Back to Journals » OncoTargets and Therapy » Volume 15
Upregulation of Cartilage Oligomeric Matrix Protein Predicts Poor Prognosis in Urothelial Carcinoma
Authors Kuo YH, Lai HY, Chan TC, Hsing CH, Huang SK, Hsieh KL, Chen TJ, Li WS, Lu JC, Li CF
Received 15 April 2022
Accepted for publication 15 June 2022
Published 30 June 2022 Volume 2022:15 Pages 727—740
DOI https://doi.org/10.2147/OTT.S370028
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Yu-Hsuan Kuo,1,2 Hong-Yue Lai,3 Ti-Chun Chan,3,4 Chung-Hsi Hsing,3,5 Steven K Huang,6,7 Kun-Lin Hsieh,6 Tzu-Ju Chen,8,9 Wan-Shan Li,9,10 Jhih-Cheng Lu,11,* Chien-Feng Li3,4,*
1Division of Hematology and Oncology, Department of Internal Medicine, Chi-Mei Medical Center, Tainan, 71004, Taiwan; 2College of Pharmacy and Science, Chia Nan University, Tainan, 71710, Taiwan; 3Department of Medical Research, Chi Mei Medical Center, Tainan, 710, Taiwan; 4National Institute of Cancer Research, National Health Research Institutes, Tainan, 704, Taiwan; 5Department of Anesthesiology, Chi Mei Medical Center, Tainan, 710, Taiwan; 6Division of Urology, Department of Surgery, Chi Mei Medical Center, Tainan, 710, Taiwan; 7Department of Medical Science Industries, College of Health Sciences, Chang Jung Christian University, Tainan, Taiwan; 8Department of Clinical Pathology, Chi Mei Medical Center, Tainan, 710, Taiwan; 9Department of Medical Technology, Chung Hwa University of Medical Technology, Tainan, Taiwan; 10Department of Pathology, Chi Mei Medical Center, Tainan, 710, Taiwan; 11Division of Urology, Department of Surgery, Chi Mei Medical Center, Liouying, 736, Taiwan
*These authors contributed equally to this work
Correspondence: Chien-Feng Li, Department of Medical Research, Chi Mei Medical Center, Tainan, 710, Taiwan, Tel +886-6-2812811, Fax +886-6-2510218, Email [email protected]
Purpose: Cartilage oligomeric matrix protein (COMP) is known as a large pentameric glycoprotein, which interacts with various extracellular matrix proteins in tissues. COMP has been reported to play a role in multiple connective tissue disorders. Recently, elevated COMP levels have been found to be associated with increased tumor size, metastases, faster recurrence of cancer, and overall poorer survival in several cancers. However, the clinical importance of COMP in urothelial carcinoma remains unclear. We investigated the association between COMP expression and clinical outcomes in urothelial carcinoma.
Patients and Methods: In this retrospective study, we collected urothelial carcinoma (UC) tissue from 340 upper urinary tract UC (UTUC) patients and 295 urinary bladder UC (UBUC) patients. Pearson’s chi-square test, Kaplan–Meier analysis, and the multivariate Cox proportional hazards model was used to examine the relationship between COMP expression and patient characteristics, pathological findings, and patient survival, such as metastasis-free survival (MFS) and disease-specific survival (DSS).
Results: A total of 295 UBUC patients and 340 UTUC patients were recruited. The COMP mRNA level was significantly higher among invasive tumors (pT2–pT4) than in noninvasive tumors (pTa-T1) in UBUC groups (P < 0.01). COMP overexpression was associated with advanced T stage, nodal metastases, vascular invasion, perineural invasion, high histological grade, and high mitotic rate in both UBUC and UTUC cohorts. COMP overexpression was predictive of shorter DSS (hazard ratio [HR] in UBUC, 3.986, P < 0.001; in UTUC, 2.283, P = 0.027] and MFS (HR in UBUC, 6.813, P < 0.001; in UTUC, 4.070, P < 0.001). Kaplan–Meier analysis demonstrated high COMP expression associated with poor DSS and MFS in UTUC and UBUC groups (all P < 0.0001).
