Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
Updates on Genital Dermatophytosis
Received 3 June 2020
Accepted for publication 12 August 2020
Published 2 October 2020 Volume 2020:13 Pages 743—750
DOI https://doi.org/10.2147/CCID.S262704
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Rameshwari Thakur,1,2 Avneet Singh Kalsi3
1Microbiology and Infection Control, Shivam Orthocare, Una, Himachal Pradesh, India; 2Department of Microbiology, Muzaffarnagar Medical College, Muzaffarnagar, Uttar Pradesh, India; 3Research, Muzaffarnagar Medical College, Muzaffarnagar, Uttar Pradesh, India
Correspondence: Rameshwari Thakur Shivam Orthocare, Hamirpur Road, Una, Himachal Pradesh, India
Tel +91-9627440337
Fax +91-9654775082
Email [email protected]
Abstract: Dermatophytes are a group of keratinophilic fungi, which normally cause superficial infection of skin, hair and nails. Based on ecology, they are classified into three groups: anthropophilic, zoophilic and geophilic. Superficial dermatophytic infection of the genital region is called genital dermatophytosis, tinea genitalis or pubo-genital dermatophytosis. In this review, we would like to discuss briefly, the various clinical presentations of genital dermatophytosis, current changes in the taxonomy and nomenclature, introduction of new diagnostic techniques and briefly describe some common dermatophytes and their sources. Also, there are serious concerns associated with the recent development of antifungal resistance among the dermatophytes. We are also facing the scenario of hard-to-treat dermatophytosis.
Keywords: Trichophyton mentagrophytes, Microsporum canis, Trichophyton benhamiae, topical corticosteroids
Introduction
Dermatophytic infection of the pubo-genital region has been reported due to anthropophilic, zoophilic and geophilic dermatophytes1–7 (Table 1). Previously, it invariably only used to be due to anthropophilic dermatophytes.8–13 Recently, it has been found to be associated more often with zoophilic dermatophytes. During the past 60 years, there has been a big change with predominance of zoophilic infections over anthropophilic infections.14
 |
Table 1 Genital Dermatophytosis Due to Different Dermatophyte Species/Genotypes |
Genital dermatophytosis has been found to be transmitted sexually,1,2,6 by autoinoculation4 and by excessive use of potent corticosteroids, topically or systemically.5 In hot and humid climates, dermatophytosis is more common. Other predisposing factors are HIV/AIDS, diabetes mellitus, immunosuppression and atopic dermatitis.
Clinical Presentations
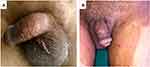
Dermatophytic infection of the genital region can present as various clinical forms: it can also be seen along with tinea cruris. In a study in Italy, by Romano et al, (2005),15 tinea genitalis was seen in 2% of cases of tinea cruris. Pandey et al, (1981),9 on the other hand had noticed 20% of genital involvement among patients with tinea cruris, and Thakur et al, (2018)2 found 22.14% of patients with tinea cruris also had concomitant tinea genitalis (Figure 1B). Genital dermatophytosis is more often seen in tropical countries due to their hot and humid climate resulting in local humidity and skin maceration.12 Other predisposing factors are diabetes mellitus.13,15,16 Individuals with immunosuppression are also prone to dermatophytic infections.17 Also, people with atopic dermatitis are likely to suffer more often with dermatophytosis.8,9
Isolated genital lesions, which are somewhat uncommon, can involve penile shaft (Figure 1A), scrotum, preputial skin, glans penis in males and mons pubis, vulva, labia majora and labia minora in females (Figure 2). The lesions are usually mild to moderately inflammatory in anthropophilic infections and generally severely inflammatory, painful, ulcerative and may involve lymph nodes, when the infection is due to zoophilic dermatophytes. Topical use of potent corticosteroid creams may mask the typical characteristic appearance of the lesions, which are usually seen as annular plaques with slightly raised erythematous scaly margins, centrifugally advancing borders and with central clearing. With the use of topical corticosteroids, atypical presentation can be there with no central clearing. An uncommon dermatophytic lesion in dermis, which is usually due to Trichophyton rubrum (T. rubrum) is called Majocchi’s Granuloma (MG).18 There is formation of the granuloma of the hair follicle. Genital shaving can also result in the development of MG.19 Also, a severe transmissible Majocchi’s granuloma has been reported in an immunocompetent traveller.20 Diagnosis of MG is confirmed by histopathology with periodic acid-Schiff (PAS) and Groccot Gomori’s methenamine Silver (GMS) methods.18
New Taxonomy, Clinical and Laboratory Diagnostic Challenges
Previously, there were three main genera of dermatophytes: Trichophyton, Epidermophyton, and Microsporum. But, now according to the new updated taxonomy by Hoog et al based on internal transcribed spacer sequencing, β-tubulin fragments, ribosomal 60S subunit and translation elongation factor-3, dermatophytes have been classified into nine genera: Trichophyton, Epidermophyton, Microsporum, Nannizzia, Lophophyton, Paraphyton, Arthroderma, Gaurromyces, and Ctenomyces.21 Four genera of dermatophytes are usually associated with genital dermatophytosis, i.e., Trichophyton, Epidermophyton, Microsporum, and Nannizzia.
