Back to Journals » OncoTargets and Therapy » Volume 13
Upregulated lncRNA THRIL/TNF-α Signals Promote Cell Growth and Predict Poor Clinical Outcomes of Osteosarcoma
Authors Xu B , Jin X, Yang T, Zhang Y, Liu S, Wu L , Ying H, Wang Z
Received 23 October 2019
Accepted for publication 18 December 2019
Published 7 January 2020 Volume 2020:13 Pages 119—129
DOI https://doi.org/10.2147/OTT.S235798
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Bo Xu,* Xinmeng Jin,* Tieyi Yang, Yan Zhang, Shuyi Liu, Liang Wu, Hui Ying, Zhi Wang
Department of Orthopaedics, Gongli Hospital of Pudong New Area, Shanghai 200135, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhi Wang
Department of Orthopaedics, Gongli Hospital of Pudong New Area, NO. 219 Miaopu Road, Pudong New Area, Shanghai 200135, People’s Republic of China
Tel +86 188 1782 1626
Email [email protected]
Background: The immunosuppressive facet and tumorigenic role of TNF-α have been revealed in osteosarcoma (OS). Long noncoding RNA THRIL is identified to regulate TNF-α expression and participates in immune response. Thus, investigations on the clinical expression pattern of THRIL/TNF-α signal in OS would provide a potential target premise for OS patients.
Methods: We collected OS (n=83), nontumor tissues (n=37) and serum samples (n=83 for OS and n=40 for healthy control) to determine the expressions and clinical significance of THRIL/TNF-α signal. Knockdown of THRIL in OS cell lines MG63 and Saos2 in vitro and in vivo was performed to confirm its function in the development of OS.
Results: Elevated expression of THRIL was associated with increased TNF-α levels in OS tissues and serum samples. Combination of THRIL and TNF-α in tissues showed a more efficient diagnostic value for OS patients than either of them. Moreover, high-expressed THRIL was associated with larger tumor size, advanced Enneking stage and lung metastasis, whereas high TNF-α expression was found in patients with high histologic grade and patients who simultaneously harbor high THRIL and TNF-α showed the worst overall survival and metastasis-free survival. TNF-α signals increased OS cell vitalities in vitro but knockdown of THRIL inhibited TNF-α expressions, leading to impaired cell vitality, increased apoptosis and also downregulated epithelial to mesenchymal transition (EMT) phenotype and the ability of invasion, but these processes were restored by the treatment of TNF-α. The oncogenic role of THRIL/TNF-α signal was also confirmed in the xenograft model of MG63 cells.
Conclusion: Overexpressed THRIL and TNF-α promoted OS progression with efficient diagnostic and prognostic value. THRIL/TNF-α signal supported cell growth and EMT phenotype of OS cells in vitro and in vivo.
Keywords: lncRNA, THRIL, TNF-α, osteosarcoma, growth
Introduction
Osteosarcoma (OS) often occurs in children and adolescents with an incidence of 4.4 per million people worldwide and is the most common malignant bone tumor. Approximately 50% of all patients at the time of diagnosis develop metastases, which results in a high mortality rate despite improvements in radiotherapy, adjuvant chemotherapy and surgery.1–3 Elucidation of the molecular basis of OS progression is conducive to developing effective therapeutic agents and improving OS patient’s prognosis.
Persistent inflammation is known to promote and exacerbate malignancy. An intrinsic (driven by genetic events that cause neoplasia) and an extrinsic (driven by inflammatory conditions which predispose to cancer) pathway link inflammation and cancer.4 Tumor necrosis factor-α (TNF-α) is a proinflammatory cytokine that coordinates tissue homeostasis by regulating cytokine production, cell survival, and cell death.5 Accumulating evidence indicated that, in addition to being produced by immune cells within the tumor-associated microenvironment, TNF-α can be produced by a wide variety of malignant cells, including OS cells.6 Li et al reported that TNF-α may enhance the adaptive immune resistance mediated by IFN-γ-induced B7-H1 in hepatocellular carcinoma cells.7 TNF-α expression in ovarian carcinoma varies by histologic subtype and provides some support for the role of inflammation in ovarian carcinogenesis.8 Besides, TNF-α also promoted OS progression by maintaining tumor cells in an undifferentiated state.9 Activation of TNF-α signal enhanced CRL4B E3 ligase activity and regulates cell cycle progression in human OS cells.10 TNF-α regulated IL-34 expression in osteosarcoma cells and contributes to OS growth by increasing the neo-angiogenesis and the recruitment of M2 macrophage.11 Thus, the regulator of TNF-α is essential for controlling its inflammation imbalance during cancer therapy of OS.
