Back to Journals » OncoTargets and Therapy » Volume 12
Upregulation of microRNA-1270 suppressed human glioblastoma cancer cell proliferation migration and tumorigenesis by acting through WT1
Authors Wei L, Li P, Zhao C, Wang N, Wei N
Received 28 October 2018
Accepted for publication 8 December 2018
Published 20 June 2019 Volume 2019:12 Pages 4839—4848
DOI https://doi.org/10.2147/OTT.S192521
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Lai Wei,1 Pan Li,2 Chunjing Zhao,1 Na Wang,1 Na Wei1
1Department of Pharmacy, The Second Affiliated Hospital of Chongqing Medical University, Chongqing 400010, China; 2Institute of Ultrasound Imaging, Chongqing Medical University, Chongqing 400010, China
Background: Glioblastoma multiforme (GBM) is one of the most aggressive brain tumors among human beings. In this study, we explored the functions of human microRNA-1270 (hsa-miR-1270) on GBM cancer cell proliferation, migration, and tumorigenesis.
Materials and methods: In GBM cell lines and clinical tissues, hsa-miR-1270 expression was probed by quantitative real-time PCR (qRT-PCR). In LN-18 and A172 cells, hsa-miR-1270 was upregulated by lentiviral transduction. The effects of hsa-miR-1270 upregulation on GBM in vitro and in vivo functions were probed by proliferation, migration, and xenograft assays, respectively. The correlation between hsa-miR-1270 and Wilms’ tumor gene (WT1) was probed by dual-luciferase activity assay, qRT-PCR, and Western blot. WT1 was then secondarily overexpressed in hsa-miR-1270-upregulated LN-18 and A172 cells, to explore its mechanisms in GBM’s association with hsa-miR-1270.
Results: Hsa-miR-1270 was significantly downregulated in both GBM cell lines and clinical tumors. Upregulating hsa-miR-1270 considerably suppressed GBM cell proliferation and migration in vitro and xenograft in vivo. WT1 was inversely correlated with hsa-miR-1270 in GBM. WT1 overexpression in hsa-miR-1270-upregulated GBM cells reversed the anticancer functions of hsa-miR-1270 on cancer proliferation and migration.
Conclusion: Hsa-miR-1270 upregulation may have suppressing effects on GBM cancer cells, likely by functionally acting through WT1.
Keywords: GBM, miRNA, hsa-miR-1270, WT1, xenograft
Introduction
Glioblastoma multiforme (GBM), or grade IV astrocytoma, is a heterogeneous, fast-growing, and aggressive type of brain tumor. Once diagnosed with GBM, cancer patients are often associated with high relapse rate and high mortality rate.1–3 Although significant progress had been made over the past decades to develop more treatment options for patients with GBM, such as antiangiogenic therapy, molecule-targeted therapies, immunotherapy, or gene therapy, the prognosis remains very poor for those patients with 5-year survival rates to be around 5%.4–7 Thus, it is very critical to explore the heterogeneous pathology, understand its underlying mechanisms, and seek effective therapeutic targets for GBM.
miRNAs are families of single-stranded, noncoding small (~18–22 nucleotide long) RNAs that attach to the complementary DNA sequences on the 3′-UTR of their downstream targeting genes, inducing gene silencing, thus yielding significantly functional regulations on molecular and cellular developments in both humans and animals.8–11 In addition, miRNAs were demonstrated to play important roles in regulating pathology among many human diseases, such as cancer.12–15 Specifically, in human brain tumor of glioma, miRNAs were shown to be aberrantly dysregulated in cancer patients’ tumor tissues or plasma, and may induce functional regulations on cancer cell development, maturation, metastasis, or drug resistance.16–18
Within the families of human cancer–related miRNAs, human mature microRNA 1270, hsa-miR-1270, was discovered to be either upregulated or downregulated in several types of human cancers, for example, upregulated in osteosarcoma or renal cancer,19,20 but downregulated in endometrial cancer.21 In addition, hsa-miR-1270 was found to be associated with several cancer-regulating genes, such as glypican 5 or histone deacetylase 4 in human non-small-cell lung cancer,22,23 and alpha fetoprotein in hepatoma.24 However, there has been little evidence directly linking hsa-miR-1270 with functional regulations in human cancers.
