Back to Journals » International Journal of Nanomedicine » Volume 17
Update on the Use of PET/MRI Contrast Agents and Tracers in Brain Oncology: A Systematic Review
Authors Smeraldo A , Ponsiglione AM , Soricelli A, Netti PA, Torino E
Received 12 February 2022
Accepted for publication 29 April 2022
Published 29 July 2022 Volume 2022:17 Pages 3343—3359
DOI https://doi.org/10.2147/IJN.S362192
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Alessio Smeraldo,1– 3,* Alfonso Maria Ponsiglione,1,* Andrea Soricelli,4 Paolo Antonio Netti,1– 3 Enza Torino1– 3
1Department of Chemical, Materials and Production Engineering, University of Naples “Federico II”, Naples, 80125, Italy; 2Interdisciplinary Research Center on Biomaterials, CRIB, Naples, 80125, Italy; 3Center for Advanced Biomaterials for Health Care, CABHC, Istituto Italiano di Tecnologia, IIT@CRIB, Naples, 80125, Italy; 4Department of Motor Sciences and Healthiness, University of Naples “Parthenope”, Naples, 80133, Italy
*These authors contributed equally to this work
Correspondence: Enza Torino, Department of Chemical, Materials and Production Engineering, University of Naples “Federico II”, Piazzale Tecchio 80, Naples, 80125, Italy, Tel +39-328-955-8158, Email [email protected]
Abstract: The recent advancements in hybrid positron emission tomography–magnetic resonance imaging systems (PET/MRI) have brought massive value in the investigation of disease processes, in the development of novel treatments, in the monitoring of both therapy response and disease progression, and, not least, in the introduction of new multidisciplinary molecular imaging approaches. While offering potential advantages over PET/CT, the hybrid PET/MRI proved to improve both the image quality and lesion detectability. In particular, it showed to be an effective tool for the study of metabolic information about lesions and pathological conditions affecting the brain, from a better tumor characterization to the analysis of metabolic brain networks. Based on the PRISMA guidelines, this work presents a systematic review on PET/MRI in basic research and clinical differential diagnosis on brain oncology and neurodegenerative disorders. The analysis includes literature works and clinical case studies, with a specific focus on the use of PET tracers and MRI contrast agents, which are usually employed to perform hybrid PET/MRI studies of brain tumors. A systematic literature search for original diagnostic studies is performed using PubMed/MEDLINE, Scopus and Web of Science. Patients, study, and imaging characteristics were extracted from the selected articles. The analysis included acquired data pooling, heterogeneity testing, sensitivity analyses, used tracers, and reported patient outcomes. Our analysis shows that, while PET/MRI for the brain is a promising diagnostic method for early diagnosis, staging and recurrence in patients with brain diseases, a better definition of the role of tracers and imaging agents in both clinical and preclinical hybrid PET/MRI applications is needed and further efforts should be devoted to the standardization of the contrast imaging protocols, also considering the emerging agents and multimodal probes.
Keywords: PET/MRI, contrast agents, radiotracers, brain oncology, medical imaging
Introduction
At present, Magnetic Resonance Imaging (MRI) is the principal diagnostic modality for evaluating patients with brain lesions to diagnose and localize brain tumors. It provides excellent soft-tissue characterization capabilities, comparatively high resolution, and high availability.1 However, on the downside, its specificity for neoplastic tissue is low, hampering the evaluation of the grade of malignancy, tumor progression or potential growth of a lesion.2 Furthermore, MRI can present limitations in assessing treatment response after surgery, chemotherapy, and radiotherapy or in quantifying tissue changes caused by inflammation, demyelination, infection, and ischemia.3
Another advanced imaging technique, which has been extensively adopted in brain cancer patients, is Positron Emission Tomography (PET), a molecular imaging technique relying on the detection of emitted photons from radiotracers to provide dynamic functional molecular imaging. PET allows the assessment of biological processes, such as glucose consumption and amino acid uptake non-invasively and quantitatively. Still, it is not suitable for revealing structural aberrations in the white and gray matters. In addition, it has a low spatial resolution, cannot be used to detect rapid changes in brain activation, and has high costs due to its complex equipment.4,5 Among the main advantages, PET emerges for the possibility to co-register medical images with other imaging modalities.