Dermatologists can also carry out dermoscopy if a patient also has tinea corporis to check involvement of vellus hair as seen on dermoscopy which indicates systemic therapy.22 Most of the laboratories depend upon the conventional methods for the identification of dermatophytes, which require great expertise and experience. It mainly consists of direct microscopy with 10–20% Potassium hydroxide (KOH) and culture on Sabouraud’s dextrose agar with addition of chloramphenicol and cycloheximide. Conventional techniques are time consuming because cultivation and physiological testing can take 2–4 weeks and the results can still be confusing.23 Moreover, the microscopically positive specimen may fail to grow in culture.24 Accurate and deeper knowledge of dermatophytes has been achieved with the help of molecular research and it has helped us to decipher the diagnostic problems associated with conventional methods.25
At times, genotyping becomes necessary for differentiating zoophilic dermatophytes from anthropophilic, because some of the zoophilic dermatophytes have been found to have human-to-human transmission, e.g., Trichophyton mentagrophytes (T. mentagrophytes) genotypes VII and VIII.1,2 Mating partners within the same species can differ in virulence and the old theory of zoophily and anthropophily may require some modifications, especially in the newly redefined species.26 During the recent epidemic of superficial dermatophytosis in India due to T. mentagrophytes genotype VIII, a study was conducted in which the initial 50 strains tested for mating type, were found to be “Plus mating type” and carried the HMG transcription factor gene at MAT locus.27
Thakur et al28 were first to publish a paper from India on an outbreak of tinea cruris and tinea genitalis due to Trichophyton interdigitale (T. interdigitale) in 2016.28 It was confirmed later that the outbreak was due to T. mentagrophytes genotype VIII. The clinical presentations of the cases were more like that of anthropophilic infection and with human-to-human transmission.
There have been changes in the taxonomy and nomenclature of T. mentagrophytes complex (Table 2).
 |
Table 2 Old and Current Nomenclature of T. mentagrophytes Complex |
Anthropophilic Infections
Genital dermatophytosis is commonly seen in countries with hot and humid climate and is usually caused by the anthropophilic dermatophytes, e.g. T. rubrum, and next in frequency being E. floccosum.5,13,22 Tinea genitalis can also be acquired due to autoinoculation from tinea pedis and onychomycosis.4
Zoonotic Infections
Trichophyton mentagrophytes/Trichophyton interdigitale species group (TMTISG) was earlier known as Trichophyton mentagrophytes complex. Now, T. interdigitale has been separated as anthropophilic species and T. mentagrophytes as zoophilic species.30 Occasionally, genital dermatophytosis can be due to Trichophyton verrucosum (T. verrucosum). T. verrucosum is also known as cattle ringworm fungus.
Luchsinger et al6 first published a paper on tinea genitalis due to T. interdigitale type III (3 patients) and T. interdigitale type IV (3 patients), in six Swiss tourists, both males and females, who had returned from Southeast Asia in 2015. The typing was in accordance with the 'Signature polymorphism' published by Heidemann et al.25 Four of the male patients gave history of having had sex with the commercial sex workers.6 Two similar reports of two individuals, each one of them having travelled to Thailand and Egypt respectively with similar lesions after having had sexual contact, was published recently.33,34 Another recent report of 14 patients with genital dermatophytosis due to T. mentagrophytes genotype Thailand Type 1 presented with severe inflammatory and abscessing infection.35
Trichophyton benhamiae
Trichophyton benhamiae (T. benhamiae) was earlier known as Arthroderma benhamiae and is a new emerging pathogen. It is a zoophilic dermatophyte, and one often acquires infection either from guinea pigs or other animals. In the past 15 years, it has been reported in animals from Japan, Europe, and the United States and recently in China in a 4-year-old girl.36–42
Previously, the identification of dermatophyte species was based on the clinical data, morphological characteristics, and physiological details. Accordingly, Arthroderma benhamiae was considered to belong to the T. mentagrophyte species complex.25,43 Infections in the genital region due to T. benhamiae have been reported from Germany.1,4
Microsporum canis
Recently, tinea genitalis due to Microsporum canis (M. canis) has been reported from Europe.4,7 M. canis is commonly found in cats and dogs and cats are known to be the most common host.44 M. canis has also been reported in many domestic and wild animals.45–47 Human-to-human infection of M. canis has been often documented and asymptomatic animals are believed to be the source of 50% human infections.48 In Europe, from 50% to 90% of dermatophyte infections are caused by M. canis and the cat is believed to be its main reservoir.49–52
In some breeds of dogs and cats, e.g., Jack Russells and Persians, possibly, the genetic factors can be responsible for increasing the risk of infection.53 The spores of M. canis are shed in the environment from the scales of infected animals that may remain infectious for 12–24 months.54 In some countries, due to some concerns, dogs and cats are usually neutered or de-sexed. This may lead to higher risk of acquiring dermatophyte infection. In a study, it was observed, that male neutered cats had a 12-fold higher risk of dermatophytosis compared to intact male cats.55
Geophilic Infections
Geophilic dermatophytes are found in soil. Nannizzia gypsea (N. gypsea) was formerly known as Microsporum gypseum (M. gypseum). This has cosmopolitan distribution.