Long noncoding RNAs (lncRNAs) are encoded by a vast less explored region of the human genome and its disruption is intrinsically linked to diverse diseases including cell growth, inflammation and tumor metastasis.12 lncRNA THRIL (TNF-α and hnRNPL related immune-regulatory lincRNA) is essential for the induction of TNF-α expression. Knockdown of THRIL caused dysregulation of TNF-α during innate activation of THP1 macrophages and was correlated with the severity of symptoms in patients with Kawasaki disease, an acute inflammatory disease of childhood.13 Upregulated THRIL was demonstrated to aggravate lipopolysaccharide (LPS)-induced osteoarthritis cell injury model via activating JAK1/STAT3 and NF-κB pathways.14 Currently, the function of THRIL/TNF-α signal in cancer is unclear. One of the most prominent features of cancer is hypoxia, which was found to increase the THRIL expression.15 Therefore, considering the tumorigenic role of TNF-α in OS, the function and its clinical significance of THRIL/TNF-α signal needed to be investigated in OS patients.
In this study, we analyzed the expression pattern of THRIL/TNF-α in OS tissues and serum and further revealed its diagnostic and prognostic value. We found that THRIL and TNF-α were co-upregulated in OS patients and predicted poor clinical outcomes. Knockdown of THRIL was performed in vitro and in vivo to confirm its role in OS growth and metastasis.
Materials and Methods
Ethics and Sample Collection
All methods used for this study were approved by the Ethics Committee of Gongli Hospital of Pudong New Area, and this study was conducted in accordance with the Declaration of Helsinki. The OS tissues and serum samples (n=83), nontumor tissues (n=37) and serum from healthy subjects were obtained from patients who had undergone surgical resection of OS in the Gongli Hospital of Pudong New Area and other hospitals in Acknowledgments. We confirmed that all patients provided written informed consent. The median age was 22 years and the median of tumor size was 8 cm, and 22 in 83 patients were found with lung metastases. The tumor stages were confirmed according to the Enneking staging system (ESS). The tissues or serum samples were isolated from surgical removal or patients’ blood and then stored at −80°C until use. All the patients were followed-up until 20 April 2019. The minimum follow-up time is 3 months and the maximum follow-up time is 58 months (median: 26 months). The high or low expressions of THRIL/TNF-α were defined by the median of its expression.
RNA Extraction and RT-Q-PCR
Total RNA was extracted from clinical specimens or cells via the TRIzol reagent (Invitrogen, USA), A260/A280 nm >1.8 was qualified for quantitative analysis. Then, RNAs were transcribed into cDNA using superscriptase II (Invitrogen, USA) according to the manufacturer’s instructions. The cDNA was subjected to real time-polymerase chain reaction using Power SYBR® Green PCR Master Mix (Invitrogen, USA). The following primers were used: THRIL-F: GAGTGCAGTGGCGTGATCTC, R: AAAATTAGTCAGGCATGGTGGTG; TNF-α-F: CCTCTCTCTAATCAGCCCTCTG; R: GAGGACCTGGGAGTAGATGAG; Bcl-2-F: GGTGGGGTCATGTGTGTGG, R: CGGTTCAGGTACTCAGTCATCC; E-cadherin-F: CGAGAGCTACACGTTCACGG, R: GGGTGTCGAGGGAAAAATAGG; Vimentin-F: GACGCCATCAACACCGAGTT, R: CTTTGTCGTTGGTTAGCTGGT. The expression levels of genes were estimated via 2−ΔΔCt method and were normalized to GAPDH for gene expression. Each sample was measured in triplicate.
