Back to Journals » Cancer Management and Research » Volume 11
Upfront whole brain radiotherapy for multiple brain metastases in patients with EGFR-mutant lung adenocarcinoma
Authors Li C, Guo J , Zhao L, Hu F, Nie W, Wang H, Zheng X, Shen Y, Gu P, Zhang Y, Zhang X
Received 3 December 2018
Accepted for publication 15 February 2019
Published 23 April 2019 Volume 2019:11 Pages 3433—3443
DOI https://doi.org/10.2147/CMAR.S196881
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Changhui Li,1 Jindong Guo,2 Lei Zhao,2 Fang Hu,1 Wei Nie,1 Huimin Wang,1 Xiaoxuan Zheng,1 Yinchen Shen,1 Ping Gu,1 Yujun Zhang,1 Xueyan Zhang1
1Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai 200030, People’s Republic of China; 2Department of Radiation Oncology, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai 200030, People’s Republic of China
Purpose: This study aimed to evaluate the efficacy of upfront whole-brain radiotherapy (WBRT) in EGFR-mutant lung adenocarcinoma patients with multiple brain metastases (BM).
Methods: In this study, 195 patients with EGFR mutations who had multiple BM at preliminary diagnosis were included and retrospectively reviewed. Patients were admitted to receive the following treatments in a multi-disciplinary setting: upfront WBRT followed by EGFR-TKI, concurrent EGFR-TKI and WBRT and upfront EGFR-TKI followed by WBRT. A disease-specific graded prognostic assessment (DS-GPA) was performed for all the patients. The treatment response and overall survival (OS) were assessed as well.
Results: The median OS of these patients was 27 months. Objective response rate (ORR) was significantly better in upfront WBRT group than other two groups (P=0.004). Moreover, patients who received upfront WBRT (n=67) had longer OS than the concomitant group (36 vs 25 months; P=0.006) and the upfront EGFR-TKI group (36 vs 25 months; P<0.0001). The prognosis of patients with different DS-GPA scores significantly differed (P<0.0001). In concomitant group and upfront EGFR-TKIs group, patients with higher DS-GPA scores of 2–3 had more favorable prognosis compared with those with lower DS-GPA scores of 0–1.5 (27 vs 25 months; P=0.023). Patients who received EGFR-TKIs concurrently with WBRT had longer OS than those received upfront EGFR-TKIs with high DS-GPA scores. (37 vs 17 months; P=0.023).
Conclusion: The use of upfront WBRT for EGFR-mutated lung adenocarcinoma patients with multiple BM can improve ORR and OS. More importantly, patients with high DS-GPA scores are recommended to receive WBRT immediately after EGFR-TKIs therapy.
Keywords: non-small cell lung cancer, brain metastases, EGFR, tyrosine kinase inhibitors, whole brain radiotherapy
Plain language summary
In developing countries, especially in China, the first-generation EGFR-TKIs and WBRT have remained the main treatments in brain metastasis (BM) patients with EGFR mutations. Some studies have shown that the treatment of WBRT plus EGFR-TKIs resulted in a higher response rate of BM. However, the effective sequence between WBRT and EGFR-TKIs has remained unclear. Our study suggested that the ORR was significantly improved and a significantly longer OS was achieved in the WBRT first group. Additionally, multiple BM patients with high DS-GPA scores should be immediately treated with WBRT after taking EGFR-TKIs.
Introduction
Non-small cell lung cancer (NSCLC) is a major type of lung cancer has associating with a high risk of brain metastasis (BM). Some studies have reported that 57% of new NSCLC patients have advanced metastases, and 20% of them have brain metastases.1,2 The patients with EGFR-mutant NSCLC showed higher diagnosis rates with BM. The median overall survival (OS) time of patients without treatment is 3–6 months or even less.3,4 Current treatment options for brain metastases include surgery, radiotherapy, or in combination with other strategies such as molecular targeted therapy and chemotherapy.