Nannizzia gypsea
This fungus has a special predilection towards black chernozemic soil, which usually has high moisture and neutral pH.56 The genital infection caused by this dermatophyte is found in males confined to the scrotum.
A detailed article on tinea genitalis due to N. gypsea and other dermatophytes has been published by Yin et al in 2018.3 The lesions due to N. gypsea can be atypical and at times, may cause pseudomembranous-like tinea of the scrotum.57 Other reports of tinea genitalis due to N. gypsea from China, Japan, and Brazil have also been published.58–66
Discussion
There is an urgent need for the mycologists and dermatologists to update themselves with the current changes in the taxonomy and nomenclature of dermatophytes.21 Previously, outbreaks and epidemics were typically due to anthropophilic dermatophytes, but now, can also be seen due to zoophilic dermatophytes, e.g., T. mentagrophytes and M. canis.67 In the past, differentiation of T. mentagrophytes (zoophilic species) from T. interdigitale (anthropophilic species) was not feasible, but molecular typing has solved this problem.
There was one more drawback due to the use of the double naming system which was used in the past when mycologists were dependent upon microscopic identification of the dermatophytes. Different naming systems were used for anamorph and teleomorph genera. Recently, two International expert symposia were organized in Amsterdam, the Netherlands, to abolish the dual naming in fungi: One Fungus=One Name symposium was held on April 19 and 20, 2011 and One Fungus = Which Name symposium was held on April 12 and 13, 2012.68
Now, the method of identification of dermatophytes has changed and depends upon genotype rather than studying the phenotypic characteristics. Moreover, the nucleic acid sequence difference now guides taxonomy and has substituted phenotype and at times, may include, sexual compatibility.69
The pubo-genital lesions due to T. mentagrophytes genotype VII, have been described to be ulcerative, inflammatory and with the involvement of lymph nodes in some cases.1 Most of these findings were in Caucasians. But, the lesions described by Thakur et al, in 201628 and 2018,2 due to T. mentagrophytes genotype VIII, were extensive with mild-to-moderate inflammation, with no ulceration or involvement of lymph nodes.2 Lesions due to N. gypsea are only seen in males and there has been no history of sexual transmission.3
In some of the cases of dermatophytosis, the source of infection may not be found. Due to expansion of cities, suburban areas may become transition zones for contact with wild animals like wild boar, foxes, river rats, mice and other rodents, and thus become channels, which allow extra urban infections to penetrate into cities.70
Treatment
There are several treatment options for topical and systemic therapy. It is very important to see whether patients have lesions confined to genitalia or have multiple lesions in different parts of the body. Involvement of groin, others parts or tinea pedis may require treatment for longer duration. Treatment is tailored according to the individual needs of the patient. Antifungal therapy is also decided based upon laboratory findings such as direct KOH mount and culture. Inflammatory component is often seen in zoonotic and geophilic infections.