In a very recent study, Sun et al demonstrated that, among human glioma subtypes, hsa-miR-1270 might be downregulated in grade IV astrocytoma or GBM.25 To follow up on this discovery, we conducted a systematic approach to explore the expression profile and functional mechanisms of hsa-miR-1270 in human GBM. The results of this study may help to identify the clinical implication of hsa-miR-1270, as to whether it may be used as an epigenetic target for future molecular therapy for patients with GBM.
Materials and methods
GBM cells and clinical tissues
Five human GBM cell lines, LN-18, LN-229, U-138MG, T98G, and A172 cells, were commercially obtained from American Type Culture Collection (Manassas, VA, USA). In addition, a normal human astrocyte (NHA) cell line was commercially obtained from the Chinese Academy of Sciences Cell Bank of Type Culture Collection (Shanghai, China). The cell lines were all cultured in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Thermo Fisher Scientific), 100 U/mL penicillin (Thermo Fisher Scientific), and 100 mg/mL streptomycin (Thermo Fisher Scientific), in a humidified environment at 37°C with 5% CO2.
Clinical tissues were obtained from eleven GBM patients who received maximal surgical resection at the Second Affiliated Hospital of Chongqing Medical University between June 2012 and November 2017. GBM tissues were identified by independent magnetic resonance imaging and histology evaluations according to the WHO classifications.26 Paired samples, including GBM tissues and adjacent normal brain tissues, were retrieved, and immediately snap-frozen in liquid nitrogen. Those tissues were then stored in a −70°C biograded freezer until further processing.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from GBM cell lines or clinical tissues using a PureLink miRNA Isolation Kit (Thermo Fisher Scientific) according to the manufacturer’s recommendation. Then, a TaqMan™ MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific) was applied to convert mRNA to cDNA. qRT-PCR was then conducted using an ABI Prism 7900 HT Fast-Real time PCR System (Applied Biosystems, Waltham, MA, USA) according to the manufacturer’s recommendation. For quantification on hsa-miR-1270 gene expression, a mirVana™ qRT-PCR miRNA Detection Kit (Invitrogen, Waltham, MA, USA) was used according to the manufacturer’s recommendation, and U6 small nuclear RNA (U6 sRNA) was used as a reaction template. Alternatively, for quantification on Wilms’ tumor gene (WT1), a PowerUp™ SYBR™ Green Master Mix kit (Invitrogen) was used according to the manufacturer’s recommendation, and glyceraldehyde 3-phosphate dehydrogenase was used as reaction template. Finally, relative gene expression levels were quantified using the 2−ΔΔCt method.
Hsa-miR-1270 overexpression assay
A lentivirus with the insertion of an hsa-miR-1270 mimics, miR-1270M, was commercially obtained from Shanghai Genepharma Co., Ltd. (Shanghai, China). Correspondingly, another lentivirus with the insertion of a nonspecific human miRNA mimics, miR-MC, was also obtained from Shanghai Genepharma. Among GBM cell lines, LN-18 and A172 cells were transduced with miR-MC or miR-1270M for 48 hours, and then selected by blasticidin (8 μg/mL, Sigma Aldrich, St Louis, MO, USA) for 72 hours. After that, healthy cell colonies were selected and passaged for three times. Then, qRT-PCR was conducted to examine hsa-miR-1270 expression in lentiviral-transduced GBM cells.
In vitro proliferation assay
LN-18 and A172 cells were seeded in 96-well plates (1.5 × 103 cells/well), and allowed to proliferate for five consecutive days. Their in vitro proliferation was examined using a CyQUANT® NF Cell Proliferation Assay Kit (Thermo Fisher Scientific) according to the manufacturer’s recommendation, and quantified as absorbance readings at 570 nm (Multiskan, Thermo Fisher Scientific).
In vitro migration assay
LN-18 and A172 cells were seeded in the upper chambers of a Transwell plate (24-well, 8 μm, VWR, USA). The Transwell membranes were precoated with Matrigel (0.1%, Sigma Aldrich). Cells in the upper chambers were maintained in culture medium without FBS (1.5 × 104 cells/well), whereas the lower chambers were filled with serum-supplemented regular culture medium. The Transwell plate was kept in a humidified environment at 37°C with 5% CO2 for 48 hours. Then, Transwell inserts, floating cells, and culture medium were all removed. LN-18 and A172 cells migrated to the bottoms of Transwell plates were fixed, stained with 0.1% crystal violet (Sigma Aldrich) for 20 minutes at room temperature, and then imaged. In each well, the numbers of migrated GBM cells were measured for five randomly selected areas (200 μM × 200 μM). GBM cancer migration was then quantified as the percentages of migrated GBM cells in each well against migrated GBM cells in control wells.