Cranial radiotherapy plays a critical role in patients with BM from NSCLC, and whole brain radiotherapy (WBRT) is a primary treatment modality for patients with multiple brain lesions.5 However, long-term results of WBRT and stereotactic radiosurgery (SRS) have been disappointing due to the limitations of radiotherapy, such as failing to improve OS, and enhancing the risk of a decline in learning, as well as memory function.6,7 EGFR tyrosine kinase inhibitor (EGFR-TKI) is an effective first-line treatment for lung adenocarcinoma, particularly those harboring EGFR sensitive mutations.8 However, due to the tight junctions between brain endothelial cells from the brain-blood barrier (BBB), it is limited that the first and second generation of EGFR-TKIs to permeate into the cerebrospinal fluid (CSF).9
Numerous studies have demonstrated that WBRT plus EGFR-TKIs led to more feasible and promising results than a single administration of EGFR-TKIs or WBRT.10–12 However, the effectiveness of the treatment strategy remains unclear for the management of BM. Hence, a retrospective analysis was performed to investigate whether there are any differential treatment outcomes among upfront WBRT followed by EGFR-TKIs, concurrent EGFR-TKIs and WBRT, and upfront EGFR-TKIs followed by WBRT.
Patients and methods
We screened patients who diagnosed with stage IV lung adenocarcinoma between June 1, 2012 and June 1, 2016 at Shanghai Chest Hospital (Shanghai, China). A total of 195 patients who met the eligibility criteria were included and retrospectively analyzed.
Eligibility criteria were as follows: (1) patients with stage IV lung cancer with BM at initial diagnosis; (2) histologically or cytologically proven adenocarcinoma and patients with EGFR sensitive mutations; (3) measurable BM identified by magnetic resonance imaging (MRI) or computed tomography (CT) of brain; (4) with multiple brain lesions (transferred to brain and >3 lesions); (5) underwent only WBRT (WBRT for more than three brain lesions in our hospital). Newly diagnosed patients with multiple BM and EGFR TKI-naive remained the basic requirements. All three groups of patients in our study received WBRT and EGFR-TKIs before intracranial progression. The exclusion criteria were as follows: patients had negative-EGFR-TKIs mutations or without EGFR mutation; patients who previously received EGFR-TKIs, especially Osimertinib during the treatment, and failed to receive EGFR-TKIs after WBRT or underwent surgical resection during initial BM. Patients who were not eligible to receive radiotherapy after the failure of EGFR-TKIs for intracranial progression or lost the follow-up for 6 months were excluded as well. All patients completed clinical evaluation as well.
Study design
Patients’ medical records and follow-up data were collected for their accurate clinical and survival information. The detailed data included age, sex, smoking history, symptomatic BM, EGFR mutation type, Eastern Cooperative Oncology Group (ECOG) performance status (PS) at the time of BM, size of the largest BM, number of BM, and extracranial metastases during brain metastases, whether the patients underwent any chemotherapy. The significant dates and time were also recorded such as the date of initial cancer diagnosis and BM diagnosis, the date of WBRT, chemotherapy and EGFR-TKIs, the time of death or the most recent follow-up. Patients were categorized by age (<60 years, ≥60 years), sex (male, female), PS (0–1,2–3), smoking history (never, current/former), symptomatic BM (yes, no),size of the largest BM (<1 cm, ≥1 cm), number of BM (4–10, >10), EGFR mutation type (exon 19 deletion or exon 21 L858R mutation), extracranial metastases (yes, no), and chemotherapy (yes, no).Patients were admitted to receive the following treatments in a multi-disciplinary setting: the use of upfront WBRT followed by EGFR-TKIs (with upfront WBRT, then applied EGFR-TKIs after 4 weeks, n=67), used concurrent EGFR-TKIs and WBRT (WBRT and EGFR-TKIs were used together/sequentially/reversely within 4 weeks, n=64) and upfront EGFR-TKIs followed by WBRT (with upfront EGFR-TKIs, and then WBRT was utilized after 4 weeks, n=64). The treatment responses were assessed during the whole-process therapy. Finally, to indicate whether the patients shared similar prognostic features, the disease-specific graded prognostic assessment (DS-GPA) was calculated. This study was carried out in accordance with the declaration of Helsinki, and approved by the Institutional Review Board of Shanghai Chest Hospital (Ethical Approval No. KS1721). Written informed consents were obtained from all patients before the collection of information.