Conclusion: COMP overexpression was linked to poor clinical prognosis and poor pathological features in UC. These results suggest COMP as a biomarker for UC.
Keywords: cartilage oligomeric matrix protein, urothelial carcinoma, extracellular matrix structure constituent, prognosis
Introduction
Urothelial carcinoma (UC) includes UC of upper urinary tract (UTUC) and UC of the urinary bladder (UBUC). Bladder cancer is the tenth most common cancer in the world, and accounts for approximately 573,000 new cases and 213,000 deaths.1 In Taiwan, bladder cancer is the 9th most common cancer in males, and 16th most common in females.2 In western countries, UBUC accounts for 90–95% of UCs. However, UTUC has a higher incidence, 30% in Taiwan, than in other parts of world.3 Non muscle-invasive bladder cancer (NMIBC) represents approximately 70% of organ-confined bladder cancer.4 The prognosis of NMIBC is favorable with a 5-year recurrence-free survival rate of 43%. However, up to 21% of NMIBC patients with high-risk disease will progress to muscle-invasive bladder cancer (MIBC).5,6 High-risk patients can be defined by several clinical features, such as T stage, lymph nodes metastases, and histology grade. However, the identification of genomics-based predictive biomarkers is warranted to define subsequent treatment policies after curative surgery.
Cartilage oligomeric matrix protein (COMP), also known as Thrombospondin-5 (TSP-5), is expressed by multiple cell types. COMP levels are elevated in a variety of musculoskeletal diseases.7 COMP levels have been reported to be elevated in breast malignancies, hepatocellular carcinoma, prostate cancers, and colon cancers. Increased tumor growth, cancer metastasis, cancer recurrence, and overall shorter survival have all been linked to COMP overexpression.8–11 The prognostic importance of COMP in UC has not been fully elucidated. The goal of this study was to assess COMP expression and prognostic value in UTUC and UBUC patients.
Materials and Methods
Data Mining of the Gene Expression Omnibus (GEO) Dataset
The NCBI Gene Expression Omnibus (GEO) database was used to obtain a transcriptome dataset (GSE31684) including 93 UBUC patients who underwent curative surgery. All probe sets without pre-selection were utilized. Raw data were then imported into Nexus Expression 3 software to calculate the level of gene expression. By comparing tumor stage (high stage vs low stage) and metastatic events (metastasis vs non-metastasis), comparative studies were carried out to determine the significantly differentially expressed genes linked to extracellular matrix structural constituent (GO:0005201). Additionally, differentially regulated genes in MIBC vs NMIBC and metastatic disease vs non-metastatic disease were identified (P < 0.001 and log ratio >1) (Figure 1 and Table 1).
Study Population
Between 1996 and 2004, the Chi Mei Medical Center enrolled 340 patients with UTUC and 295 patients with UBUC who underwent curative surgery. The study had been approved by the Institutional Review Board (IRB) of Chi Mei Medical Center (Address: 901 Chunghwa Road, Yung Kang Dist., Tainan City 710, TAIWAN) with the approval number of 10,501,005. All subjects gave their informed consent. Retrospective data on demographics and clinical information, such as pathological features, oncological follow-up, and cause of death, were obtained. Patients who had received neoadjuvant radiotherapy or chemotherapy, had acute blood disorders, bone marrow abnormalities, a concomitant muscle-invasive bladder tumor, or had inadequate clinical data were excluded from the study. The tumor stage was assessed using the Tumor, Node, Metastasis (TNM) system developed by the American Joint Committee on Cancer (AJCC) in 2002. Using the seventh edition of the AJCC staging system, two pathologists examined tumor specimens and classified them as low grade or high grade. In general, all patients were treated with curative surgery. Cisplatin-based adjuvant treatment was given to all UBUC patients with pT3 or pT4 cancers or nodal involvement.