ELISA
The serum levels of TNF-α were qualified by Human TNF-α ELISA kit (Cat#:EHC103a) according to the manufacturer’s instructions. The detection sensitivity was 7.8 pg/mL. All samples were tested by duplication, and absorbency was measured at 620 nm by a microplate reader.
Cell Culture and Reagents
Human OS cell lines MG63 and Saos2 were obtained from the American Type Culture Collection (ATCC) and cultured in DMEM supplement with 10% FBS (GIBCO) and 1% penicillin/streptomycin. All the cells were cultured at 37°C water-saturated 5% CO2 atmosphere. siRNA-THRIL or negative control was conducted by RiboBio (Guangzhou, china), siRNA-THRIL-1: 5ʹ- GCTCTATGCACAGATAAATTT -3ʹ; siRNA-THRIL-2: 5ʹ- GCTCAGTCGTGTTTATTAATT -3ʹ; siRNA-TNFR1: 5ʹ-CAAAGGAACCUACUUGUACUU-3ʹ. Human recombinant TNF-α was purchased from Preoteintech (#HZ-1014, WUHAN, China). The antibody to E-cadherin and Vimentin were obtained from Abcam (USA).
Estimation of Cell Growth
MG63 and Saos2 cells were transfected of siRNA-THRIL with/without the treatment of TNF-α (50 ng/mL) for 48 h, the cell vitality was determined by CCK-8. Briefly, cells were harvested and washed with PBS, and then cell counting kit-8 (Kumamoto, Japan) mixed with DMEM was used for cell viability assay according to the manufacturer’s instructions., and the absorbance was measured at 450 nm by a microplate reader. In addition, apoptosis was determined by flow cytometry. Briefly, cells were stained with 2 μL of annexin V mixed with 2 μL of propidium iodide (PI, eBioscience) were used according to the manufacturer’s instructions and were analyzed using a flow cytometry.
Transwell Assay
To determine the role of THRIL in invasion of OS cells, 2×104 cells after transfection were suspended with 200 µL of serum-free medium. The Corning Polycarbonate Membrane Insert transwell chambers (Product #3422, Corning Costar Corp, Cambridge, MA, USA) were used to perform invasion assays. The Transwell membranes were pre-coated with Matrigel (BD Biosciences, USA) at 37°C for at least 2 hrs to form a reconstructed basement membrane. After incubation, cells at the top chamber were removed with cotton swabs, and those on the lower surface were fixed with methanol, stained by crystal violet and counted under an inverted microscope.
Immunofluorescence Assays
MG63 and Saos2 cells with THRIL knockdown were cultured in a plastic chamber on microscope glass slides (Millicell EZ Slide, Millipore, Billerica, MA, USA) with TNF-α (50 ng/mL) stimulation for 48 hrs. The cells were then washed in PBS, fixed in 4% paraformaldehyde for 10 mins at room temperature, permeabilized with triton X-100 0.1% for 20 mins. The cells were incubated with primary antibody against E-cadherin and Vimentin for 120 mins. After washings, Alexa Fluor 488/594 secondary antibody was added for 90 mins at room temperature. Nuclei were stained with DAPI, which was observed under an FV10i confocal microscope (OLYMPUS, Tokyo, Japan).
Tumor Model
To investigate the role of THRIL in the OS development, the xenograft model of human MG63 cells was established, MG63 cells were transfected with lentivirus vector of siRNA-THRIL, or negative control, 2×106 conditional MG63 cells were subcutaneously injected in rear flank of nude mice (6 per group), TNF-α (500 ng/mice) was treated i.p. twice a week for 3 weeks. The tumor sizes were measured 3 days apart and the tumor volumes were calculated: V (cm3) = width2 (cm2) * length (cm)/2. The animal study was approved by institutional animal research committee of Gongli Hospital of Pudong New Area and that animals were cared for following the guidelines for use and care of laboratory animals.