The integration of these two techniques for the development of simultaneous multimodal imaging has become particularly relevant in the oncology field, where several diagnostic biomarkers can be combined to assess tumor microenvironment.6–10 Moreover, in recent years, an increase in the utilization of hybrid PET/MRI scanners has been registered, allowing comparative metabolic and anatomical imaging at high resolution.2,5,11 Indeed, PET/MRI is a tool that combines simultaneously the high resolution provided by MRI for anatomical details and the high functional sensitivity of PET. These coupled features appear to be significantly advantageous over independent PET and MRI examinations in better understanding tumor characteristics that could be useful for surgery and radiation therapy.7,8,11–13
Studies show that PET/MRI and PET/CT perform equally well in oncology or that PET/MRI has minor to moderate advantages over PET/CT.14 In addition, compared to PET/CT, hybrid PET/MRI systems present higher costs for purchase, installation, and maintenance and usually require longer scanning time.14 However, besides the main advantages of the PET/MRI lying in the decreased radiation dose and improved motion,15 in the application to the brain pathologies, it has been proved that PET/MRI offers an increased contrast of soft-tissue compared to PET/CT allowing to distinguish between grey matter, white matter and cerebral spinal fluid, providing a better anatomic contrast and boundaries definition.6 Moreover, through the use of specific sequences, complementary biological information such as cell density and apoptosis (diffusion-weighted (DW) MRI) or angiogenesis (perfusion-weighted (PW) MRI) can be obtained.3,6,7,10,13 In addition, the abnormal uptake of a paramagnetic contrast agent (CA) can highlight possible pathological blood-brain-barrier (BBB) dysfunctions.6,7 Instead, PET offers high sensitivity and specificity thanks to the possibility of using a wide range of tracers. According to the tumor properties to analyse, a proper radiopharmaceutical should be used. [18F]fluorodeoxyglucose ([18F]FDG) is the most commonly used PET tracer due to the higher glucose metabolism that tumor cells exhibit compared to the surrounding healthy tissues.6,8,10 [18F]FDG crosses the BBB, being trapped in cancer cells after phosphorylation.16 Indeed, in the early 1970s, researchers proved the ability of beta-emitting 14Cdeoxyglucose (DG) to cross the BBB.17 Similarly to glucose, the [18F]FDG is transported into cells via glucose transporters, and it is phosphorylated by the hexokinase system, but it cannot be metabolized and, therefore, it persists in the tissue for an extended period of time as a polar metabolite.18,19 This behavior allows both mapping of regional function in the brain and visualizing tumor on FDG-PET scans. However, healthy brain tissues have a high metabolism, leading to low tumor-brain contrast.6 Despite the [18F]FDG is widely used in clinical practice, it has a relatively low specificity and shows high background uptake by the normal brain. These limitations have driven the development of amino acid PET tracers.6,8,13 In fact, the unregulated protein synthesis in malignant tumors, a symptom of an increased cell proliferation activity, can be highlighted by the elevated uptake of these amino acid tracers.6,8,10 Typical examples are O-(2-[18F]fluoroethyl)-L-tyrosine ([18F]FET), 3,4-dihydroxy-6-[18F]-fluoro-L-phenylalanine ([18F]FDOPA), 3’-deoxy-3’-[18F]fluorothymidine ([18F]FLT), and [11C]methionine ([11C]MET).3,6,8,13,20 In particular, the [18F]FET is emerging as an optimal radiotracer to differentiate between low- and high-grade tumors with high sensitivity (94%) and specificity (100%).3,6,8,10,21,22
PET/MRI is becoming a well-established technique for brain tumor imaging thanks to the above-mentioned advantages. Consequently, the choice of suitable PET tracers is essential for the specific clinical purpose. Simultaneously, MRI, both with and without CAs, allows the investigation of the tumor also from a morphological perspective.
In the present work, we aim to provide a systematic review of the growing use of PET/MRI in the brain oncology area, focusing the attention on the trend of PET tracers and MRI CAs, which are usually employed to perform hybrid PET/MRI studies of brain tumors.