Immunohistochemistry and Scoring
Specimens were prepared by standard procedure. The sections were incubated for 1 hour with the primary COMP antibody (Clone: EPR22857-38, Abcam, dilution 1:200). A DAKO ChemMate EnVision Kit (K5001, Carpinteria, CA, USA) was then used to detect antibodies. Positive controls were cell blocks collected from cell lines known to express COMP. Negative controls were sections processed without the primary anti-COMP antibody. The H-score was calculated using the following equation by two pathologists to estimate COMP immunoreactivity: H-score = SPi (i + 1), where Pi represents the percentage of stained tumor cells in various intensities ranging from 0% to 100%, and i represents the staining intensity (0 to 3+). If scoring differences occurred, the two pathologists analyzed the slides at the same time and came to an H-score agreement. Based on the median H-score, the immunostain was divided into low and high expression levels.
Real-Time RT-PCR
Pure tumor cells were isolated using laser capture microdissection in 20 snap-frozen UB samples to determine mRNA expression of COMP. Total RNA was extracted and sent for reverse-transcription to quantify transcript level. As previously described, mRNA abundance of COMP (Hs00164359_m1) was measured with pre-designed TaqMan assay reagents (Applied Biosystems) using the the ABI StepOnePlus™ System. COMP expression relative to normal urothelium was calculated by the comparative Ct method after normalization to POLR2A (Hs01108291_m1) as the internal control.
Statistical Analysis
Pearson’s chi-square test was used to assess the relationship between COMP expression and various clinicopathological features. We looked at two outcomes: metastasis-free survival (MFS) and disease-specific survival (DSS). Relevant COMP expression and clinicopathological characteristics were identified as predictors of DSS (measured from curative surgery to the time of cancer mortality) and MFS (measured from curative surgery to the first metastasis) using univariate and multivariate analysis. The Kaplan–Meier method with a Log rank test was used to create survival curves. To find the independent variables, all significant parameters from the univariate analysis were incorporated in the multivariate Cox proportional hazards model. For statistical analysis, IBM’s SPSS Statistics V.17.0 software (Armonk, NY, USA) was used. The cutoff for statistical significance was set at P < 0.05.
Results
Upregulation of COMP Gene Links to Extracellular Matrix Structure in the UBUC Transcriptome
A published UBUC transcriptome dataset (GSE31684) for data mining was used that included 93 patients who had a radical cystectomy. A total of 78 patients were diagnosed with invasive illness (pT2–pT4), and 15 were diagnosed with noninvasive or superficial disease (pTa and pT1). We discovered 13 probes that covered 13 transcripts related to extracellular matrix structural constituents (GO:0005201). COMP was shown to be significantly elevated in invasive UC when compared to non-invasive UC (Table 1 and Figure 1). Table 1 shows that in advanced UC, the COMP gene (Probe: 205713 s at) was upregulated by up to 1.8386-fold log ratios (P < 0.0001). COMP was similarly elevated in metastatic UC with 1.1823-fold log ratios (P = 0.0001) compared with non-metastatic UC.
Associations of COMP mRNA Expression with pT Status
In the UBUC groups, the level of COMP transcripts was considerably higher in tumors with a high pT status (pT2–pT4) compared with noninvasive tumors (pTa-T1) (P < 0.01) (Figure 2).
Clinicopathological Features of the UC Cohorts
A total of 340 UTUC and 295 UBUC patients were included in this study (Table 2). The average age was 65.8 years. A total of 159 patients (46.8%) in the UTUC cohort had advanced T stage (pT2-4) tumors, 28 patients (8.2%) had lymph node metastases, and 284 patients (83.5%) presented with high-grade tumors; in addition, 106 patients (31.2%) had vascular invasion (VI) and 19 patients (5.9%) had perineural invasion (PNI). A total of 167 patients (49.1%) had malignancies with a high mitotic rate. In the UBUC cohort 123 patients (41.7%) had muscle invasive bladder cancer (pT2-4). At the time of diagnosis, the majority of patients (81%) had a high-grade tumor. Only 29 patients (7.8%) were found to have lymph node metastases. A total of 156 lesions had a high mitotic rate (52.9%). In addition, VI and PNI were discovered in 20 (6.8%) and 49 (16.6%) lesions, respectively.