Statistical Analyses
The results are analyzed by the Statistical Package for Social Sciences version 16.0 (SPSS 16.0, SPSS Inc., Chicago, IL, USA) and the Prism statistical software package (Version 5.0, Graphpad Software Inc.). Kolmogorov–Smirnov and Shapiro–Wilk tests showed that the expression of THRIL/TNF-α did not follow a normal distribution. The Mann–Whitney U-test was used to compare the two groups (e.g., normal versus OS) and the differences between more than two groups were analyzed by the Kruskal–Wallis test. The receiver operating characteristic (ROC) curve was used to analyze its diagnostic value. Correlations between the expression of THRIL/TNF-α were analyzed by Spearman’s Rho analysis. Correlations between THRIL/TNF-α and clinicopathological characters were analyzed by Pearson Chi-square. Kaplan–Meier survival curves and the log-rank statistic were used to analyze the prognostic significance of THRIL/TNF-α. Cox proportional hazard regression was used for univariate and multivariate analysis of overall survival according to THRIL/TNF-α expression. P<0.05 was considered statistically significant. All experiments were performed at least 3 times.
Results
THRIL Is Upregulated in OS and Positively Correlates with TNF-α
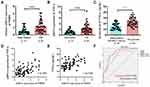
We first determined the expression of THRIL and TNF-α during the development of OS. THRIL and TNF-α were found to be upregulated in OS tissues (n=83) when compared to which in nontumor tissues (n=37) (Figure 1A and B). The enhanced TNF-α level was confirmed in the serum derived from OS patients (n=83) when compared with independent healthy subjects (n=40) (Figure 1C). Since THRIL could regulate TNF-α expression, we analyzed the correlation between THRIL and TNF-α. The results showed that THRIL is positively associated with TNF-α in OS tissues and serum (Figure 1D and E), suggesting that THRIL/TNF-α signal participated in the OS development.
The receiver operating characteristic (ROC) curve suggests that THRIL (AUC=0.773) and TNF-α (AUC=0.794) are good diagnostic markers to discriminate OS patients from healthy people, but combined with two factors showed more predominant diagnostic value (AUC=0.852) (Figure 1F).
Elevated Expression of THRIL and TNF-α Occurs in OS Patients with Advanced Tumor Progression
Considering the upregulated THRIL/TNF-α signal in OS samples, we next determined the clinical significance of THRIL/TNF-α in OS. The expression of THRIL/TNF-α was divided into High and Low groups by median. The results showed that patients with larger tumor size, advanced Enneking stage and distant metastasis have higher THRIL levels in OS tissues. However, THRIL showed no relationships with gender, age, anatomic site and histology grade. Elevated TNF-α level was found in patients with high histology grade, which was consistent with the previous findings that TNF-α promoted OS progression by maintaining tumor cells in an undifferentiated state (Table 1).
 |
Table 1 Correlations Between the Expressions of THRIL and TNF-α with Clinicalpathologic Characteristics in OS Patients |
When the combination of THRIL and TNF-α was used for analysis, we found that the patients whose simultaneously high-expressed THRIL and TNF-α (THRILHigh/TNF-αHigh) showed more advanced OS progression with a higher incidence of lung metastasis than patients with low-expressed THRIL and TNF-α (THRILLow/TNF-αLow) (Table 2).
 |
Table 2 Clinical Significance of Simultaneously High-Expressed or Low-Expressed THRIL and TNF-α in OS Patients |
High THRIL and TNF-α Levels Predict Short Overall Survival and Metastasis-Free Time
The prognostic role of THRIL/TNF-α signal was also analyzed. The results showed that high expression of THRIL predicted shorter overall survival time (P=0.024) and metastasis-free survival (MFS) (P=0.033) (Figure 2A and B), but TNF-α showed no impacts on overall survival time and metastasis-free time of OS patients (Figure 2C and D).