Treatments and evaluation criteria
Intracranial and extracranial disease statuses were ascertained by a systemic examination, including chest CT scan, MRI of brain, bone scanning, and abdominal ultrasound examination. A small number of patients underwent positron emission tomography/CT (PET/CT) in lieu of the above-mentioned examination to evaluate metastasis. The tumor responses to the whole-process treatments were assessed by the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (vesion 1.1), and classified into the complete response (CR), partial response (PR), stable disease (SD) and progression of disease (PD). CR and PR were included in the objective response rate (ORR). Amplification refractory mutation system (ARMS) was used to detect the patient’s DNA with ADx-EGFR Mutation Detection Kit (Amoy Diagnostics Co., Ltd., Xiamen, China) using 10–15×3-5 um slides. The kit utilizes the principle of ARMS to cover the mutations in 18–21 exons of the EGFR gene. The first-generation EGFR-TKIs was given orally at a dose of 150 mg (Erlotinib) daily, 250 mg (Gefitinib) daily or 125 mg (Icotinib) three times daily, respectively. WBRT was delivered using megavoltage machines with parallel-opposed 6 MV photon fields or 5-degree RAO–LAO fields that covered the entire cranial content. The eyes were excluded from the beam by either field arrangement or shielding. A dose of 300cGy was given daily for 10 days over 2 weeks, which yielded a total dose of 3000 cGy.
The major aim of the present study was assess the responses of combined treatment, and OS was estimated from the date of BM diagnosis to death or the most recent follow-up (June 1, 2018).
Statistical analysis
Characteristics of patients and the treatment response were compared using χ2 test for categorical variables. OS was analyzed by using the Kaplan–Meier method and the differences between the curves were used for the log-rank test. Finally, the Cox proportional-hazards model was used for performing univariate and multivariate analyses to determine the independent prognostic factor, and the correlation was statistically significant at 0.05 level. All statistical analyses were carried out using SPSS 23.0 software (IBM Corporation, Armonk, NY, USA).
Results
Patients’ baseline characteristics
A total of 29,680 medical records were screened, and 1,357 patients were diagnosed with stage IV lung adenocarcinoma and BM. Among them, 1,162 patients were excluded as they did not meet the inclusion criteria (negative-EGFR was found in 682 cases; 138 patients had used EGFR-TKIs before the diagnose of BM; EGFR mutation was identified in 68 patients; 71 patients had oligometastatic brain lesions; 49 cases were treated with SRS; 118 cases had incomplete medical records; and 36 patients used Osimertinib during the treatment). Finally, 195 eligible multiple BM patients harboring EGFR mutations were included and reviewed in this study. The patients’ selection flowchart is shown in Figure 1.
 | Figure 1 The patients’ selection flowchart. Abbreviations: BM, brain metastases; TKI, tyrosine kinase inhibitor; WBRT, whole-brain radiotherapy; SRS, stereotactic radiosurgery. |
Of these 195 patients, the median follow-up was 27 months (range, 1 to 72 months). Besides, 67 (34%) patients received WBRT, then applied EGFR-TKIs after 4 weeks, 64 (33%) cases received WBRT and EGFR-TKIs together/sequentially/reversely within 4 weeks and 64 (33%) patients received EGFR-TKIs, and WBRT was undertaken after 4 weeks. The median age during the diagnosis of BM among upfront WBRT group, EGFR-TKIs concurrently with WBRT group, and upfront EGFR-TKIs group were 59, 57, and 58 years old, respectively. The three groups were well-balanced with respect to age, sex, ECOG PS, smoking history, symptomatic BM, size of the largest BM, number of BM, EGFR mutations, extracranial metastases during BM, whether the patients had underwent any other chemotherapy and DS-GPA score. Table 1 shows the patients’ baseline characteristics.
 | Table 1 Patient characteristics |
Treatment responses
We assessed the treatment responses for different treatment strategies as the first-line treatment after BM. In the ORR assessment, the values of the upfront WBRT group, concurrent group and upfront TKI group were 82%, 64%, and 63%, respectively. In addition, ORR was significantly improved in patients with upfront WBRT (P=0.004) (Figure 2).
 | Figure 2 The treatment responses were evaluated among the groups of upfront WBRT, TKI+WBRT, and upfront TKI. |
Survival outcomes
During analysis, 35 patients were alive. For the entire cohort, the median OS after BM was 27 months (95% CI: 24.6–29.4 months), and the last follow-up was carried out at June 1, 2018. Patients treated with upfront WBRT had a significantly longer OS (36 months) than that of the concurrent group (25 months, P=0.006) and upfront EGFR-TKIs group (25 months, P<0.0001) (Figure 3A). There was no significant difference in the OS between the concurrent group and the upfront EGFR-TKIs group (25 vs 25 months; P=0.480).