 |
Table 2 Correlation Between COMP Expression and Other Important Clinicopathological Features in Urothelial Carcinomas |
Correlations Between COMP Expression and Pathological Features in UC
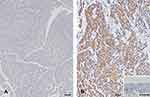
As determined using immunohistochemistry, muscle invasive UC had greater COMP immunoreactivity than non-muscle invasive UC (Figure 3). Table 2 summarizes the relationships between COMP expression levels and clinicopathological parameters in UC cases. In UTUC cohorts, COMP overexpression was associated with advanced T stage (P < 0.001), nodal metastases (P = 0.002), high histological grade (P < 0.001), vascular invasion (P < 0.001), perineural invasion (P = 0.002), and high mitotic rate (P = 0.007). In the UBUC cohorts, a significant correlation between high COMP immunoexpression and advanced T stage (P < 0.001), nodal metastases (P < 0.001), high histological grade (P = 0.007), vascular invasion (P < 0.001), perineural invasion (P = 0.006), and high mitotic rate (P = 0.006) were identified.
Prognostic Significance of COMP Expression
A total of 113 UC patients died, including 61 UTUC patients, and 52 UBUC patients. In addition, 146 individuals, including 70 UTUC patients and 76 UBUC patients, developed metastases. The predictive effects of COMP expression on cancer metastasis and patient survival in UTUC were assessed using univariate and multivariate analyses (Table 3). Tumor site, multi-focal tumors, T stage, nodal metastases, vascular invasion, high-grade tumor, perineural invasion, and COMP overexpression were all revealed to be significant predictive variables for DSS in a univariate analysis of UTUC. Poor MFS was linked with multilocality, lymph node metastases, T stage, histological grade, perineural invasion, vascular invasion, and COMP expression. Nodal metastases, high histological grade, perineural invasion, and COMP expression were all significantly associated with worse DFS, whereas nodal metastases, vascular invasion, perineural invasion, and COMP expression were all significantly correlated with worse MFS in multivariate Cox regression analysis.
 |
Table 3 Univariate Log Rank and Multivariate Analyses for Disease-Specific and Metastasis-Free Survival in Upper Urinary Tract Urothelial Carcinoma |
COMP overexpression was found to be substantially linked with poor DFS (hazard ratio [HR], 2.283; 95% confidence interval [CI], 1.100–4.735; P = 0.027) and poor MFS (HR, 4.070; 95% CI, 1.945–8.516; P < 0.001) in multivariate analysis.
In the UBUC cohorts (Table 4), T stage, nodal metastases, vascular invasion, perineural invasion, high tumor grade, and COMP expression were all associated with poor prognostic variables for DSS and MFS in the univariate analysis. T stage, vascular invasion, perineural invasion, and COMP expression (HR, 3.986; 95% CI, 1.815–8.754; P < 0.001) were all substantially linked with poorer DSS in multivariate Cox regression analysis. Only T stage, nodal metastases, and COMP overexpression (HR, 6.813; 95% CI, 3.406–13.625; P < 0.001).
 |
Table 4 Univariate Log Rank and Multivariate Analyses for Disease-Specific and Metastasis-Free Survival in Urinary Bladder Urothelial Carcinoma |
Survival Analysis in UTUC and UBUC
In the UTUC group, Kaplan–Meier analysis showed COMP overexpression was correlated with poorer DSS (Figure 4A; P < 0.0001) and MFS (Figure 4B; P < 0.0001). COMP overexpression was also associated with poorer DSS (Figure 4C; P < 0.0001) and MFS (Figure 4D; P < 0.0001) in UBUC patients.
