nchembio.64
11. van Bockstaele EJ, Menko AS, Drolet G. Neuroadaptive responses in brainstem noradrenergic nuclei following chronic morphine exposure. Mol Neurobiol. 2011;23:155–171. doi:10.1385/MN:23:2-3:155
12. Fairbanks CA, Stone LS, Wilcox GL. Pharmacological profiles of alpha 2 adrenergic receptor agonists identified using genetically altered mice and isobolographic analysis. Pharmacol Ther. 2009;123:224–238. doi:10.1016/j.pharmthera.2009.04.001
13. Malmberg AB, Hedley LR, Jasper JR, Hunter JC, Basbaum AI. Contribution of alpha(2) receptor subtypes to nerve injury-induced pain and its regulation by dexmedetomidine. Br J Pharmacol. 2001;132:1827–1836. doi:10.1038/sj.bjp.0704032
14. Shimode N, Fukuoka T, Tanimoto M, Tashiro C, Tokunaga A, Noguchi K. The effects of dexmedetomidine and halothane on Fos expression in the spinal dorsal horn using a rat postoperative pain model. Neurosci Lett. 2003;343:45–48. doi:10.1016/S0304-3940(03)00309-4
15. Xu M, Kontinen VK, Kalso E. Effects of radolmidine, a novel alpha2-adrenergic agonist compared with dexmedetomidine in different pain models in the rat. Anesthesiology. 2000;93:473–481. doi:10.1097/00000542-200008000-00027
16. Hayashi Y, Guo TZ, Maze M. Hypnotic and analgesic effects of the alpha 2-adrenergic agonist dexmedetomidine in morphine-tolerant rats. Anesth Analg. 1996;83:606–610. doi:10.1213/00000539-199609000-00030
17. Gursoy S, Ozdemir E, Bagcivan I, Altun A, Durmus N. Effects of alpha 2-adrenoceptor agonists dexmedetomidine and guanfacine on morphine analgesia and tolerance in rats. Ups J Med Sci. 2011;116(4):238–246. doi:10.3109/03009734.2011.597889
18. Naik BI, Nemergut EC, Kazemi A, et al. The effect of dexmedetomidine on postoperative opioid consumption and pain after major spine surgery. Anesth Analg. 2016;122(5):1646–1653. doi:10.1213/ANE.0000000000001226
19. Storkson RV, Kjorsvik A, Tjolsen A, Hole K. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods. 1996;65:167–172. doi:10.1016/0165-0270(95)00164-6
20. Zhang M, Wang K, Ma M, Tian S, Wei N, Wang G. Low-dose cannabinoid type 2 receptor agonist attenuates tolerance to repeated morphine administration via regulating μ-opioid receptor expression in walker 256 tumor-bearing rats. Anesth Analg. 2016;122:1031–1037. doi:10.1213/ANE.0000000000001129
21. Corder G, Tawfik VL, Wang D, et al. Loss of μ-opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat Med. 2017;23(2):164–173. doi:10.1038/nm.4262
22. von Zastrow M. Mechanisms regulating membrane traffi cking of G protein-coupled receptors in the endocytic pathway. Life Sci. 2003;74:217–224. doi:10.1016/j.lfs.2003.09.008
23. Chorbov VM, Todorov AA, Lynskey MT, Cicero TJ. Elevated levels of DNA methylation at the oprm1 promoter in blood and sperm from male opioid addicts. J Opioid Manag. 2011;7:258–264. doi:10.5055/jom.2011.0067
24. Luger NM, Sabino MA, Schwei MJ, et al. Efficacy of systemic morphine suggests a fundamental difference in the mechanisms that generate bone cancer vs inflammatory pain. Pain. 2002;99(3):397–406.