Materials and Methods
Eligibility Criteria
The literature review presented in this study was carried out in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2015 guidelines. Only studies illustrating, at the same time, all the following aspects were included in this review:
- use of a hybrid PET/MRI diagnostic system;
- description of the PET/MRI protocol;
- focus on oncology applications;
- focus on brain oncology and related topics (eg studies on brain metastases generated by other types of tumors, or phantom studies of brain tumors).
These inclusion criteria were used as the basis for the literature screening. Then, further refinements to the search strategy and specific exclusion criteria were applied, as detailed in the study selection paragraph.
Information Sources and Search Strategy
The following three electronic databases were used for an extensive literature search: PubMed, Scopus, and Web of Science. All the mentioned databases were explored by using the following search strategy:
Title and Abstract containing the following keywords: (“pet-mr” OR “pet mr” OR “pet/mr” OR “pet-mri” OR “pet mri” OR “pet/mri”) AND “brain” AND (“tumor” OR “tumors” OR “cancer” OR “cancers” OR “oncology”).
In addition, duplicate publications were removed, the search was limited to article-type publications only (reviews, conference proceedings, book chapters and other types of publication were excluded), language was restricted to English publications only.
Moreover, the search was defined in a specific time-frame: from 01 January 2012 to 31 January 2021. This is due to the fact that the use of hybrid PET/MRI scanners has recently increased in the clinical Nuclear Medicine field, with the first commercially available whole-body PET/MRI systems introduced and certified for routine clinical use in January 2011, four years after the development of the prototype designed for brain imaging in 2007.23,24 Even though studies based on sequential PET and MRI acquisitions have been widely performed in the past, we aimed to investigate only the most recent works focused on synchronous PET/MRI acquisitions, and therefore we decided to start the search just one year after the establishment of hybrid PET/MRI scanners in the clinical practice. No further studies have been collected from other external sources.
Study Selection
The study selection process was carried out in accordance with the PRISMA flow diagram. After the screening of the databases, the duplicate publications removal, the selection of year, language, and publication type as described in the previous paragraph, the full text of the selected articles were examined in order to check their eligibility according to the criteria previously defined. In this phase, the full-text assessment was determined by the distinction between those articles using a hybrid PET/MRI system and those acquiring sequential PET and MRI images for post-processing. The latter were then excluded.
In addition, the full-text examination allowed us to discard further duplicates, non-English papers, conference proceedings, and review articles that were not identified in the previous phases of the search.
Finally, a more in-depth reading of the full-texts enabled the exclusion of those studies not focused on brain oncology and related topics (eg studies on brain metastases generated by other types of tumors, or phantom studies of brain tumors), without providing accurate descriptions of the PET/MRI protocols, and with final aims being out of the scope of this systematic review.
Data Collection
Based on a customized Microsoft Excel form, data were collected linking to each paper the following information: Name of the First Author, the Title of the Article, Year of Publication, Digital Object Identifier (DOI) and Abstract. The total number of papers, found at the end of the filtering phase, was equally divided between review authors. Possible doubts about their categorization were discussed until a consensus was reached. Studies (such as conference papers, reviews) were wrongly categorized by the electronic database as “articles” and so identified and excluded. Successively, a second screening was carried out on the full-text articles to collect further information about the study phase, tracers administered to perform PET/MRI and the oncological pathology of patients involved in the study. In particular, exploring the full-text articles allowed us to understand if PET/MRI brain images were acquired simultaneously or not to discard cases where the inclusion criteria were not respected (for example, fusion images after the acquisition with a single modality). No contact with the authors of the records for complementary information was necessary.