We further analyzed the survival time of patients with THRILHigh/TNF-αHigh, the results indicated that, compared with THRIL alone, combination of THRIL and TNF-α was more efficient to predict the decreased survival time of OS patients (Figure 2E and F).
THRIL Is an Independently Prognostic Factor for OS Patients
We further performed univariate and multivariate analyses, the results found that gender, age, anatomic site, Enneking stage, histologic grade and TNF-α were not independent prognostic indicators for overall survival time. In addition, the tumor size, lung metastasis and the expression of THRIL were independent prognostic factors for the overall survival time of patients with OS (Table 3). However, THRIL was not an independent prognostic factor for metastasis-free survival of patients with OS (Table 4).
 |
Table 3 Prognostic Factors in the Cox Proportional Hazard Model for Overall Survival |
 |
Table 4 Prognostic Factors in the Cox Proportional Hazard Model for Metastasis-Free Survival |
Knockdown of THRIL Inhibits OS Growth and Invasion via TNF-α in vitro and in vivo
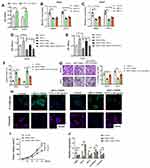
The function of TNF-α alone was investigated and found that the treatment of TNF-α (50 ng/mL) elevated the cell vitality of OS cells, which was consistent with previous reports16 (Figure 3A). Then, we performed knockdown of THRIL in two OS cell lines MG63 and Saos2 to confirm its pro-tumor function. The results indicated that knockdown of THRIL inhibited the expression of TNF-α by two siRNA. SiRNA-1 was selected for further study (Figure 3B and C). To confirm the role of TNF-α signal for THRIL function, we inhibited TNF receptor (TNFR) in OS cells in response to TNF-α treatment and THRIL knockdown. The results demonstrated that the OS cells with THRIL knockdown showed impaired cell vitality, but the treatment of TNF-α (50 ng/mL) to the OS cells with THRIL knockdown could restore its cell vitality, which was dependent on TNFR signals. Inhibition of TNFR by siRNA suppressed the function of TNF-α in the OS cells with THRIL knockdown. Thus, the function of THRIL is dependent on TNF-α/TNFR signals (Figure 3D and E).
Thus, we further investigated the role of THRIL/TNF-α signal in cell apoptosis and metastasis of two OS cell lines. Inhibition of THRIL induced apoptosis in 48 h, which could be restored by the treatment of TNF-α (50 ng/mL), indicating that THRIL promoted OS growth via TNF-α (Figure 3F). The ability of invasion was also determined by Transwell assay, we found that knockdown of THRIL inhibited OS invasion but the addition of TNF-α abrogated this suppression and upregulated the ability of invasion (Figure 3G). This process might be attributed to changed EMT phenotype of OS cells. Inhibition of THRIL downregulated the expression of vimentin but TNF-α (50 ng/mL) could restore the levels of vimentin in MG63 and Saos2 cells (Figure 3H).
We also investigated the role of THRIL in vivo. The results showed that the mice with THRIL knockdown by lentivirus have smaller tumor volume than that with a negative control. However, treatment of TNF-α (500 ng/mice) could restore the antitumor function of THRIL knockdown (Figure 3I). In line with this, the antiapoptosis gene Bcl-2 and Vimentin were downregulated by THRIL knockdown, but were recovered by the treatment of TNF-α in vivo (Figure 3J).