Available antifungal agents are topical azoles and allylamines. Topical azoles are preferred over allylamines because of its broad-spectrum antimycotic activity plus antibacterial and anti-inflammatory activity in naïve cases of tinea cruris and tinea corporis. They are well tolerated and have no adverse effects.71,72 Systemic antifungals are required in case the patient has widespread lesions, tinea cruris and extensive lesions like tinea cruris et corporis and tinea pedis. The current treatment regimen includes terbinafine 250 mg daily for 2–3 weeks, or itraconazole 200 mg daily, for about a month.73–75 In naïve cases, terbinafine 250 mg/day ought to be prescribed for 2–4 weeks, while in recalcitrant cases, itraconazole 200–400 mg/day for 4 weeks is the drug of choice.76 Systemic use of antifungal drugs during pregnancy is not advised according to the Food and Drug administration of the US. Pregnancy category C has been assigned to fluconazole, itraconazole, and griseofulvin. Terbinafine has been categorized as pregnancy category B. The safety of these antifungal drugs for systemic use during pregnancy is not known. Moreover, it is also secreted in breast milk.77
In case of tinea incognito, systemic antifungal therapy such as itraconazole 200–400 mg daily, for 4–6 weeks or longer, should be preferred. Topical corticosteroids should be stopped immediately.76 Topical corticosteroid can only be considered in inflammatory dermatophytosis.78 For patients who do not get clinical cure with terbinafine or itraconazole after 4 weeks, the therapy should be extended for longer duration or they should be started on other systemic antifungals such as griseofulvin 250–500 mg twice daily for 4–6 weeks.79 Recently, resistance to terbinafine has been reported in dermatophytes.80–82
Some dermatologists prescribe topical antifungal-corticosteroid combinations especially when there is severe inflammation in the groin. It is recommended at the initial stage of therapy for 1–2 weeks, followed by monotherapy.83–85 It reduces inflammatory component, pruritis, and reduces spread of infection.86 Such combinations should only be prescribed if there is a strong indication. Inappropriate use of such combinations are associated with adverse effects, e.g., cutaneous atrophy, tinea incognito, Majocchi’s granuloma including suppression of the hypothalamus-pituitary-adrenal axis, especially when potent corticosteroid combinations are prescribed.86
Conclusion
Changing lifestyles, frequent visits to health clubs, close-fitting synthetic garments, promiscuous culture, keeping small pets such as cats and dogs are the possible contributing factors leading to increased incidence of dermatophytosis.
As the numbers of cases of genital dermatophytosis are being reported with increased frequency, especially due to zoophilic dermatophytes, appropriate diagnosis involving molecular typing becomes mandatory to trace the source of infection either in animals or humans and treat the patients accordingly. An easy access to the highly potent topical corticosteroid creams, such as clobetasol propionate, with combination of antibacterial and antifungal agents as over-the-counter drugs, has resulted in the spread of lesions, atypical appearance and various side-effects.2,5 Treating such lesions poses a great difficulty to the dermatologists. Sale of topical corticosteroid creams, as over-the-counter drugs should be made illegal.
Before bringing the pet to the house, there should be thorough check-up from the veterinary doctor. The house should be sanitized thoroughly on a regular basis, especially with antifungal disinfectants, such as sodium hypochlorite (household bleach), enilconazole, accelerated hydrogen peroxide (AHP), potassium peroxymonosulfate, and even essential oils.87 There should be closer and more frequent collaboration between human dermatologists and veterinary doctors.70
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kupsch C, Czaika VA, Deutsch C, Gräser Y. Trichophyton mentagrophytes – a new genotype of zoophilic dermatophyte causes sexually transmitted infections. J Dtsch Dermatol Ges. 2019;17(5):493–501. doi:10.1111/ddg.13776
2. Thakur R, Kushwaha P, Kalsi AS, Singh P. Tinea genitalis in a rural tertiary care hospital of Western U.P. India. Indian J Clin Exp Dermatol. 2018;4(4):266–273. doi:10.18231/2581-4729.2018.0056
3. Yin S, Xie X, Li M, et al. Prevalence of low inflammatory tinea genitalis in southern China. Mycoses. 2019;62(3):284–290. doi:10.1111/myc.12859
4. Ginter-Hanselmayer G, Nenoff P, Kurrat W, Propst E, Durrant-Finn U, Uhrlaß S. Tinea in genital area: A diagnostic and therapeutic challenge. Hautarzt. 2016;67(9):688–699. doi:10.1007/s00105-016-3848-5
5. Verma SB, Vasani R. Male genital dermatophytosis - clinical features and the effects of the misuse of topical steroids and steroid combinations - an alarming problem in India.. Mycoses. 2016;59(10):606–614. doi:10.1111/myc.12503
6. Luchsinger I, Bosshard PP, Kasper RS, Reinhardt D, Lautenschlager S. Tinea genitalis: a new entity of sexuality transmitted infection? Case series and review of literature. Sex Transm Infect. 2015;91(7):493–496. doi:10.1136/sextrans-2015-052036
7. Prohić A, Krupalija-Fazlić M, Jovović Sadiković T. Incidence and etiological agents of genital dermatophytosis in males. Med Glas (Zenica). 2015;12(1):52–56.