Data Clustering
The data were initially divided into subgroups according to the study and validation phases: clinical, preclinical, and phantom. However, a few were counted twice due to the presence in the same research of data belonging to two of the previous subcategories. In addition, a further criterion was used to cluster studies depending on PET and MRI tracers. Firstly, a division was created based on the use or not of the MRI CA. Despite the introduction of the PET/MRI hybrid technology for more than 10 years, a double injection is performed using an agent for each diagnostic technique. In particular, while the use of a tracer is essential for PET analysis, this is not true for MRI functioning since this latter diagnostic technique is usually used as an anatomical reference to support PET modality. Among MRI CAs, gadolinium-based ones are widely used in clinical practice covering almost the totality of the studies. Successively, both PET tracers and MRI CAs were split into different categories. In the case of PET tracers, a distinction was made based on the nuclide (mainly fluorine-18, carbon-11 and gallium-68) and their labeling, while for MRI, more specifically for gadolinium-based CAs, the chelating agent was the clustering criterion.
Risk of Bias
Data derived from the studies were standardized using an Excel form agreed by reviewers after the exploration of full-text articles in order to reduce possible biases. Moreover, a specific comments area for each article was created to allow reviewers to report and discuss any doubts about the collocation in a specific category or possible exclusions. In addition, a standardization was performed to group together the same agents differently named across studies (chemical name, trade name or other synonyms).
Results Synthesis and Analysis
As previously described, two macro-categories were created based or not on the use of the MRI CA. In the presence of the MRI CA, an additional group called “not specified” was created after widely noticing that in many studies the MRI CA was not mentioned in the clinical protocol. The analysis of the collected data was carried out to bring out possible variations or trends in the use of PET tracers, in combination or not with specific MRI CAs, also highlighting different time intervals. The analysis was graphically represented, using OriginPro v2017 software, through histograms that optimally show the distribution of numerical data. Moreover, these latter were often organized in panels with the aim to help readers make comparisons between several data.
Results
Literature Search
The number of records found through the computer-based search is reported in Table 1.
 |
Table 1 Database Distribution of Found Records |
A total number of 534 records were found from PubMed, Scopus, and Web of Science databases. The effective number of records after duplicates removal was 257.
A computer-aided screening of the remaining records was carried out in three main steps, as briefly reported in the methodological section and outlined in Figure 1: (i) screening by year; (ii) screening by language; (iii) screening by publication type.
 |
Figure 1 Article selection process through the PRISMA flow diagram. |
After the automated screening, 129 full-text publications were examined by the reviewers to check the eligibility. Among these publications, 75 articles were removed due to the following reasons:
- 46 papers did not use a hybrid PET/MRI system;
- 17 papers did not sufficiently specify or describe the adopted PET/MRI protocol;
- 5 papers were out of the scope of this systematic review (no focus on brain oncology and related topics, eg studies on brain metastases generated by other type of tumors, or phantom studies of brain tumors);
- 5 papers were conference proceedings (not identified in the previous computer-aided screening);
- 1 paper was a duplicate (not identified in the previous computer-aided screening);
- 1 paper was written in non-English language (not identified in the previous computer-aided screening).
At the end of the selection process, the remaining 54 articles were included in both the qualitative and quantitative syntheses.
The included articles present both clinical and preclinical studies as well as works on phantoms, as detailed in the following Tables 2–4, respectively, which show the results of the literature search in alphabetical order (by the last name of the first author) with details on the year of the study, PET tracers, and MRI CAs used in the presented PET/MRI protocol, as well as the focus of the study.
 |
Table 2 Clinical Studies Included in the Database |
 |
Table 3 Preclinical Studies Included in the Database |
 |
Table 4 Phantom Studies Included in the Database |
The overall distribution of the studies, divided into clinical, preclinical, and phantom, is shown in Figure 2 (additional graphs regarding the types of study included and their geographical distribution are reported in the Supplementary Materials, Figures S1–S3).
 |
Figure 2 Articles distribution based on the type of study in the whole (A) and in three specific time frames (B) (2012–2014, 2015–2017, 2018–2021). |
While Figure 2A shows the overall distribution of the selected studies, Figure 2B presents the same studies grouped into three-time intervals (2012–2014, 2015–2017, 2018–2021) to reveal the increasing trend in the number of brain oncology PET/MRI studies within the first years from the introduction of the hybrid PET/MRI system (2012–2014) up to the most recent works on this topic.
PET/MRI Studies with and without MRI Contrast Agents
The selected articles have been divided making a distinction between PET/MRI studies where the only PET tracer is included in the protocol from those where an MRI CA is additionally used (Figure 3).
