Back to Journals » International Medical Case Reports Journal » Volume 9
Upper eyelid granuloma: a rare delayed-onset complication secondary to cosmetic filler injection on forehead
Authors Pao S, Lin S, Chang Y, Chiang S
Received 29 September 2015
Accepted for publication 19 February 2016
Published 23 June 2016 Volume 2016:9 Pages 155—157
DOI https://doi.org/10.2147/IMCRJ.S97356
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Shu-I Pao,1 Shih-Min Lin,1,2 Yun-Hsiang Chang,1 Shang-Yi Chiang,1
1Department of Ophthalmology, Tri-Service General Hospital, National Defense Medical Center, Taipei, 2Department of Ophthalmology, Kaohsiung Armed Forces General Hospital, Kaohsiung
Abstract: A 72-year-old Taiwanese woman had the history of cosmetic cryopreserved autologous fat injection on her forehead ~21 years ago and was referred to our oculoplastic clinic and presented with multiple painless mass, which she had for 4 years, on both upper eyelids. Histopathology confirmed the diagnosis of foreign body granuloma on both upper eyelids. Clinicians should be aware of the potential complication of granuloma reaction and migration even many years after the injection. We advise that autologous fat injection should be performed solely by trained physicians and it should be made known that there is a possible occurrence of migration.
Keywords: upper eyelid granuloma, cosmetic filler, excisional biopsy
Introduction
We present a case of foreign body granuloma on the eyelids secondary to cosmetic filler injection on the forehead, which developed several years after the injection. Filler migration and the clinical significance and microscopic features of granuloma lesions are also discussed. Written informed consent was obtained from the patient and this study was approved by the Tri-Service General Hospital review board (affiliated to the National Defense Medical Center).
Case report
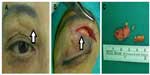
A 72-year-old Taiwanese woman was referred to our oculoplastic clinic, with swelling on both upper eyelids, with multiple painless nonmovable palpable masses, without blepharoptosis, which she had for 4 years. The best-corrected visual acuity of both eyes was 6/8.6, and intraocular pressure was normal. She had cosmetic cryopreserved autologous fat injection on her forehead ~21 years ago. She did not receive the injection around both eyelids, and there were no other specific problems after the injections. The masses were found below the eyebrow border, sized ~2.5 and ~2.0 cm in diameter in the right and left eyes, respectively, with well-defined, irregular-shaped borders firm on palpation. There was no sign of acute inflammation of the overlying skin (Figure 1A). Excisional biopsy was performed 1 week after the first visit. The excised mass was yellowish with an irregular border and had a rubbery consistency (indicated by arrows in Figure 1A and B). The size of the mass detected in the subcutaneous region in the right eye was 2 cm × 1 cm (Figure 1B and C). Microscopic examination showed that foreign body granuloma was composed of multiple nodules characterized by basophilic amorphous materials surrounded by an irregular fibrous border (Figure 2A; hematoxylin and eosin [H&E], ×100). The fibrous border was composed of histiocytes, multinucleated giant cells, and fibroblasts (Figure 2B; H&E, ×400). After 1 year postoperative follow-up, the patient recovered well without obvious disfigurement and had only slight scar from the surgery.
Discussion
Eyelid problems range from benign, self-resolving processes to malignant, possibly metastatic, tumors. In the past few decades, more and more people have been resorting to facial enhancement with dermal fillers for cosmetic purposes. Clinically, dermal fillers are composed of autologous fat, bovine collagen, paraffin, fluid silicone, polytetrafluoroethylene (Teflon; DuPont, Wilmington, DE, USA), and polymer and silicone particles. These fillers have been used to correct soft tissue defects as well as to fill in soft tissue volume around the lips and nasolabial region. For this reason, dermal fillers should have properties of biocompatibility, safety, and stability at the implant site and the abilities to maintain their volume, remain pliable, induce minimal foreign body reactions, and not cause foreign body granuloma.1 Autologous fat is a nonallergenic, well-tolerated, supple, versatile implant material. Autogenous fat injection (AFI) into the periorbital or midface region is a common type of cosmetic surgery for rejuvenation in middle-aged and elderly Western subjects.2 Common complications of facial AFI include unrealistic patient expectations, bruising, hematoma, undercorrection, overcorrection, and asymmetry contour of injection site.3 Often these adverse reactions result from improper use of products performed by unskilled (or unlicensed) practitioners.4 The complication of lipogranuloma formation at the periorbital area away from the AFI site is rarely reported.5 It is plausible that the injected fat tissue on the forehead could move down to the periorbital area away from the injection site because of the movement of the frontalis muscle and gravity, as in the case of our patient. Furthermore, the fat tissue harvested for AFI is usually stored frozen at −20°C, but during such cryopreservation, ice crystals form inside the fat cell and many fat cells lose viability. Also, if the fat tissue without viability is injected, it can increase the risk for inflammation by foreign body reaction.5 The patient in our report was confirmed to have used the cryopreserved AFI for the second time, and we consider this to be the cause of the lipogranuloma.
Conclusion
In conclusion, there is evidence present to show that potential complications after tissue injection of autologous fat still exist. Immediate injection of harvested fat tissue can be helpful for prevention of lipogranuloma. Also, we advise that AFI should be performed solely by trained physicians. As we treat similar cases, we should also be concerned with a possible occurrence of migration. In our study, the patient had palpable and visible masses near the upper eyelid that resulted from AFI on the forehead 21 years ago. Therefore, tracking patient’s detailed history and performing a thorough physical examination are necessarily important. If any unknown mass is detected, excisional biopsy should be immediately conducted by experienced clinicians for diagnosis to avoid severe facial disfigurement afterward.
Disclosure
The authors report no financial or conflicts of interest in this work.
References
Sankar V, McGuff HS. Foreign body reaction to calcium hydroxylapatite after lip augmentation. JADA. 2007;138(8):1093–1096. | |
Paik JS, Cho WK, Park GS, Yang SW. Eyelid-associated complications after autogenous fat injection for cosmetic forehead augmentation. BMC Ophthalmol. 2013;13:32. | |
Niamtu J 3rd. Complications in fillers and botox. Oral Maxillofac Surg Clin North Am. 2009;21:13–21. | |
Schwartzfarb EM, Hametti JM, Romanelli P, Ricotti C. Foreign body granuloma formation secondary to silicone injection. Dermatol Online J. 2008;14(7):20. | |
Ryeung Park Y, Choi JA, Yoon La T. Periorbital lipogranuloma after cryopreserved autologous fat injection at forehead. Can J Ophthalmol. 2013;48:e166–e168. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.