25. Mohan S, Davis RL, DeSilva U, Stevens CW. Dual regulation of mu opioid receptors in SK-N-SH neuroblastoma cells by morphine and interleukin-1beta: evidence for opioid-immune crosstalk. J Neuroimmunol. 2010;227:26–34. doi:10.1016/j.jneuroim.2010.06.007
26. Kristi Stafford A, Gomes AB, Shen J, Yoburn BC. Mu-opioid receptor downregulation contributes to opioid tolerance in vivo. Pharmacol Biochem Behav. 2001;69(1):233–237. doi:10.1016/S0091-3057(01)00525-1
27. Sullivan AF, Kalso EA, McQuay HJ, Dickenson AH. Evidence for the involvement of the mu but not delta opioid receptor subtype in the synergistic interaction between opioid and alpha 2 adrenergic antinociception in the rat spinal cord. Neurosci Lett. 1992;139:65–68. doi:10.1016/0304-3940(92)90859-6
28. Aghajanian GK, Wang YY. Common α2- and opiate effector mechanisms in the locus coeruleus: intracellular studies in brain slices. Neuropharmacology. 1987;26:793–799. doi:10.1016/0028-3908(87)90054-2
29. Taylor DA, Fleming WW. Unifying perspectives of the mechanisms underlying the development of tolerance and physical dependence to opioids. J Pharmacol Exp Ther. 2001;297:11–18.
30. He Y, Yang C, Kirkmire CM, Wang ZJ. Regulation of opioid tolerance by let-7 family MicroRNA targeting the opioid receptor. J Neurosci. 2010;30(30):10251–10258. doi:10.1523/JNEUROSCI.2419-10.2010
31. Brodsky M, Elliott K, Hynansky A, Inturrisi CE. CNS levels of mu opioid receptor (MOR-1) mRNA during chronic treatment with morphine or naltrexone. Brain Res Bull. 1995;38(2):135–141. doi:10.1016/0361-9230(95)00079-T
32. Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154(2):384–396. doi:10.1038/bjp.2008.100
33. Williams JT, Ingram SL, Henderson G, et al. Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013;65(1):223–254.
34. Meuser T, Giesecke T, Gabriel A, et al. Mu-opioid receptor mRNA regulation during morphine tolerance in the rat peripheral nervous system. Anesth Analg. 2003;97(5):1458–1463. doi:10.1213/01.ANE.0000081721.75663.87
35. Delestiennem N, Wauquier C, Soin R, Dierick JF, Gueydan C, Kruys V. The splicing factor ASF/SF2 is associated with TIA-1-related/TIA-1-containing ribonucleoproteic complexes and contributes to post-transcriptional repression of gene expression. FEBS J. 2010;277(11):2496–2514. doi:10.1111/j.1742-4658.2010.07664.x
36. Mittal N, Roy N, Babu MM, Janga SC. Dissecting the expression dynamics of RNA-binding proteins in posttranscriptional regulatory networks. Proc Natl Acad Sci USA. 2009;106(48):20300–20305. doi:10.1073/pnas.0906940106
37. Wang X, Loram LC, Ramos K, et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci USA. 2012;109:6325–6330. doi:10.1073/pnas.1200130109
38. Tanga FY, Nutile-McMenemy N, DeLeo J. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA. 2005;102:5856–5861. doi:10.1073/pnas.0501634102
39. Hutchinson MR, Zhang Y, Shridhar M, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi:10.1016/j.bbi.2009.08.004
40. Lewis SS, Hutchinson MR, Rezvani N, et al. Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta. Neuroscience. 2010;165:569–583. doi:10.1016/j.neuroscience.2009.10.011
41. Eidson LN, Murphy AZ. Blockade of Toll like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. J Neurosci. 2013;33(40):15952–15963.
42. Ji D, Zhou Y, Li S, et al. Anti-nociceptive effect of dexmedetomidine in a rat model of monoarthritis via suppression of the TLR4/NF-κB p65 pathway. Exp Ther Med. 2017;14(5):4910–4918.
43. Fairbanks CA, Nguyen HO, Grocholski BM, Wilcox GL. Moxonidine, a selective imidazoline-alpha2-adrenergic receptor agonist, produces spinal synergistic antihyperalgesia with morphine in nerve-injured mice. Anesthesiology. 2000;93:765–773. doi:10.1097/00000542-200009000-00026
44. Tajerian M, Millecamps M, Stone LS. Morphine and clonidine synergize to ameliorate low back pain in mice. Pain Res Treat. 2012;150842.
45. Liu HJ, Gao XZ, Liu XM, Xia M, Li WY, Jin Y. Effect of intrathecal dexmedetomidine on spinal morphine analgesia in patients with refractory cancer pain. J Palliat Med. 2014;17(7):837–840. doi:10.1089/jpm.2013.0544
46. Fairbanks CA, Wilcox GL. Spinal antinociceptive synergism between morphine and clonidine persists in mice made acutely or chronically tolerant to morphine. J Pharmacol Exp Ther. 1999;288:1107–1116.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.