Taking into account the overall distribution of articles without any distinction between clinical, preclinical and phantom studies, there seems to be a slight tendency to use the only PET tracers (see Figure 3A). However, looking in detail at the three time intervals (Figure 3B), an increasing trend in the use of both agents to support the clinical analysis comes out. In fact, the ratio has grown from 6 to 0.86 in the last years in favor of “PET and MRI tracers” category, demonstrating the importance for clinicians to enhance image contrast for both modalities to better visualize possible functional and anatomical alterations.
PET Tracers Used in PET/MRI with and without MRI Contrast Agents
A more focused analysis of the PET tracers has been successively carried out to highlight possible trends in the use of specific tracers in the presence or not of an MRI CA (Figure 4).
Firstly, a distinction has been made according to the PET molecule’s radioisotope. Indeed, as shown in Figure 4A and C, it is evident that, in both figures, fluorine-18 is the most widely used (around 77% in Figure 4A and around 69% in Figure 4C). The remaining percentage is shared between gallium-68 and carbon-11 radioisotopes, the former being more used in the absence of MRI CAs (Figure 4A) while the latter in the presence of MRI CAs (Figure 4C). In fact, the gallium-68 radioisotope is more present in studies based only on PET tracers to the detriment of the carbon-11 radioisotope (Figure 4C). The introduction of an MRI CA (Figure 4A) produces percentage changes from 7.5% to 19.23% and from 15% to 7.69% for carbon-11 and gallium-68, respectively. A special mention is made for the [64Cu]Cu-NOTA-IO-MAN tracer that represents the only multimodal one, among all the selected studies, able to provide contrast for both PET and MRI modalities at the same time.73 It represents an example of the growing research trend moving towards the design of multimodal imaging probes.
An insight into each radioisotope category has been performed in order to understand the ligands mostly employed in protocols (see Figure 4B and D). Regarding the fluorine-18 radioisotope, [18F]FDG is the most widely used ligand, followed by [18F]FET. However, a growing use of the latter over time can be observed in Figure 5.
 |
Figure 5 Categorization of PET tracers used in three specific time frames both in presence and not of the MRI CA. |
In particular, in the “PET tracers without MRI CA” category, the [18F]FDG remains the most used tracer in the examined studies over time. On the other side, in the presence of MRI CAs, the temporal trend shows how [18F]FET is increasingly used, in step with [18F]FDG within the most recent time window (2018–2021). In the case of carbon-11, the MET ligand covers almost all the studies (only one protocol with [11C]temozolomide, [11C]TMZ, has been found). For the gallium-68, a uniform distribution of different ligands can be observed over time.
MRI Contrast Agents Used in PET/MRI
Gadolinium-based CAs are the most widely used MRI contrast enhancers even in hybrid PET/MRI protocols, as displayed in Figure 6.
As from Figure 6A, 62.5% of the overall MRI CAs used in PET/MRI studies include gadolinium-based CAs. In particular, Dotarem is present in selected studies in a percentage equal to 29.1%, followed by Gadovist at 16.7%, and then Magnevist at 12.5%. In addition to the [64Cu]Cu-NOTA-IO-MAN, previously mentioned, only one protocol with an iron-based MRI CA (Feraheme) has been found.52 Moreover, when looking at Figure 6A, a slice of around 29% is labeled as “Not specified” since it refers to those studies where the MRI CA was not explicitly defined in the protocol. From an insight into the temporal trend, again divided into three-time windows (Figure 6B), it does not emerge a preference for the use of MRI CAs in PET/MRI protocols over the years.
Finally, in Figure 7, the three leading MRI CAs (Dotarem, Magnevist and Gadovist) are related to the corresponding PET tracer used in the same study protocol. When looking at column colors, the radioisotope fluorine-18 is largely employed, with a slightly higher preference for [18F]FET rather than for the [18F]FDG, widely used in studies without MRI CAs. In addition, carbon-11 is used in three studies, while nowhere else the radioisotope gallium-68 is used in combination with one of these three MRI CAs.