In vivo xenograft assay
GBM LN-18 cells, after lentiviral transduction, were xenografted into the subcutaneous compartments of male athymic nude mice (5-week old, 1 × 106 cells/injection). Twelve mice received xenografts transduced with miR-MC lentivirus. Fourteen mice received xenografts transduced with miR-1270M lentivirus. Every week, length (L, mm) and width (W, mm) of LN-18 xenografts were measured, and their volumes (V, mm3) were estimated based on a formula, V=L*W*W/2. After 5 weeks, mice were killed. LN-18 xenografts were removed from mice bodies and compared under the microscope.
Dual-luciferase reporter assay
The 3′-UTR of human WT1 gene was inserted into a pmiR-REPORT luciferase vector to generate a wild-type luciferase plasmid, WT1-WT (Shanghai Genepharma). Alternatively, at position 910–916 and position 2329–2335 of human WT1 DNA sequence, the sequences were mutated to nullify hsa-miR-1270 binding. The mutant WT1 3′-UTR was also inserted into pmiR-REPORT to generate another luciferase plasmid, WT1-MU (Shanghai Genepharma). In a dual-luciferase reporter assay, HEK293T cells were pretransduced with miR-MC or miR-1270M, followed by secondary transfection of WT1-WT or WT1-MU for 24 hours. Relative luciferase activities were measured using a Multiskan plate-reader (Thermo Fisher Scientific) according to the manufacturer’s recommendation.
Western blotting assay
LN-18 and A172 cells were lysed with a Pierce™ IP Lysis Buffer (Thermo Fisher Scientific). Equal amount of extracted protein samples were then blotted onto a nitrocellulose membrane (Shanghai Genepharma). A WT1 polyclonal antibody (1:500, Invitrogen) and a polyclonal beta-actin antibody (1:10,000 Invitrogen) were used for primary Western blot reaction, and horseradish peroxidase-conjugated secondary antibodies (Invitrogen) were used for secondary Western blot reaction. Finally, the blots were visualized using a Fusion Solo chemiluminescence system (PEQLAB Biotechnologie GmbH, Germany) according to the manufacturer’s recommendation.
WT1 overexpression assay
The whole sequence of human WT1 gene was inserted into a mammalian overexpression vector, pcDNA/3.1+, to generate a WT1 overexpression plasmid, pc/WT1 (Shanghai Genepharma). An empty pc/DNA/3.1+ vector was applied as control plasmid, pc/C (Shanghai Genepharma). In LN-18 and A172 cells with hsa-miR-1270 overexpression, they were secondarily transfected with pc/C or pc/WT1 for 72 hours. After that, healthy cell colonies were selected and passaged for three times. Then, qRT-PCR was conducted to examine WT1 expression in GBM cells.
Statistical analyses
In this study, all statistical analyses were conducted using a SPSS software (SPSS, version 16.0, IBM Corporation, Armonk, NY, USA). Data were averaged from at least three biologic repeats, and demonstrated as means ± SEM. A one-way ANOVA, along with Turkey’s post hoc test, was conducted across this study. A P-value <0.05 was considered as statistically significant.
Ethical statement
The approval to conduct this study was granted by the Human Research and Ethical Committees at all participating institutes, including The Second Affiliated Hospital of Chongqing Medical University and Chongqing Medical University, both in Chongqing City, China. Consent forms were signed by all participating patients. In addition, all protocols were conducted in accordance with the principles of the Declaration of Helsinki. All animal experiments were approved by the Ethical Committees prior to the commencement of the study, and conducted according to the institutional and national guidelines and regulations.
Results
Hsa-miR-1270 is downregulated in both GBM cell lines and clinical tumors
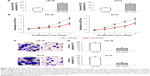
We examined hsa-miR-1270 gene expression in GBM cell lines. Through qRT-PCR analysis, we found that hsa-miR-1270 was significantly downregulated in LN-18, N-229, U-138MG, T98G, and A172 cells, compared with a NHA cell line (Figure 1A, *P<0.05). In addition, in eleven patients diagnosed with GBM, qRT-PCR showed that hsa-miR-1270 was significantly downregulated in GBM tissues, compared with paired normal tissues, in nine patients (Figure 1B, *P<0.05).