8. Pillai KG, Singh G, Sharma BM. Trichophyton rubrum infection of the penis. Dermatologica. 1975;150:252–254. doi:10.1159/000251438
9. Pandey SS, Chandra S, Guha PK, Kaur P, Singh G. Dermatophyte infection of penis. Association with a particular undergarment. Int J Dermatol. 1981;20(2):112–114. doi:10.1111/j.1365-4362.1981.tb00419.x
10. Kumar B, Talwar P, Kaur S. Penile tinea. Mycopathologia. 1981;11(3):169–177. doi:10.1007/BF00482812
11. Vora SN, Mukhopadhyay A. Incidence of dermatophytosis of the penis and scrotum. Indian J Dermatol Venereol Leprol. 1994;60(2):89–91.
12. Mukhopadhyay AK. Trichophyton rubrum infection of prepuce. Indian J Dermatol Venereol Leprol. 2005;71(2):130–131. doi:10.4103/0378-6323.14004
13. Das JK, Sengupta S, Gangopadhyay A. Dermatophyte infection of the male genitalia. Indian J Dermatol. 2009;54(5):21–23. doi:10.4103/0019-5154.45436
14. Zhan P, Li D, Wang C, et al. Epidemiological changes in tinea capitis over the sixty years of economic growth in China. Med Mycol. 2015;53(7):691–698. doi:10.1093/mmy/myv057
15. Romano C, Ghilardi A, Papini M. Nine male cases of tinea genitalis. Mycoses. 2005;48:202–204. doi:10.1111/j.1439-0507.2005.01127.x
16. Pielop J, Rosen T. Penile dermatophytosis. J Am Acad Dermatol. 2001;44(5):864–867. doi:10.1067/mjd.2001.112923
17. Aly R, Berger T. Common superficial fungal infections in patients with AIDS. Clin Infect Dis. 1996;22(Suppl. 2):128–132.
18. Boral H, Durdu M, Ilkit M. Majocchi’s granuloma: current perspectives. Infect Drug Resist. 2018;11:751–760. doi:10.2147/IDR.S145027
19. Wagenknecht D, Simon JC, Treudler R, Nenoff P. Pubogenital tinea after intimate shaving with isolation of Trichophyton benhamiae. J der Deutschen Dermatologischen Gesellschaft. 2018;16(5):596–598. doi:10.1111/ddg.13500
20. Gallo JG, Woods M, Graham RM, Jennison AV. A severe transmissible Majocchi’s granuloma in an immunocompetent returned traveler. Med Mycol Case Rep. 2017;18:5–7. doi:10.1016/j.mmcr.2017.07.003
21. de Hoog GS, Dukik K, Monod M, et al. Toward a Novel Multilocus Phylogenetic Taxonomy for the Dermatophytes. Mycopathologia. 2017;182(1–2):5–31. doi:10.1007/s11046-016-0073-9
22. Gómez-Moyano E, Crespo-Erchiga V. Tinea of vellus hair: an indication for systemic antifungal therapy. Br J Dermatol. 2010;163(3):603–606. doi:10.1111/j.1365-2133.2010.09811.x
23. Mochizuki T, Ishizaki H, Barton RC, et al. Restriction fragment length polymorphism analysis of ribosomal DNA intergenic regions is useful for differentiating strains of Trichophyton mentagrophytes. J Clin Microbiol. 2003;41(10):4583–4588. doi:10.1128/jcm.41.10.4583-4588.2003
24. Mügge C, Haustein UF, Nenoff P. Causative agents of onychomycosis–a retrospective study. J Dtsch Dermatol Ges. 2006;4(3):218–228. doi:10.1111/j.1610-0387.2006.05877.x
25. Heidemann S, Monod M, Gräser Y. Signature polymorphism in the internal transcribed spacer region relevant for the differentiation of zoophilic and anthropophilic strains of Trichophyton interdigitale and other species of T. mentagrophytes sensu lato. Br J Dermatol. 2010;162(2):282–295. doi:10.1111/j.1365-2133.2009.09494.x
26. Gräser Y, Monod M, Bouchara JP, et al. New insight in dermatophyte research. Med Mycol. 2018;56(suppl_1):2–9. doi:10.1093/mmy/myx141
27. Nenoff P, Verma SB, Vasani R, et al. The current Indian epidemic of superficial dermatophytosis due to Trichophyton mentagrophytes – A molecular study. Mycoses. 2019;62(4):336–356. doi:10.1111/myc.12878
28. Thakur R, Kushwaha P, Kumar H, Singh P. Tinea cruris and Tinea genitalis due to Trichophyton interdigitale in and around Muzaffarnagar (Western UP), India: possibly an Outbreak. Int J Curr Microbiol App Sci. 2016;5(9):468–473. doi:10.20546/ijcmas.2016.509.051
29. Neves H, Canovaxavier N. The transmission tinea cruris. Br J Dermatol. 1964;76(10):429–436. doi:10.1111/j.1365-2133.1964.tb14467.x
30. Taghipour S, Pchelin IM, Mahmoudabadi AZ, et al. Trichophyton mentagrophytes and T. interdigitale genotypes associated with particular geographic area and clinical manifestations. Mycoses. 2019:1–8. doi:10.1111/myc