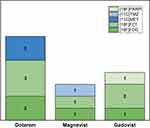 |
Figure 7 PET tracers used in protocols where one of the three most used MRI CAs is present. |
Discussion and Conclusions
We have systematically reviewed the use of the PET tracers and MRI CAs as employed in hybrid PET/MRI imaging studies of the brain, with a specific focus on the oncology field. The most widely used PET imaging approaches target the glycolytic flux using [18F]FDG, highly used for neurological applications. However, the increasing understanding of tumorigenesis has fostered the development of several imaging strategies intended to visualize tumor burden more specifically. Many radiotracers better delineate malignant cells than [18F]FDG, which does not detect malignant tissue with a high degree of sensitivity or specificity and has high background brain uptake. Nevertheless, those radiotracers that have been evaluated after chemoradiation also have shown uptake in nonmalignant processes, and their specificity for cancer is currently estimated to be between 60% and 90%.
In recent years, interest has increased towards the use of amino acid tracers, such as [18F]FET, for tumor grading, treatment planning, biopsy guidance, and glioma imaging for prognosis and treatment response assessment.12 The amino acid tracer FET was used in 27.8% of the total reviewed works. It may play a more critical role than FDG in imaging gliomas and meningioma because it can identify tumor borders with superior tumor-to-background contrast providing clearer borders of lesions.12,65 A major advantage of these tracers is their ability to cross the intact BBB through amino acid transport, as confirmed by several recent studies, revealing that areas with increased FET uptake correspond to the tumor cell distribution.64 Another amino acid tracer is the [18F]FDOPA, which has a 5.55% response in the articles viewed. The uptake of [18F]FDOPA in the normal brain is relatively low, improving visualization of low-grade tumors, delineating the extent of the tumor, differentiating neoplastic from non-neoplastic tissue and predicting response to therapy. Tumor cell uptake of [18F]FDOPA utilizes a transporter upregulated in brain tumors.37 Among the different amino acid tracers [11C]MET PET is well characterized for the evaluation of glioma, especially for hypo- or isometabolic lesions, and has been available for the last decades in clinical routine (found in 12.96% of papers) as it is convenient and efficient in its radiochemical production. The uptake of [11C]MET is mediated by the neutral L-amino acid transporter that serves the increased demand for amino acids in tumor cells. The distribution of [11C]MET has potential to characterise primary brain tumor/metastases, assess the efficacy of oncological treatment and differentiate radionecrosis from tumor recurrence. [11C]MET PET has been shown in previous studies to identify suspected/recurrent glioma with high sensitivity (range 96–100%), specificity (range 60–88%) and diagnostic accuracy (range 82–94%).33
Compared with [18F]FDG, amino acid PET tracers, such as [11C]MET, [18F]FET and [18F]FDOPA, exhibit lower uptake in a healthy brain, do not depend on the compromise of the BBB and present clearer tumor borders with higher tumor-to-background contrast. In particular, the half-life of fluorine-18 (110 min) is longer than that of carbon-11 (20 min), making [18F]FET more suitable for routine clinical applications in neuro-oncology. Furthermore, FET has high in vivo stability and is efficiently synthesized by nucleophilic reactions. In addition, unlike contrast-enhanced MRI, radiolabeled amino acid tracers can visualize both contrast-enhancing and non-enhancing brain tumors. These biological properties, improving estimation and delineation of tumor margins, have important implications for resection, biopsy, and radiation treatment.