Hsa-miR-1270 upregulation suppressed GBM in vitro functions of proliferation and migration
To understand the biologic functions of hsa-miR-1270 in GBM, we transduced LN-18 and A172 cells with lentivirus of miR-1270M. Analysis of qRT-PCR confirmed that, in miR-1270M-transduced GBM cells, their endogenous hsa-miR-1270 expressions were significantly upregulated, compared with miR-MC-transduced GBM cells (Figure 2A, *P<0.05).
Lentiviral-transduced LN-18 and A172 cells were examined by an in vitro proliferation assay for five consecutive days. It demonstrated that in vitro cancer cell growth was significantly suppressed in miR-1270M-transduced cells compared with miR-MC-transduced cells (Figure 2B, *P<0.05).
Then, lentiviral-transduced LN-18 and A172 cells were examined by an in vitro migration assay for 24 hours. GBM cells migrated into the lower chambers of Transwell assay were stained by crystal violet. The imaging results showed that significantly less miR-1270M-transduced cells were observed than miR-MC-transduced cells (Figure 2C). In addition, quantification on cancer migration demonstrated that hsa-miR-1270 upregulation markedly inhibited GBM migrating capability in vitro (Figure 2D, *P<0.05).
Thus, our data clearly demonstrated that hsa-miR-1270 upregulation suppressed GBM in vitro functions of proliferation and migration.
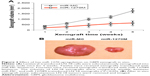
Hsa-miR-1270 upregulation suppressed GBM in vivo xenograft
GBM LN-18 cells, after lentiviral transduction, were xenografted into the subcutaneous compartment of male athymic nude mice. The xenografts were allowed to grow for 5 weeks in vivo. Their subcutaneous tumor volumes were estimated on weekly basis. It showed that LN-18 xenografts transduced with miR-1270M grew much more slowly than those transduced with miR-MC (Figure 3A, *P<0.05). After 5 weeks, subcutaneous GBM xenografts were removed from mice, and imaged. It demonstrated that hsa-miR-1270 upregulation significantly suppressed GBM in vivo xenograft (Figure 3B).
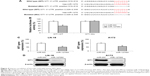
WT1 was regulated by hsa-miR-1270 in GBM
Next, we asked whether other signaling pathways were correlated with hsa-miR-1270 to regulate GBM functions. By studying several online miRNA targeting algorithms (TargetScan, www.targetscan.org and miRDB, www.mirdb.org), we found that human WT1 was a possible downstream molecular target of hsa-miR-1270 with two potential DNA binding sites on its 3′-UTR (Figure 4A). Then, through the examination of a dual-luciferase reporter assay, we confirmed that hsa-miR-1270 did physically bind human WT1 gene (Figure 4B, *P<0.05). In addition, analysis of qRT-PCR showed that, in lentiviral-transduced LN-18 and A172 cells, WT1 gene expressions were significantly downregulated by forced hsa-miR-1270 upregulation (Figure 4C, *P<0.05). Moreover, Western blot analysis showed that, in lentiviral-transduced LN-18 and A172 cells, WT1 protein expressions were also significantly reduced by forced hsa-miR-1270 upregulation (Figure 4D).
Thus, our data clearly showed that WT1 was regulated by hsa-miR-1270 in GBM.
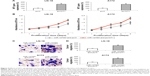
WT1 upregulation reversed hsa-miR-1270-induced suppression on GBM in vitro functions
Finally, in hsa-miR-1270-upregulated LN-18 and A172 cells, we transduced them with a mammalian overexpression plasmid, pc/WT1. Analysis of qRT-PCR showed that, in cells transfected with pc/WT1, their endogenous WT1 expressions were strongly upregulated, as compared to cells transfected with a control overexpression plasmid, pc/C (Figure 5A, *P<0.05).
The mechanistic implications of WT1 upregulation on GBM in vitro functions were explored. First, secondary-transfected LN-18 and A172 cells were examined by the in vitro proliferation assay for five consecutive days. It showed that, in hsa-miR-1270-upregulated GBM cells, in vitro cancer cell growth was significantly augmented in pc/WT1-transfected cells compared with pc/C-transfected cells (Figure 5B, *P<0.05).
Then, secondary-transfected LN-18 and A172 cells were examined by an in vitro migration assay. Twenty four hours later, the imaging results showed that, in hsa-miR-1270-upregulated GBM cells, significantly more pc/WT1-transfected cells were observed than pc/C-transfected cells (Figure 5C). In addition, quantification on cancer migration demonstrated that, in hsa-miR-1270-upregulated GBM cells, WT1 upregulation significantly increased GBM migrating capability in vitro (Figure 5D, *P<0.05).