Discussion
COMP is a large pentameric cartilage protein with five identical monomers linked by disulfide bridges at the N-terminus. Four epidermal growth factor (EGF) domains, eight calcium-binding type 3-repeat domains, and a globular carboxyl-terminal are contained in each subunit. COMP is a member of the thrombospondin gene family12 and is found in articular cartilage, ligaments, and tendons. The protein can also be found in the skin, breast tissues, and liver tissues.9,13,14 Although the role of COMP glycoprotein has been well documented in multiple connective tissue disorders,15 its role in carcinogenesis remains uncertain. Recently, evidence has demonstrated that COMP upregulation correlates with poor prognosis and metastases in breast cancer, prostate cancer, thyroid cancer, colon cancer, and hepatocellular carcinoma.8,9,11,16–18
In order to find molecular biomarkers, we use the NCBI Gene Expression Omnibus (GEO) database to obtain a transcriptome dataset (GSE31684) including 93 UBUC patients who underwent curative surgery. In our one unpublished study, we evaluated the genes associated with the nucleobase-containing compound metabolic process (GO:0006139) and found DPYSL3 was the only transcript that showed a significant survival impact. Moreover, we performed cell line study to clarify the biological functions of this novel biomarker.34 Based on this, we believe the pathogenesis of UC is complex and all biomarkers are different in nature and biology. Searching other possible molecular biomarkers is mandatory. In this study, we use the same transcriptome dataset, the genes related to extracellular matrix structure constituent (GO:0005201) was assessed to better understand the role of COMP in urothelial cancer. COMP was substantially elevated in invasive UC compared with non-invasive UC across these genes. Thus, COMP was chosen as a candidate for additional testing considering the purpose was to determine the most upregulated gene relating to tumor invasiveness and metastases. To our knowledge, this is the first study to demonstrate COMP overexpression associated with poor survival in urothelial carcinoma.
There are several theories to explain the role of COMP in cancer development and prognosis: (1) COMP can promote tumor EMT.17,19 (2) COMP activates the Jagged1-Notch 3 signaling pathway, which leads to the initiation of cancer stem cells.20 (3) COMP prevents apoptosis by inducing the expression of apoptosis inhibitors.21 (4) COMP expressing cancer cells switch to aerobic glycolysis to generate energy.16 As previously reported, the epithelial to mesenchymal transition (EMT) is a critical biological step in the migration and invasion of malignant tumor cells.22 It is crucial to know the molecular mechanism of EMT regulation in malignant tumor cells and its role in the occurrence, growth, and metastasis of malignant cells. COMP can stimulate tumor EMT, although the mechanism is unknown.17,19 COMP glycoprotein has been demonstrated to be co-expressed with many EMT genes, and a clear association between high COMP expression and poor colon cancer survival has also been reported.19,23 On the other hand, extracellular matrices (ECMs) are multi-component networks that surround cells in tissues. Cell survival, proliferation, and differentiation, as well as tissue organization, are all dependent on these networks. In addition to providing support, the ECM supra-structure may process and convey a variety of signals to cells, which ultimately regulate their behavior. Importantly, ECM-derived signals play a key role in the EMT process during carcinogenesis. COMP did not affect breast cancer cell adhesion or migration, but it did boost invasiveness by upregulating MMP 9 and enhancing cancer cells’ ability to destroy surrounding ECM stroma in a breast cancer cell model.9 COMP expression was increased in malignant breast and prostate tissues, according to gene expression analysis.16,24 This observation is consistent with immunostaining results, which indicated that COMP is expressed not only in stroma but also in epithelial tumor cells. COMP can stimulate proliferation by interacting with collagen molecules, which are known for their function in maintaining the extracellular matrix and driving cell proliferation via the PI3K/Akt/mTOR/p70S6K pathways.10,25–28
COMP is stored in platelet α-granules, released and synthesized following platelet activation.29 COMP binds to thrombin and inhibits thrombin-induced platelet aggregation, activation, and retraction, as well as thrombin-mediated fibrinogen cleavage.30 Increased plasma fibrinogen levels and the high platelet-to-lymphocyte ratio (PLR) have been linked to worse clinical outcomes in UC.31–33 A recent retrospective study reported that high PLR was associated with a poor initial tumor response to pembrolizumab.31 The hypothesis that high fibrinogen and high PLR are associated with poor prognosis in UC through COMP is quite interesting and worth further exploration.