31. Kwon-Chung KJ, Bennett JE. Medical Mycology. Philadelphia: Lea and Febiger; 1992.
32. Nenoff P, Herrmann J, Gräser Y. Trichophyton mentagrophytes sive interdigitale? A dermatophyte in the course of time. J Dtsch Dermatol Ges. 2007;5(3):198–202. doi:10.1111/j.1610-0387.2007.06180.x
33. Wendrock-Shiga G, Metche D, Uhrlaß S, Koch D, Krüger C, Nenoff P. Tinea barbae profunda durch Trichophyton mentagrophytes nach Thailand-Reise. Der Hautartz. 2017;68:639–648. doi:10.1007/s00105-017-4008-2
34. Nenoff P, Schubert K, Jarsumbeck R, Uhrlaß S, Krüger C. Tinea genitalis profunda durch Trichophyton mentagrophytes nach Ägyten-Reise. Aktuelle Dermatol. 2017;43(04):146–153. doi:10.1055/s-0043-106149
35. Nenoff P, Uhrlaß S, Wendrock-Shiga G, et al. Trichophyton mentagrophytes genotype Thailand Type 1 as causative pathogen of abscessing dermatophytoses - tinea genitalis and barbae - in Germany. In:
36. Nakamura Y, Kano R, Nakamura E, Saito K, Watanabe S, Hasegawa A. Case report. First report on human ringworm caused by Arthroderma benhamiae in Japan transmitted from a rabbit. Mycoses. 2002;45(3–4):129–131. doi:10.1046/j.1439-0507.2002.00732.x
37. Drouot S, Mignon B, Fratti M, Roosje P, Monod M. Pets as the main source of two zoonotic species of the Trichophyton mentagrophytes complex in Switzerland, Arthroderma vanbreuseghemii and Arthroderma benhamiae. Vet Dermatol. 2009;20(1):13–18. doi:10.1111/j.1365-3164.2008.00691.x
38. Nenoff P, Schulze I, Uhrlaß S, Krüger C. Kerion caused by the zoophilic dermatophyte Trichophyton species of Arthroderma benhamiae in a child. A new emerging pathogen of dermatomycoses in Germany. Hautarzt. 2013;64(11):846–849. doi:10.1007/s00105-013-2665-3
39. Sieklucki U, Oh SH, Hoyer LL. Frequent isolation of Arthroderma benhamiae from dogs with dermatophytosis. Vet Dermatol. 2014;25(1):39–e14. doi:10.1111/vde.12095
40. Sabou M, Denis J, Boulanger N, et al. Molecular identification of Trichophyton benhamiae in Strasbourg, France: a 9-year retrospective study. Med Mycol. 2018;56(6):723–734. doi:10.1093/mmy/myx100
41. White SD, Guzman DS, Paul-Murphy J, Hawkins MG. Skin diseases in companion guinea pigs (Cavia porcellus): a retrospective study of 293 cases seen at the Veterinary Medical Teaching Hospital, University of California at Davis (1990–2015). Vet Dermatol. 2016;27(5):395–e100. doi:10.1111/vde.12348
42. Tan J, Liu X, Gao Z, Yang H, Yang L, Wen H. A case of Tinea Faciei caused by Trichophyton benhamiae: first report in China. BMC Infect Dis. 2020;20(1):171. doi:10.1186/s12879-020-4897-z
43. Kawasaki M. Verification of a taxonomy of dermatophytes based on mating results and phylogenetic analyses. Med Mycol J. 2011;52(4):291–295. doi:10.3314/mmj.52.291
44. Pasquetti M, Min ARM, Scacchetti S, Dogliero A, Peano A. Infection by Microsporum canis in paediatric patients: A veterinary perspective. Vet Sci. 2017;4(3):E46. doi:10.3390/vetsci4030046
45. Gallo MG, Tizzani P, Peano A, Rambozzi L, Meneguz PG. Eastern cottontail (Sylvilagus floridanus) as carrier of dermatophyte fungi. Mycopathologia. 2005;160(2):163–166. doi:10.1007/s11046-005-6619-x
46. Gallo MG, Lanfranchi P, Poglayen G, et al. Seasonal 4-year investigation into the role of the alpine marmot (Marmota marmota) as a carrier of zoophilic dermatophytes. Med Mycol. 2005;43(4):373–379. doi:10.1080/13693780400008282
47. Pal M, Dave P. Tinea faciei in a goat handler due to Microsporum canis. Rev Iberoam Micol. 2005;22(3):181–182. doi:10.1016/s1130-1406(05)70039-5
48. Mancianti F, Nardoni S, Corazza M, D’Achille P, Ponticelli C. Environmental detection of Microsporum canis arthrospores in the households of infected cats and dogs. J Feline Med Surg. 2003;5(6):323–328. doi:10.1016/S1098-612X(03)00071-8
49. Sparkes AH, Gruffydd-Jones TJ, Shaw SE, Wright AI, Stokes CR. Epidemiological and diagnostic features of canine and feline dermatophytosis in the United Kingdom from 1956 to 1991. Vet Rec. 1993;133(3):57–61. doi:10.1136/vr.133.3.57
50. Marchisio VF, Gallo MG, Tullio V, Nepote S, Piscozzi A, Cassinelli C. Dermatophytes from cases of skin disease in cats and dogs in Turin, Italy. Mycoses. 1995;38(5–6):239–244. doi:10.1111/j.1439-0507.1995.tb00059.x
51. Breuer-Strosberg R. [Reported frequency of dermatophytes in cats and dogs in Austria].. Dtsch Tierarztl Wochenschr. 1993;100(12):483–485.