Since 2018, new emerging tracers have been added to those most commonly used. With about 18% use of tracers, [68Ga]Ga-Citrate, a Fe3+ biomimetic that binds to apo-transferrin in the blood, can detect high-grade glioma in adults and children.72 Moreover, this latter can also be used to develop targeted internal radiation therapies.25 Cancer cells generally have an elevated demand for Fe3+, an essential nutrient required for various biochemical processes associated with cell growth and proliferation.28 Among the most recently used PET tracers, [68Ga]Ga-Pentixafor targets specifically the CXCR4 receptor and has been applied to lymphoma, leukemia, and myeloma. Although [68Ga]Ga-Pentixafor cannot penetrate the intact BBB, the latter is impaired in patients with brain tumors.66
In light of what has been outlined above, despite the availability of different PET tracers, both in combination with MRI CAs or alone, it emerges that [18F]FDG remains the most important tracer for PET/MRI, as also confirmed in previous investigations rating it among the top 3 tracers used in clinical practice and especially in cancer imaging, despite some limitations for specific cancer types.9,23,78 Moreover, an additional point emerges from the analysis of the selected studies: the lack of multimodal contrast media. In fact, to the best of our knowledge, the use of the PET/MRI system is rarely associated with a hybrid compound able to provide image contrast for both PET and MRI at the same time. Despite the development of multimodal contrast media as one of the main research topics in the biomedical area, it appears that multimodal probes for hybrid PET/MRI in the brain are not mature enough. In this regard, the literature on the design of multimodal imaging agents goes in two main directions: the first one consists of the elaboration of new chemical formulations; the second one aims to synthesize nanovectors able to simultaneously encapsulate and carry two or more CAs or tracers that are used in the clinical practice. Examples of such nanosystems for the encapsulation of a specific contrast medium are widely available in the literature, with particular regard to MRI CAs, and showed to bring additional advantages like the improvement of the contrast-enhancing properties and the targeting capability obtained by means of surface decoration and functionalization.79–89 More efforts are now devoted to developing nanocarriers for multimodal imaging purposes, especially for MRI/optical imaging, MRI/CT, and PET/MRI applications.84,90–97 However, in the case of PET/MRI, the development of these hybrid contrast media is particularly limited by the short half-life of PET tracers. In fact, the need for a cyclotron or a linear accelerator is already a problem in a single PET modality. Consequently, the development of more complex nanosystems exacerbates these difficulties. This consideration could lead to the choice of radionuclides with a longer half-life, such as copper-64, without forgetting that a prolonged circulation time in the human body could be harmful as well.
In conclusion, taking into account the technical advancements in hybrid PET-MRI and its growing clinical value in the neuro-oncology area, it can be observed that there is still variability in the use of PET tracers and MRI CAs, alone or in combination, during PET/MRI protocols for brain tumor investigation, despite standardized protocols can be identified for specific diseases and diagnostic questions. Furthermore, while most widely used PET tracers can be identified in the two categories of [18F]FDG and [18F]FET, the temporal evolution of the acquisition techniques and the clinical and research advancements over the years have left space for different additional tracers. As far as the MRI CAs, while the gadolinium-based ones, remain mainly used also in PET/MRI studies, there seems to be no preferred combination of PET tracers in hybrid PET/MRI studies. Finally, the present study suggests that perspective research efforts could be devoted to a better definition of the role of tracers and CAs in both clinical and preclinical hybrid PET/MRI applications, also given the newly emerging imaging agents and the need for novel multimodal nanoprobes.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lohmann P, Kocher M, Ruge MI, et al. PET/MRI radiomics in patients with brain metastases. Front Neurol. 2020;11:1. doi:10.3389/fneur.2020.00001