Conclusion
COMP upregulation correlated with worse clinicopathological features. COMP is also associated with worse survival in UC, suggesting its role as a prognostic biomarker for UC. To our knowledge, this is the first study to elucidate the role of COMP in UC. Further research is needed to fully understand the mechanism and apply these findings to clinical practice.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. May 2021;71(3):209–249. doi:10.3322/caac.21660
2. Hung CF, Yang CK, Ou YC. Urologic cancer in Taiwan. Jpn J Clin Oncol. 2016;46(7):605–609. doi:10.1093/jjco/hyw038
3. Li CC, Chang TH, Wu WJ, et al. Significant predictive factors for prognosis of primary upper urinary tract cancer after radical nephroureterectomy in Taiwanese patients. Eur Urol. 2008;54(5):1127–1134. doi:10.1016/j.eururo.2008.01.054
4. Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66(6 Suppl 1):4–34. doi:10.1016/j.urology.2005.07.062
5. Ritch CR, Velasquez MC, Kwon D, et al. Use and validation of the AUA/SUO risk grouping for nonmuscle invasive bladder cancer in a contemporary cohort. J Urol. 2020;203(3):505–511. doi:10.1097/JU.0000000000000593
6. van den Bosch S, Alfred Witjes J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. Eur Urol. 2011;60(3):493–500. doi:10.1016/j.eururo.2011.05.045
7. Briggs MD, Hoffman SM, King LM, et al. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat Genet. 1995;10(3):330–336. doi:10.1038/ng0795-330
8. Norman GL, Gatselis NK, Shums Z, et al. Cartilage oligomeric matrix protein: A novel non-invasive marker for assessing cirrhosis and risk of hepatocellular carcinoma. World J Hepatol. Jul 18 2015;7(14):1875–83. doi:10.4254/wjh.v7.i14.1875
9. Englund E, Bartoschek M, Reitsma B, et al. Cartilage oligomeric matrix protein contributes to the development and metastasis of breast cancer. Oncogene. 2016;35(43):5585–5596. doi:10.1038/onc.2016.98
10. Liu -T-T, Liu X-S, Zhang M, et al. Cartilage oligomeric matrix protein is a prognostic factor and biomarker of colon cancer and promotes cell proliferation by activating the Akt pathway. J Cancer Res Clin Oncol. 2018;144(6):1049–1063. doi:10.1007/s00432-018-2626-4
11. Nfonsam VN, Jecius HC, Janda J, et al. Cartilage oligomeric matrix protein (COMP) promotes cell proliferation in early-onset colon cancer tumorigenesis. Surg Endosc. 2020;34(9):3992–3998. doi:10.1007/s00464-019-07185-z
12. Oldberg A, Antonsson P, Lindblom K, Heinegard D. COMP (cartilage oligomeric matrix protein) is structurally related to the thrombospondins. J Biol Chem. 1992;267(31):22346–22350. doi:10.1016/S0021-9258(18)41677-8
13. Farina G, Lemaire R, Korn JH, Widom RL. Cartilage oligomeric matrix protein is overexpressed by scleroderma dermal fibroblasts. Matrix Biol. 2006;25(4):213–222. doi:10.1016/j.matbio.2006.01.007
14. Zachou K, Gabeta S, Shums Z, et al. COMP serum levels: a new non-invasive biomarker of liver fibrosis in patients with chronic viral hepatitis. Eur J Intern Med. 2017;38:83–88. doi:10.1016/j.ejim.2017.01.007
15. Tseng S, Reddi AH, Di Cesare PE. Cartilage Oligomeric Matrix Protein (COMP): a biomarker of arthritis. Biomark Insights. 2009;4:33–44. doi:10.4137/bmi.s645
16. Englund E, Canesin G, Papadakos KS, et al. Cartilage oligomeric matrix protein promotes prostate cancer progression by enhancing invasion and disrupting intracellular calcium homeostasis. Oncotarget. 2017;8(58):98298–98311. doi:10.18632/oncotarget.21176