After controlling significant co-variables in a multivariable model, the absence of extracranial metastases was independently associated with improved OS (adjusted HR: 0.554; 95% CI: 0.385–0.797; P=0.001; Figure 4). Additionally, the prognosis was independently correlated with management strategy of BM among these three groups (upfront WBRT vs WBRT+TKIs adjusted HR: 1.373, 95% CI: 1.093–1.724, P=0.006; upfront WBRT vs upfront EGFR-TKIs adjusted HR: 1.917, 95% CI: 1.234–2.980, P=0.004).
Subgroup analyses
To identify potential differences in the benefits of the entire cohort by varied prognoses, we subdivided patients by DS-GPA: upfront WBRT with DS-GPA score of 0 to 1.5 (n=36; 18%); upfront WBRT with DS-GPA score of 2.0 to 3 (n=31; 16%); concurrent EGFR-TKIs and WBRT with DS-GPA GPA score of 0 to 1.5 (n=38; 19%); concurrent EGFR-TKIs and WBRT with DS-GPA scores of 2.0 to 3 (n=26; 13%); upfront EGFR-TKI with DS-GPA scores of 0 to 1.5 (n=45; 23%); and upfront EGFR-TKI with DS-GPA scores of 2.0 to 3 (n=19; 10%). Statistically significant differences in the median survival times (MST, in months) were noted for all the groups by DS-GPA score (P<0.0001, Figure 3B). Patients in the concurrent group and upfront EGFR-TKIs group with DS-GPA scores 2–3 had a significantly longer OS rate than those with DS-GPA scores of 0–1.5 (P=0.023, Figure 3C). Patients in the concurrent group at DS-GPA scores of 2–3showed a trend of a longer median OS rate than that in patients in upfront EGFR-TKIs group (P=0.023, Figure 3D). There was no significant difference in the OS rate at DS-GPA scores of 0–1.5 between concurrent group and upfront EGFR-TKIs group (P=0.141, Figure 3E).
Discussion
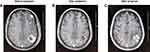
Our study explored the relationship between WBRT and EGFR-TKIs in lung adenocarcinoma patients with sensitive EGFR mutations and multiple BM. To reveal the real clinical practice, we collected data of 195 eligible patients in this study. The results showed that the ORR in the upfront WBRT group was significantly higher than the concurrent group and the upfront EGFR-TKIs group. Furthermore, the treatment of early WBRT prolonged OS of the patients. There was no significant difference in the OS between the concurrent group and the upfront EGFR-TKIs group. However, through the grading analysis of GPA classes, patients treated with concurrent EGFR-TKIs and WBRT or upfront EGFR-TKIs with DS-GPA scores of 2-3 had a significantly longer OS than those with scores of 0–1.5. As shown in Figure 5, 57–year-old man (ECOG PS=0) had no extracranial metastases and no symptoms at the time of BM. He also was diagnosed with lung adenocarcinoma and multiple BM before treatment, and treated with upfront WBRT followed by EGFR-TKIs, with an efficacy evaluation for PR. After 36 months of maintenance, the first intracranial progression occurred.