52. Cabañes FJ, Abarca ML, Bragulat MR. Dermatophytes isolated from domestic animals in Barcelona, Spain. Mycopathologia. 1997;137(2):107–113. doi:10.1023/a:1006867413987
53. Scott D, Miller W, Griffin C. Fungal Skin Diseases. Muller & Kirk’s Small Animal Dermatology.
54. Sparkes AH, Werrett G, Stokes CR, Gruffydd-Jones TJ. Microsporum canis: inapparent carriage by cats and the viability of arthrospores. J Small Anim Pract. 1994;35(8):397–401. doi:10.1111/j.1748-5827.1994.tb03861.x
55. Boyanowski KJ, Ihrke PJ, Moriello KA, Kass PH. Isolation of fungal flora from the hair coats of shelter cats in the Pacific coastal USA. Vet Dermatol. 2000;11(2):143–150. doi:10.1046/j.1365-3164.2000.00161.x
56. Chmel L, Buchvald J. Ecology and transmission of Microsporum gypseum from soil to man. Sabouraudia. 1970;8(2):149–156. doi:10.1080/00362177085190791
57. Aridogan IA, Izol V, Ilkit M. Superficial fungal infections of the male genitalia: A review. Crit Rev Microbiol. 2011;37(3):
58. Lu S, Xi LY, Zhang JM, Lu CM. Pseudomembranous-like tinea of the scrotum: report of six cases. Mycoses. 2009;52(3):282–284. doi:10.1111/j.1439-0507.2008.01557.x
59. Huan T, Lan XM, Zhou CJ, Yang XC. Pseudomembranous-like tinea of the scrotum Infected by Microsporum gypseum in a young man. Indian J Dermatol. 2015;60(4):422. doi:10.4103/0019-5154.160531
60. Chen Z, Bi X, Gu J, Xu X, Wang Y, Wu J. White paint dot-like lesions of the scrotum: microsporum gypseum infection. Australas J Dermatol. 2013;54(4):e95–6. doi:10.1111/j.1440-0960.2012.00954.x
61. Qianggiang Z, Limo Q, Jiajun W, Li L. Report of two cases of tinea infection with scutula-like lesions caused by Microsporum gypseum. Int J Dermatol. 2002;41(6):372–373. doi:10.1111/j.1365-4632.2002.1505_2.x
62. Li J, Li Y, Liu X, et al. Pseudomembranous-like tinea of the scrotum: two cases. Eur J Dermatol. 2013;23(3):416–417. doi:10.1684/ejd.2013.2061
63. Dekio S, Li-mo Q, Kawasaki Y, Jidoi J. Tinea of the scrotum–report of a case presenting as lichenified plaques. J Dermatol. 1990;17(7):448–451. doi:10.1111/j.1346-8138.1990.tb01673.x
64. Miranda MF, de Brito AC, Zaitz C, de Carvalho TN, Carneiro FR. Microsporum gypseum infection showing a white-paint-dot appearance. Int J Dermatol. 1998;37(12):956–957. doi:10.1046/j.1365-4362.1998.00562.x
65. Prochnau A, de Almeida HL, Souza P. Scutular tinea of the scrotum: report of two cases. Mycoses. 2005;48(3):162–164. doi:10.1111/j.1439-0507.2005.01097.x
66. Bakos L, Bonamigo RR, Pisani AC, Marianti JC, Mallmann R. Scutular favus-like tinea cruris et pedis in a patient with AIDS. J Am Acad Dermatol. 1996;34(6):1086–1087. doi:10.1016/s0190-9622(96)90296-0
67. Thakur R, Kalsi AS. Outbreaks and epidemics of superficial dermatophytosis due to Trichophyton mentagrophytes complex and Microsporum canis: global and Indian scenario. Clin Cosmet Investig Dermatol. 2019;12:887–893. doi:10.2147/CCID.S220849
68. Hawksworth DL, Crous PW, Redhead SA, et al. The Amsterdam declaration on fungal nomenclature. IMA Fungus. 2011;2(1):105–112. doi:10.5598/imafungus.2011.02.01.14
69. de Hoog GS, Chaturvedi V, Denning D, et al. ISHAM Working Group on Nomenclature of Medical Fungi. Name changes in medically important fungi and their implications for clinical practice. J Clin Microbiol. 2015;53(4):1056–1062. doi:10.1128/JCM.02016-14
70. Moretti A, Agnetti F, Mancianti F, et al. Dermatophytosis in animals: epidemiological, clinical and zoonotic aspects. G Ital Dermatol Venereol. 2013;148(6):563–572.