Discussion
Continuous exposure to inflammatory cytokines (such as TNF-α, IL-6, IL-8 and IL-1β, etc.) is known to cause tumorigenesis.4 TNF-α can be produced by leukemia cells to mediate cell survival and higher TNF-α concentrations in leukemia patients were correlated with heavier fatigue and poorer quality of life.17 TNF-α also promoted OS progression by maintaining tumor cells in an undifferentiated state.9 Many lncRNAs have been reported to regulate and control the balance of inflammation during several diseases. Li et al reported that THRIL bound specifically to heterogenous nuclear ribonucleoprotein L (hnRNPL) and formed a functional THRIL–hnRNPL complex that regulated transcription of the TNF-α gene by binding to its promoter. Transcriptome analysis revealed that THRIL was required for expression of many immune-response genes, including TNF-α13 We here identified the positive regulatory role of THRIL to TNF-α in OS cells. Knockdown of THRIL inhibited TNF-α expression and TNF-α-induced OS cell growth. Similarly, NF-κB-interacting lncRNA (NKILA) was highly induced in response to IL-1β and TNF-α stimulation.18 lncRNA Mirt2 functioned as a checkpoint to prevent aberrant activation of inflammation and was a potential regulator of macrophage polarization via attenuating Lys63 (K63)-linked ubiquitination of TRAF6, thus inhibiting activation of NF-κB and MAPK pathways.19 lnc-IL7R was capable of diminishing the LPS-induced inflammatory response via repressing LPS-induced E-selectin, VCAM-1, IL-6, and IL-8.20 However, the inflammatory lncRNAs in during the development of OS remains unclear. TGF-β or TNF-α treatment could enhance the expression of oncogenic lncRNA UCA1 for cell growth, migration and invasion of OS cells.21 We also found that TNF-α-induced EMT phenotype could be restored by THRIL knockdown via upegulation of E-cadherin and downregulation of Vimentin. Thus, controlling chronic inflammation is crucial to prevent tumor progression.
Currently, the researches on the role of THRIL focused on inflammation-related diseases. Upregulated THRIL was found to predict increased risk of acute respiratory distress syndrome and positively correlates with disease severity, inflammation, and mortality in sepsis patients.22 In this study, we found that lncRNA THRIL upregulated TNF-α in tumor tissue and serum of OS patients, which could be efficient diagnostic and prognostic biomarker. THRIL was also positively associated with CRP, PCT, TNF-α, and IL-1β levels in sepsis patients.22 In Kawasaki disease, an acute inflammatory disease of childhood, THRIL was correlated with the severity of symptoms of patients.13 In hypoxia-induced injuries, THRIL inhibition represents a protective effect against hypoxia-induced injuries in H9C2 cells.22 However, THRIL RNA expression was significantly decreased in autism spectrum disorder (ASD) children, although TNF-α, IL-1β and IL-17 were significantly increased in ASD children.23 THRIL was significantly decreased in patients with type 2 diabetes mellitus compared to control subjects, which were positively correlated with poor glycemic control, insulin resistance.24 But the investigation of THRIL in cancer is limited. We found that the expression of THRIL was increased in OS patients. High-expressed THRIL was positively associated with advanced OS progression including tumor size, clinical stage and lung metastasis. Patients with upregulated THRIL have shorter overall survival time and metastasis-free time. Esfandi et al investigated the role of THRIL in lung cancer, but the expression of THRIL was not changed in lung cancer samples and their corresponding adjacent noncancerous tissues.25 Similar results were also reported in bladder cancer.26 Interestingly, Wang et al found a significantly decreased risk of precancerous cervical lesions for the THRIL rs7133268 AG genotype (odds ratio adjusted=0.63).27 These findings suggested the content-dependent role of THRIL during disease progression.
The function of THRIL was related to its regulatory role of TNF-α. We here confirmed that THRIL was positively associated with upregulated TNF-α in OS tissues and serum. Combination of THRIL and TNF-α showed prominent diagnostic value for OS patients. However, TNF-α exerts diverse functions in cancer, other than the role of promoting tumor death. For example, TNF-α could promote cell growth, migration and invasion of MCF-7 estrogen receptor (ER)-positive and MDA-MB-231 (triple-negative) breast cancer cell lines, partially by inducing the expression of MMPs.28 Consistently, we here found that knockdown of THRIL in vivo could inhibit cell growth and invasion, which was abrogated by the treatment of TNF-α. The clinical analysis also showed that patients with high expression of TNF-α have high histology grade and THRILHigh/TNF-αHigh predicted worst overall survival and metastasis-free survival of OS patients.