17. Li Q, Wang C, Wang Y, et al. HSCs-derived COMP drives hepatocellular carcinoma progression by activating MEK/ERK and PI3K/AKT signaling pathways. J Exp Clin Cancer Res. 2018;37(1):231. doi:10.1186/s13046-018-0908-y
18. Zhang J, Wang H, Lv C, et al. Cartilage oligomeric matrix protein affects the biological behavior of papillary thyroid carcinoma cells by activating the PI3K/AKT/Bcl-2 pathway. J Cancer. 2021;12(6):1623–1633. doi:10.7150/jca.49144
19. Nfonsam VN, Nfonsam LE, Chen D, et al. COMP gene coexpresses with EMT genes and is associated with poor survival in colon cancer patients. J Surg Res. 2019;233:297–303. doi:10.1016/j.jss.2018.08.021
20. Papadakos KS, Bartoschek M, Rodriguez C, et al. Cartilage oligomeric matrix protein initiates cancer stem cells through activation of Jagged1-Notch3 signaling. Matrix Biol. 2019;81:107–121. doi:10.1016/j.matbio.2018.11.007
21. Verma P, Dalal K. Serum cartilage oligomeric matrix protein (COMP) in knee osteoarthritis: a novel diagnostic and prognostic biomarker. J Orthop Res. 2013;31(7):999–1006. doi:10.1002/jor.22324
22. Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29(3):212–226. doi:10.1016/j.tcb.2018.12.001
23. Jandova J, Xu W, Nfonsam V. Sporadic early-onset colon cancer expresses unique molecular features. J Surg Res. 2016;204(1):251–260. doi:10.1016/j.jss.2016.04.068
24. Barrett T, Suzek TO, Troup DB, et al. NCBI GEO: mining millions of expression profiles–database and tools. Nucleic Acids Res. 2005;33(Database issue):D562–6. doi:10.1093/nar/gki022
25. Tsai KS, Kao SY, Wang CY, Wang YJ, Wang JP, Hung SC. Type I collagen promotes proliferation and osteogenesis of human mesenchymal stem cells via activation of ERK and Akt pathways. J Biomed Mater Res A. 2010;94(3):673–682. doi:10.1002/jbm.a.32693
26. Halasz K, Kassner A, Morgelin M, Heinegard D. COMP acts as a catalyst in collagen fibrillogenesis. J Biol Chem. 2007;282(43):31166–31173. doi:10.1074/jbc.M705735200
27. Somaiah C, Kumar A, Mawrie D, et al. Collagen promotes higher adhesion, survival and proliferation of mesenchymal stem cells. PLoS One. 2015;10(12):e0145068. doi:10.1371/journal.pone.0145068
28. Senoo H, Hata R. Extracellular matrix regulates cell morphology, proliferation, and tissue formation. Kaibogaku Zasshi. 1994;69(6):719–733.
29. Metharom P, Berndt MC. COMP: an endogenous thrombin inhibitor. Blood. 2015;126(7):831–832. doi:10.1182/blood-2015-06-650846
30. Liang Y, Fu Y, Qi R, et al. Cartilage oligomeric matrix protein is a natural inhibitor of thrombin. Blood. 2015;126(7):905–914. doi:10.1182/blood-2015-01-621292
31. Kurashina R, Ando K, Inoue M, et al. Platelet-to-lymphocyte ratio predicts the efficacy of pembrolizumab in patients with urothelial carcinoma. Anticancer Res. 2022;42(2):1131–1136. doi:10.21873/anticanres.15576
32. Bao Y, Wang Y, Li X, et al. Prognostic significance of platelet-to-lymphocyte ratio in urothelial carcinoma patients: a meta-analysis. Cancer Cell Int. 2019;19(1). doi:10.1186/s12935-019-1032-6.
33. Tanaka N, Kikuchi E, Matsumoto K, et al. Prognostic value of plasma fibrinogen levels in patients with localized upper tract urothelial carcinoma. BJU Int. 2013;111(6):857–864. doi:10.1111/j.1464-410X.2012.11353.x
34. Peir-In L, Hong-Yue L, Ti-Chun C, et al. Upregulation of dihydropyrimidinase-like 3 protein predicts poor prognosis in urothelial carcinoma. Onco Targets Ther. 2022;9:1059.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.