Radiotherapy plays a critical role in the treatment of patients with BM. WBRT and SRS are the mainly treatment options.5 SRS is also used as a mainly treatment option for oligo-BM, which is less invasive, as well as minimizing the unintended irradiation of the adjacent normal tissue. This treatment should be considered for patients with two or three BM (oligometastatic NSCLC).13–15 However, BM is mainly accompanied by blood transfer. Typically,there is a polka dot appearance of widespread small lesions in the brain. The WBRT has been regarded as the standard treatment for those patients.5,16,17
In addition to WBRT, the first and second line of EGFR-TKIs demonstrated a distinct therapeutic potential against BM from NSCLC, and also improved the median OS by 9–13.5 months.18–21 In patients with untreated EGFR-mutant advanced NSCLC, Osimertinib could be more efficacious than the first or the second line of EGFR-TKIs at reducing the risk of CNS progression. Recently, Osimertinib has been approved as the first-line treatment of EGFR-mutant NSCLC with BM.22,23 However, according to the cost-effectiveness thresholds presented by the World Health Organization (WHO), Osimertinib is not cost-effective as the first-line therapy of EGFR-mutant NSCLC, and that is rarely used in China.24,25 Thus, the conventional treatment is currently EGFR-TKIs (Gefitinib or Erlotinib) in developing countries. In particular, in China, the first-generation EGFR-TKIs remained the main treatment option in BM patients with EGFR mutations. Several studies have compared the effectiveness of WBRT and EGFR-TKIs, and confirmed that first-generation of EGFR-TKIs combined with WBRT is more effective than TKIs alone or WBRT alone.
Numerous researches have shown that the treatment with WBRT plus EGFR-TKIs achieved a higher response rate of BM, that significantly improved the intracranial progression-free survival (iPFS) compared with EGFR-TKIs monotherapy.10–12,26 In contrast, opposite findings were reported. For example, a retrospective analysis reported that TKI+WBRT had no survival benefit compared with EGFR-TKIs alone.27 However, it was revealed that the patient proportions between the first-line EGFR-TKIs group (78.4%) and TKIs+WBRT group (58.8%) was unbalanced. The same result was obtained in another study, in which those findings may be due to the small sample sizes.28 Therefore, EGFR-TKIs combined with WBRT is still an effective treatment choice, while the specific relationship between EGFR-TKIs and WBRT needs to be further studied. A multi-institutional analysis demonstrated that the use of upfront WBRT, and deferral of EGFR-TKIs, is associated with longer OS.15 However, this study did not determine the appropriate timing of these treatments. In our study, we can conclude that early WBRT can prolong OS. We also recommend that patients receive upfront WBRT then applied EGFR-TKIs after 4 weeks. We clarified the time sequence between WBRT and EGFR-TKIs, and subsequently analyzed the probable reasons as well.
Preclinical results demonstrated that even with the small molecular weight, the permeation ability of the first and second-generation EGFR-TKIs into CSF seems to be limited.9,29 Therefore, continuous improvement of possible therapeutic strategies to improve overall disease control of life is becoming more critical. The combination therapy of WBRT and EGFR-TKIs showed a promising treatment option. The probable interacting mechanisms between radiotherapy and EGFR-TKIs include the radiosensitizing effect of EGFR-TKIs and the opening of BBB by radiation.30–35 A number of studies have indicated that the drug CSF concentration could be increased up to one month after WBRT and there might be a window extending from 1 week after the initiation of radiotherapy to 1 month after the completion of treatment.36,37 These studies reasonably confirmed conclusion achieved by our study that EGFR-TKIs can result in excellent effect after 4 weeks of WBRT, thus the best time to receive treatment of EGFR-TKI was over 4 weeks after WBRT.
In addition,DS-GPA is an objective, quantitative and the easiest prognostic indexes for lung cancer patients with BM.38 Patient cohorts stratified by GPA classes were analyzed for finding survival differences. It was shown that the OS of patients in different GPA groups significantly differed. Since the three groups were balanced with respect to DS-GPA scores of patients, we concluded that our results improved outcomes based on the WBRT sequences, which were not confounded by improved control of DS-GPA scores. Our research demonstrated that multiple BM patients with high DS-GPA scores should be treated with WBRT immediately after taking EGFR-TKIs. As mentioned in a study that for high age and low KPS patients, WBRT showed no significant effect on the OS,39 which was consistent with our conclusions, and there was no significant difference between EGFR-TKIs concurrent with WBRT and upfront EGFR-TKIs with low DS-GPA scores (including old age and low KPS).
However, our study has several limitations that should be described. Firstly, this is a retrospective analysis conducted in a single institution and a non-randomized study, which included unrecognized biases and confounding factors. Secondly, we did not account for the potential toxicities related to brain therapies and their impacts on the patients’ quality of life. Finally, due to the long interval of time between BM and the follow-up, some patients could not provide details time by telephone follow-up. Out of the rigor of the data, iPFS has not been measured.