In sum, we here investigated the clinical role and function of upregulated THRIL/TNF-α signal in OS and found that patients with THRILHigh/TNF-αHigh showed poor clinical outcomes and short metastasis-free survival time, which was involved with THRIL/TNF-α signal-supported cell growth and EMT phenotype for OS progression.
Acknowledgment
We would like to thank Shanghai Changzheng Hospital, Shanghai Ninth People’s Hospital and Shanghai Ruijin Hospital for their precious OS samples.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Toki S, Kobayashi E, Yoshida A, et al. A clinical comparison between dedifferentiated low-grade osteosarcoma and conventional osteosarcoma. Bone Joint J. 2019;101-B(6):745–752. doi:10.1302/0301-620X.101B6.BJJ-2018-1207.R1
2. Ahmed G, Zamzam M, Kamel A, et al. Effect of timing of pulmonary metastasis occurrence on the outcome of metastasectomy in osteosarcoma patients. Journal of Pediatric Surgery. 2019;54(4):775–779. doi:10.1016/j.jpedsurg.2018.06.019
3. Kumar R, Kumar M, Malhotra K, Patel S. Primary osteosarcoma in the elderly revisited: current concepts in diagnosis and treatment. Curr Oncol Rep. 2018;20(2):13. doi:10.1007/s11912-018-0658-1
4. Capece D, Verzella D, Tessitore A, Alesse E, Capalbo C, Zazzeroni F. Cancer secretome and inflammation: the bright and the dark sides of NF-kappaB. Semin Cell Dev Biol. 2018;78:51–61. doi:10.1016/j.semcdb.2017.08.004
5. Salomon BL, Leclerc M, Tosello J, Ronin E, Piaggio E, Cohen JL. Tumor necrosis factor alpha and regulatory T cells in oncoimmunology. Front Immunol. 2018;9:444. doi:10.3389/fimmu.2018.00444
6. Ciebiera M, Wlodarczyk M, Zgliczynska M, et al. The role of tumor necrosis factor alpha in the biology of uterine fibroids and the related symptoms. Int J Mol Sci. 2018;19:12. doi:10.3390/ijms19123869
7. Li N, Wang J, Zhang N, et al. Cross-talk between TNF-alpha and IFN-gamma signaling in induction of B7-H1 expression in hepatocellular carcinoma cells. Cancer Immunol Immunother. 2018;67(2):271–283. doi:10.1007/s00262-017-2086-8
8. Gupta M, Babic A, Beck AH, Terry K. TNF-alpha expression, risk factors, and inflammatory exposures in ovarian cancer: evidence for an inflammatory pathway of ovarian carcinogenesis?. Human pathol. 2016;54:82–91.
9. Mori T, Sato Y, Miyamoto K, et al. TNFalpha promotes osteosarcoma progression by maintaining tumor cells in an undifferentiated state. Oncogene. 2014;33(33):4236–4241. doi:10.1038/onc.2013.545
10. Zhang C, Chen B, Jiang K, Lao L, Shen H, Chen Z. Activation of TNF-alpha/NF-kappaB axis enhances CRL4B(DCAF)(11) E3 ligase activity and regulates cell cycle progression in human osteosarcoma cells. Mol Oncol. 2018;12(4):476–494. doi:10.1002/1878-0261.12176
11. Segaliny AI, Mohamadi A, Dizier B, et al. Interleukin-34 promotes tumor progression and metastatic process in osteosarcoma through induction of angiogenesis and macrophage recruitment. Int J Cancer 2015;137(1):73–85. doi:10.1002/ijc.29376
12. Lin C, Yang L. Long noncoding RNA in cancer: wiring signaling circuitry. Trends Cell Biol. 2018;28(4):287–301. doi:10.1016/j.tcb.2017.11.008
13. Li Z, Chao TC, Chang KY, et al. The long noncoding RNA THRIL regulates TNFalpha expression through its interaction with hnRNPL. Proc National Acad Sci USA. 2014;111(3):1002–1007. doi:10.1073/pnas.1313768111
14. Liu G, Wang Y, Zhang M, Zhang Q. Long non-coding RNA THRIL promotes LPS-induced inflammatory injury by down-regulating microRNA-125b in ATDC5 cells. Int Immunopharmacol. 2019;66:354–361. doi:10.1016/j.intimp.2018.11.038
15. Xia J, Jiang N, Li Y, Wei Y, Zhang X. The long noncoding RNA THRIL knockdown protects hypoxia-induced injuries of H9C2 cells through regulating miR-99a. Cardiol J. 2018;26:564–574.