Thus, our data clearly showed that WT1 upregulation reversed hsa-miR-1270-induced suppression on GBM in vitro functions.
Discussion
It is well documented that miRNAs are aberrantly expressed, and play important roles, such as prognostic biomarker and functional regulators in human glioma or GBM.16–18 A very recent report showed that hsa-miR-1270 was particularly downregulated in grade IV astrocytoma or GBM.25 Thus, in this study, we first probed the gene expression of hsa-miR-1270 in both GBM cell lines and GBM tumors. We found that hsa-miR-1270 was universally downregulated in examined GBM cell lines. In addition, in nine of eleven paired clinical samples of GBM patients, hsa-miR-1270 was found to be downregulated in GBM tumors compared with adjacent normal tissues. Interestingly, in the other two paired clinical samples, although it was not significant, hsa-miR-1270 expression levels did seem to be lower in GBM tumors than in adjacent normal tissues (Figure 1B). Thus, the meaning of our results could be twofold. First, hsa-miR-1270 may be predominantly downregulated in both immortal GBM cell lines and GBM tumors, which makes it a potential candidate to be used as a prognostic biomarker in GBM diagnosis. Second, the exact hsa-miR-1270 expression profile in GBM patients could be heterogeneous. There could be subtypes of GBM tumors with less-downregulated hsa-miR-1270 expression levels. Or, hsa-miR-1270 may not be downregulated, or even upregulated in GBM tumors. Therefore, more clinical examinations with a large size of patient pool would be necessary to elucidate the exact hsa-miR-1270 expression profile in GBM.
Second, we applied lentiviral transduction to create two GBM cell lines, LN-18 and A172 cells, with stable hsa-miR-1270 overexpression. Then, by comparing them with GBM cells without hsa-miR-1270 overexpression, we were able to discover that hsa-miR-1270 upregulation had significant antitumor effect in GBM by suppressing cancer cell proliferation and migration in vitro, as well as tumor growth of xenograft in vivo. In several previous cancer studies regarding miR-1270, researchers were able to identify its aberrant expression profile among human cancers, but failed to reveal its functional mechanism.19,21 Only in a very recent report, Zhong et al showed that inhibiting miR-1270 could yield anticancer mechanisms in osteosarcoma.20 Thus, the results of our study were among the very first groups to identify functional mechanisms of hsa-miR-1270 in human cancers. Specifically, our data suggested that upregulating hsa-miR-1270 might be an effective biologic target for GBM molecular therapy.
Also in our study, we discovered that human WT1 gene was actively involved in hsa-miR-1270-mediated cancer-suppressing effects in GBM. Through a dual-luciferase reporter assay, qRT-PCR, and Western blot analyses, WT1 was shown to be directly bound by hsa-miR-1270, and inversely downregulated by hsa-miR-1270 upregulation in GBM cells. Most importantly, we demonstrated that WT1 overexpression reversed the anticancer effects of hsa-miR-1270 upregulation on GBM proliferation and migration in vitro. In a previous report, WT1 was shown to be strongly expressed in glial tumors, hinting its direct correlation with glioma proliferation.27 In another report, immunotherapy utilizing WT1 peptide vaccine was demonstrated to be very effective among WT1-positive GBM patients, further suggesting an oncogenic nature of WT1 in GBM.28 While the results of our study were generally in line with those previous reports, our study was the first one to correlate WT1 with microRNA epigenetic regulation in GBM.
Conclusion
Overall, our study presented strong evidence showing that hsa-miR-1270 was predominantly downregulated in GBM. Upregulating hsa-miR-1270 had significant tumor-suppressing effects on GBM functions, possibly through inverse regulation on WT1 gene. Thus, the miR-1270/WT1 signaling pathway may be a potential therapeutic target for GBM treatment. Moreover, given the fact that GBM contains subsets of GBM stem-like cells, it would be interesting to learn, possibly through future study, any possible effects of miR-1270/WT1 signaling pathway in neurogenesis or tumorigenesis in human brain.
Disclosure
The authors report no conflicts of interest in this work.