In conclusion, our study suggested that the ORR was significantly improved in the WBRT first group, and a significantly longer OS was achieved than those initially treated with EGFR-TKIs or EGFR-TKIs concurrently with WBRT. More importantly, multiple BM patients with high DS-GPA scores should be treated with WBRT immediately after taking EGFR-TKIs. Further prospective studies are required to validate these findings and determine the optimal timing.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (Grant No. 81502450) and Science and Technology Commission of Shanghai Municipality, China (Grant No. 18441904700).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi:10.3322/caac.21387
2. Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan detroit cancer surveillance system. J Clin Oncol. 2004;22:2865–2872. doi:10.1200/JCO.2004.12.149
3. Nussbaum ES, Djalilian HR, Cho KH, et al. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer. 1996;78:1781–1788.
4. Shin D-Y, Na II, Kim CH, Park S, Baek H, Yang SH. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol. 2014;9:195–199. doi:10.1097/JTO.0000000000000069
5. Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295–1304. doi:10.1200/JCO.2005.04.6185
6. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi:10.1016/S1470-2045(09)70263-3
7. Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole- brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. doi:10.1200/JCO.2010.30.1655
8. Khozin S, Blumenthal GM, Jiang X, et al. U.S. food and drug administration approval summary: erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist. 2014;19:774–779. doi:10.1634/theoncologist.2014-0089
9. Zhang J, Yu J, Sun X, Meng X. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of central nerve system metastases from non-small cell lung cancer. Cancer Lett. 2014;351:6–12. doi:10.1016/j.canlet.2014.04.019
10. Zhang J, Shi X, Cai D, et al. MINI01.11: radiotherapy plus EGFR TKIs for brain metastasis in EGFR-mutant non-small cell lung cancer: a retrospective analysis of a single institution: topic: medical oncology. J Thorac Oncol. 2016;11:S263. doi:10.1016/j.jtho.2016.09.026
11. Jiang T, Min W, Li Y, Yue Z, Wu C, Zhou C. Radiotherapy plus EGFR TKIs in non‐small cell lung cancer patients with brain metastases: an update meta‐analysis. Cancer Med. 2016;5:1055–1065. doi:10.1002/cam4.673
12. Chen Y, Yang J, Li X, et al. First-line epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor alone or with whole-brain radiotherapy for brain metastases in patients with EGFR-mutated lung adenocarcinoma. Cancer Sci. 2016;107:1800–1805. doi:10.1111/cas.13079
13. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2:210–225. doi:10.1016/j.prro.2011.12.004
14. Yang W-C, Xiao F, Shih J-Y, et al. Epidermal growth factor receptor mutation predicts favorable outcomes in non-small cell lung cancer patients with brain metastases treated with stereotactic radiosurgery. Radiother Oncol. 2018;126:368–374. doi:10.1016/j.radonc.2017.10.010
15. Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of brain metastases in tyrosine kinase inhibitor-naive epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017;35:1070–1077. doi:10.1200/JCO.2016.69.7144
16. Wilhelm I, Molnar J, Fazakas C, Haskó J, Krizbai IA. Role of the blood-brain barrier in the formation of brain metastases. Int J Mol Sci. 2013;14:1383–1411. doi:10.3390/ijms14011383
17. Minchom A, Yu KC, Bhosle J, et al. The diagnosis and treatment of brain metastases in EGFR mutant lung cancer. J Clin Oncol. 2017;35:1070–1077. doi:10.1200/JCO.2016.69.7144
18. Ceresoli GL, Cappuzzo F, Gregorc V, Bartolini S, Crinò L, Villa E. Gefitinib in patients with brain metastases from nonsmall-cell lung cancer: a prospective trial. Ann Oncol. 2004;15:1042–1047. doi:10.1093/annonc/mdh276
19. Hotta K, Kiura K, Ueoka H, et al. Effect of gefitinib (‘Iressa’, ZD1839) on brain metastases in patients with advanced non-small-cell lung cancer. Lung Cancer. 2004;46:255–261. doi:10.1016/j.lungcan.2004.04.036