16. Tan W, Luo X, Li W, et al. TNF-alpha is a potential therapeutic target to overcome sorafenib resistance in hepatocellular carcinoma. EBio Med. 2019;40:446–456. doi:10.1016/j.ebiom.2018.12.047
17. Zhou X, Li Z, Zhou J. Tumor necrosis factor alpha in the onset and progression of leukemia. Exp Hematol. 2017;45:17–26. doi:10.1016/j.exphem.2016.10.005
18. Lu Z, Chen Z, Li Y, et al. TGF-beta-induced NKILA inhibits ESCC cell migration and invasion through NF-kappaB/MMP14 signaling. J Mol Med (Berl). 2018;96(3–4):301–313. doi:10.1007/s00109-018-1621-1
19. Du M, Yuan L, Tan X, et al. The LPS-inducible lncRNA Mirt2 is a negative regulator of inflammation. Nature Commun. 2017;8(1):2049. doi:10.1038/s41467-017-02229-1
20. Cui H, Xie N, Tan Z, et al. The human long noncoding RNA lnc-IL7R regulates the inflammatory response. Eur J Immunol. 2014;44(7):2085–2095. doi:10.1002/eji.201344126
21. Li Q, Xing W, Gong X, Wang Y. Long non-coding RNA urothelial carcinoma associated 1 promotes proliferation, migration and invasion of osteosarcoma cells by regulating microRNA-182. Cell Physiol Biochem. 2018;51(3):1149–1163. doi:10.1159/000495493
22. Wang Y, Fu X, Yu B, Ai F. Long non-coding RNA THRIL predicts increased acute respiratory distress syndrome risk and positively correlates with disease severity, inflammation, and mortality in sepsis patients. J Clin Lab Anal. 2019;e22882.
23. Xie J, Huang L, Li X, et al. Immunological cytokine profiling identifies TNF-alpha as a key molecule dysregulated in autistic children. Oncotarget. 2017;8(47):82390–82398. doi:10.18632/oncotarget.19326
24. Sathishkumar C, Prabu P, Mohan V, Balasubramanyam M. Linking a role of lncRNAs (long non-coding RNAs) with insulin resistance, accelerated senescence, and inflammation in patients with type 2 diabetes. Human Genomics. 2018;12(1):41. doi:10.1186/s40246-018-0173-3
25. Esfandi F, Taheri M, Omrani MD, et al. Expression of long non-coding RNAs (lncRNAs) has been dysregulated in non-small cell lung cancer tissues. BMC Cancer. 2019;19(1):222. doi:10.1186/s12885-019-5435-5
26. Abdolmaleki F, Ghafoui-Fard S, Taheri M, et al. Expression analysis of a panel of long non-coding RNAs (lncRNAs) revealed their potential as diagnostic biomarkers in bladder cancer. Genomics. 2019. doi:10.1016/j.ygeno.2019.04.020
27. Wang Y, Liu Y, Li Z, et al. Association between MALAT1 and THRIL polymorphisms and precancerous cervical lesions. Genetic Testing Mol Biomarkers. 2018;22(9):509–517. doi:10.1089/gtmb.2018.0097
28. Wolczyk D, Zaremba-Czogalla M, Hryniewicz-Jankowska A, et al. TNF-alpha promotes breast cancer cell migration and enhances the concentration of membrane-associated proteases in lipid rafts. Cell Oncol (Dordr). 2016;39(4):353–363. doi:10.1007/s13402-016-0280-x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.