71. El-Gohary M, van Zuuren EJ, Fedorowicz Z, et al. Topical antifungal treatments for tinea cruris and tinea corporis. Cochrane Database Syst Rev. 2014;8:CD009992.
72. Van Zuuren EJ, Fedorowicz Z, El-Gohary M. Evidence-based topical treatments for tinea cruris and tinea corporis: a summary of a Cochrane systematic review. Br J Dermatol. 2015;172(3):616–641.
73. Sahoo AK, Mahajan R. Management of tinea corporis, tinea cruris, and tinea pedis: a comprehensive review. Indian Dermatol Online J. 2016;7(2):77–86.
74. Sardana K, Mahajan K, Mrig PA. Fungal Infections: Diagnosis and Treatment.
75. Nenoff P, Krüger C, Paasch U, et al. Mycology- an update part 3: dermatomycoses: topical and systemic therapy. J Dtsch Dermatol Ges. 2015;13(5):387–410.
76. Expert Consensus on the management of dermatophytosis in India (ECTODERM India). BMC Dermatol. 2018;18:6. doi:10.1186/s12895-018-0073-1
77. Pilmis B, Jullien V, Sobel J, Lecuit M, Lortholary O, Charlier C. Antifungal drugs during pregnancy: an updated review. J Antimicrob Chemother. 2015;70:14–22.
78. Schaller M. Dermatomycoses and inflammation: the adaptive balance between growth, damage, and survival. J Mycol Med. 2015;25:e44–58.
79. Rengasamy M, Chellam J, Ganapati S. Systemic therapy of dermatophytosis: practical and systematic approach. Clin Dermatol Rev. 2017;1:S19–23.
80. Singh A, Masih A, Khurana A, et al. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses. 2018;61(7):477–484. doi:10.1111/myc.12772
81. Yamada T, Maeda M, Alshahni MM, et al. Terbinafine resistance of Trichophyton clinical isolates caused by specific point mutations in the squalene epoxidase gene. Antimicrob Agents Chemother. 2017;61(7):e00115–17.
82. Hsieh A, Quenan S, Riat A, Toutous-Trellu L, Fontao L. A new mutation in the SQLE gene of Trichophyton mentagrophytes associated to terbinafine resistance in a couple with disseminated tinea corporis. J Mycol Med. 2019;29(4):352–355. doi:10.1016/j.mycmed.2019.100903
83. Czalka VA, Zuberbler T. Lokale Kombinationstherapie bei entzundlichen Dermatomykosen. Hautarzt. 2015;66:360–369.
84. Fungal skin infection – foot. 2014.http://cks.nice.org.uk/fun-gal-skin-infection-foot#!scenario.
85. Fungal skin infection – body and groin. 2014. http://cks.niceorg.uk/fungal-skin-infection-body-and-groin#!scenario.
86. Schaller M, Friedrich M, Papini M, Pujol RM, Veraldi S. Topical antifungal‐corticosteroid combination therapy for the treatment of superficial mycoses: conclusions of an expert panel meeting. Mycoses. 2016;59:365–373. doi:10.1111/myc.12481
87. Moriello KA, Coyner K, Paterson S, Mignon B. Diagnosis and treatment of dermatophytosis in dogs and cats. Clinical consensus guidelines of the World Association for Veterinary Dermatology. Vet Dermatol. 2017;28:266–e68. doi:10.1111/vde.12440
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.