20. Takahashi H, Ohrui T, Ebihara S, Yamada M, Sasaki H. Effect of gefitinib (ZD1839) on metastatic brain tumour. Lung Cancer. 2004;43:371–372. doi:10.1016/j.lungcan.2003.09.017
21. Kim J-E, Lee DH, Choi Y, et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer. 2009;65:351–354. doi:10.1016/j.lungcan.2008.12.011
22. Yi-Long W, Ahn M-J, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non–small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol. 2018;36:2702–2709. doi:10.1200/JCO.2018.77.9363
23. Cho BC, Chewaskulyong B, Lee KH, et al. Osimertinib versus standard of care EGFR TKI as first-line treatment in patients with EGFRm advanced NSCLC: FLAURA Asian subset. J Thorac Oncol. 2018;18:
24. Aguiar PN
25. Wu B, Gu X, Zhang Q. Cost-effectiveness of osimertinib for EGFR mutation–positive non–small cell lung cancer after progression following first-line EGFR TKI therapy. J Thorac Oncol. 2018;13:184–193. doi:10.1016/j.jtho.2017.10.012
26. Sung S, Lee S-W, Kwak Y-K, et al. Intracranial control and survival outcome of tyrosine kinase inhibitor (TKI) alone versus TKI plus radiotherapy for brain metastasis of epidermal growth factor receptor-mutant non-small cell lung cancer. J Neurooncol. 2018;139:205–213. doi:10.1007/s11060-018-2861-1
27. Jiang T, Su C, Li X, et al. EGFR TKIs plus WBRT demonstrated no survival benefit other than that of TKIs alone in patients with NSCLC and EGFR mutation and brain metastases. J Thorac Oncol. 2016;11:1718–1728. doi:10.1016/j.jtho.2016.05.013
28. Magnuson WJ, Yeung JT, Guillod PD, Gettinger SN, Yu JB, Chiang VL. Impact of deferring radiation therapy in patients with epidermal growth factor receptor-mutant non-small cell lung cancer who develop brain metastases. Int J Radiat Oncol Biol Phys. 2016;95:673–679. doi:10.1016/j.ijrobp.2016.01.037
29. Chen Y, Wang M, Zhong W, Zhao J. Pharmacokinetic and pharmacodynamic study of Gefitinib in a mouse model of non-small-cell lung carcinoma with brain metastasis. Lung Cancer. 2013;82:313–318. doi:10.1016/j.lungcan.2013.08.013
30. Chinnaiyan P, Huang S, Vallabhaneni G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res. 2005;65:3328–3335. doi:10.1158/0008-5472.CAN-04-3547
31. Bianco C, Tortora G, Bianco R, et al. Enhancement of antitumor activity of ionizing radiation by combined treatment with the selective epidermal growth factor receptor-tyrosine kinase inhibitor ZD1839 (Iressa). Clin Cancer Res. 2002;8:3250–3258.
32. Huang SM, Li J, Armstrong EA, et al. Modulation of radiation response and tumor-induced angiogenesis after epidermal growth factor receptor inhibition by ZD1839 (Iressa). Cancer Res. 2002;62:4300–4306.
33. d’Avella D, Cicciarello R, Albiero F, et al. Quantitative study of blood-brain barrier permeability changes after experimental whole-brain radiation. Neurosurgery. 1992;30:30–34.
34. Qin D, Ma J, Xiao J, et al. Effect of brain irradiation on blood-CSF barrier permeability of chemotherapeutic agents. Am J Clin Oncol. 1997;20:263–265.
35. Qin DX, Zheng R, Tang J, et al. Influence of radiation on the blood-brain barrier and optimum time of chemotherapy. Int J Radiat Oncol Biol Phys. 1990;19:1507–1510.
36. Zhou L, He J, Xiong W, et al. Impact of whole brain radiation therapy on CSF penetration ability of Icotinib in EGFR-mutated non-small cell lung cancer patients with brain metastases: results of phase I dose-escalation study. Lung Cancer. 2016;96:93–100. doi:10.1016/j.lungcan.2016.04.003
37. Cao Y, Tsien CI, Shen Z, et al. Use of magnetic resonance imaging to assess blood-brain/blood-glioma barrier opening during conformal radiotherapy. J Clin Oncol. 2005;23:4127–4136. doi:10.1200/JCO.2005.07.144
38. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi:10.1200/JCO.2011.38.0527
39. Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388:2004–2014. doi:10.1016/S0140-6736(16)30825-X
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.