References
Thakkar JP, Dolecek TA, Horbinski C, et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014;23(10):1985–1996. | ||
Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee S. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 2017;18(1):3–9. | ||
Johnson KJ, Schwartzbaum J, Kruchko C, et al. Brain tumor epidemiology in the era of precision medicine: the 2017 Brain Tumor Epidemiology Consortium meeting report. Clin Neuropathol. 2017;36(6):255–263. | ||
Zhang M, Ye G, Li J, Wang Y. Recent advance in molecular angiogenesis in glioblastoma: the challenge and hope for anti-angiogenic therapy. Brain Tumor Pathol. 2015;32(4):229–236. | ||
Delgado-López PD, Corrales-García EM. Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol. 2016;18(11):1062–1071. | ||
Lombardi G, Pambuku A, Bellu L, et al. Effectiveness of antiangiogenic drugs in glioblastoma patients: a systematic review and meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol. 2017;111:94–102. | ||
Alifieris C, Trafalis DT. Glioblastoma multiforme: pathogenesis and treatment. Pharmacol Ther. 2015;152:63–82. | ||
Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9(2):102–114. | ||
Chekulaeva M, Filipowicz W. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr Opin Cell Biol. 2009;21(3):452–460. | ||
Shelton SB, Reinsborough C, Xhemalce B. Who watches the watchmen: roles of RNA modifications in the RNA interference pathway. PLoS Genet. 2016;12(7):e1006139. | ||
Mohr A, Mott J. Overview of microRNA biology. Semin Liver Dis. 2015;35(1):3–11. | ||
Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. Adv Exp Med Biol. 2013;774:1–20. | ||
O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol. 2018;9:402. | ||
Jafri MA, Al-Qahtani MH, Shay JW. Role of miRNAs in human cancer metastasis: implications for therapeutic intervention. Semin Cancer Biol. 2017;44:117–131. | ||
Rivera-Barahona A, Pérez B, Richard E, Desviat LR. Role of miRNAs in human disease and inborn errors of metabolism. J Inherit Metab Dis. 2017;40(4):471–480. | ||
Beyer S, Fleming J, Meng W, Singh R, Haque SJ, Chakravarti A. The role of miRNAs in angiogenesis, invasion and metabolism and their therapeutic implications in gliomas. Cancers. 2017;9(7):E85:85. | ||
Rolle K. miRNA multiplayers in glioma. From bench to bedside. Acta Biochim Pol. 2015;62(3):353–365. | ||
Nikaki A, Piperi C, Papavassiliou AG. Role of microRNAs in gliomagenesis: targeting miRNAs in glioblastoma multiforme therapy. Expert Opin Investig Drugs. 2012;21(10):1475–1488. | ||
Weng L, Wu X, Gao H, et al. MicroRNA profiling of clear cell renal cell carcinoma by whole-genome small RNA deep sequencing of paired frozen and formalin-fixed, paraffin-embedded tissue specimens. J Pathol. 2010;222(1):41–51. | ||
Zhong L, Zheng C, Fang H, Xu M, Chen B, Li C. MicroRNA-1270 is associated with poor prognosis and its inhibition yielded anticancer mechanisms in human osteosarcoma. IUBMB Life. 2018;70(7):625–632. | ||
Bao W, Wang HH, Tian FJ, et al. A TrkB-STAT3-miR-204-5p regulatory circuitry controls proliferation and invasion of endometrial carcinoma cells. Mol Cancer. 2013;12(1):155. | ||
Ma R, Wang C, Wang J, Wang D, Xu J. miRNA-mRNA interaction network in non-small cell lung cancer. Interdiscip Sci. 2016;8(3):209–219. | ||
Zhao Z, Han C, Liu J, Wang C, Wang Y, Cheng L. GPC5, a tumor suppressor, is regulated by miR-620 in lung adenocarcinoma. Mol Med Rep. 2014;9(6):2540–2546. | ||
Zhang C, Li H, Jiang W, Zhang X, Li G. Icaritin inhibits the expression of alpha-fetoprotein in hepatitis B virus-infected hepatoma cell lines through post-transcriptional regulation. Oncotarget. 2016;7(50):83755–83766. | ||
Sun Y, Tian Y, Wang GZ, et al. Overexpression of transforming acidic coiled coil-containing protein 3 reflects malignant characteristics and poor prognosis of glioma. Int J Mol Sci. 2017;18(3):235. | ||
Feiden S, Feiden W. WHO classification of tumours of the CNS: revised edition of 2007 with critical comments on the typing und grading of common-type diffuse gliomas. Pathologe. 2008;29(6):411–421. | ||
Hashiba T, Izumoto S, Kagawa N, et al. Expression of WT1 protein and correlation with cellular proliferation in glial tumors. Neurol Med Chir. 2007;47(4):165–170. | ||
Izumoto S, Tsuboi A, Oka Y, et al. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg. 2008;53(5):963–971. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.