2. Lohmann P, Werner J, Shah NJ, Fink GR, Langen KJ, Galldiks N. Combined amino acid positron emission tomography and advanced magnetic resonance imaging in glioma patients. Cancers. 2019;11(2):153. doi:10.3390/cancers11020153
3. Marner L, Henriksen OM, Lundemann M, Larsen VA, Law I. Clinical PET/MRI in neurooncology: opportunities and challenges from a single-institution perspective. Clin Transl Imaging. 2017;5(2):135–149. doi:10.1007/s40336-016-0213-8
4. Mier W, Mier D. Advantages in functional imaging of the brain. Front Hum Neurosci. 2015;9:249. doi:10.3389/fnhum.2015.00249
5. Overcast WB, Davis KM, Ho CY, et al. Advanced imaging techniques for neuro-oncologic tumor diagnosis, with an emphasis on PET-MRI imaging of malignant brain tumors. Curr Oncol Rep. 2021;23(3):34. doi:10.1007/s11912-021-01020-2
6. Puttick S, Bell C, Dowson N, Rose S, Fay M. PET, MRI, and simultaneous PET/MRI in the development of diagnostic and therapeutic strategies for glioma. Drug Discov Today. 2015;20(3):306–317. doi:10.1016/j.drudis.2014.10.016
7. Catana C, Drzezga A, Heiss W-D, Rosen BR. PET/MRI for neurologic applications. J Nucl Med. 2012;53(12):1916–1925. doi:10.2967/jnumed.112.105346
8. Ferda J, Ferdová E, Hes O, Mraček J, Kreuzberg B, Baxa J. PET/MRI: multiparametric imaging of brain tumors. Eur J Radiol. 2017;94:A14–A25. doi:10.1016/j.ejrad.2017.02.034
9. Rosenkrantz AB, Friedman K, Chandarana H, et al. Current status of Hybrid PET/MRI in oncologic imaging. Am J Roentgenol. 2016;206(1):162–172. doi:10.2214/AJR.15.14968
10. Lopci E, Franzese C, Grimaldi M, et al. Imaging biomarkers in primary brain tumors. Eur J Nucl Med Mol Imaging. 2015;42(4):597–612. doi:10.1007/s00259-014-2971-8
11. Heiss W. The potential of PET/MR for brain imaging. Eur J Nucl Med Mol Imaging. 2009;36(1):105–112. doi:10.1007/s00259-008-0962-3
12. Rausch I, Rischka L, Ladefoged CN, et al. PET/MRI for oncologic brain imaging: a comparison of standard MR-based attenuation corrections with a model-based approach for the siemens mMR PET/MR system. J Nucl Med. 2017;58(9):1519–1525. doi:10.2967/jnumed.116.186148
13. Nandu H, Wen PY, Huang RY. Imaging in neuro-oncology. Ther Adv Neurol Disord. 2018;11:1756286418759865. doi:10.1177/1756286418759865
14. Mayerhoefer M, Prosch H, Beer L, et al. PET/MRI versus PET/CT in oncology: a prospective single-center study of 330 examinations focusing on implications for patient management and cost considerations. Eur J Nucl Med Mol Imaging. 2020;47(1):51–60. doi:10.1007/s00259-019-04452-y
15. Ehman EC, Johnson GB, Villanueva-Meyer JE, et al. PET/MRI: where might it replace PET/CT? J Magn Reson Imaging. 2017;46(5):1247–1262. doi:10.1002/jmri.25711
16. Quartuccio N, Laudicella R, Vento A, et al. The additional value of 18F-FDG PET and MRI in patients with glioma: a review of the literature from 2015 to 2020. Diagnostics. 2020;10(6):357. doi:10.3390/diagnostics10060357
17. Alavi A, Reivich M. Guest editorial: the conception of FDG-PET imaging. Semin Nucl Med. 2002;32(1):2–5. doi:10.1053/snuc.2002.29269
18. Wong TZ, Khandani AH, Sheikh A. Chapter 11 - Nuclear medicine. In: Gunderson LL, Tepper JE, editors. Clinical Radiation Oncology.
19. Verberne SJ, Temmerman OPP. 12 - Imaging of prosthetic joint infections. In: Arts JJC, Geurts J, editors. Management of Periprosthetic Joint Infections (Pjis). Woodhead Publishing; 2017:259–285.
20. Glaudemans AWJM, Enting RH, Heesters MAAM, et al. Value of 11C-methionine PET in imaging brain tumors and metastases. Eur J Nucl Med Mol Imaging. 2013;40(4):615–635. doi:10.1007/s00259-012-2295-5
21. Pöpperl G, Kreth FW, Mehrkens JH. FET PET for the evaluation of untreated gliomas: correlation of FET uptake and uptake kinetics with tumor grading. Eur J Nucl Med Mol Imaging. 2007;34(12):1933–1942. doi:10.1007/s00259-007-0534-y
22. Henriksen OM, Larsen VA, Muhic A, et al. Simultaneous evaluation of brain tumor metabolism, structure and blood volume using [18F]-fluoroethyltyrosine (FET) PET/MRI: feasibility, agreement and initial experience. Eur J Nucl Med Mol Imaging. 2016;43(1):103–112. doi:10.1007/s00259-015-3183-6
23. Fendler WP, Czernin J, Herrmann K, Beyer T. Variations in PET/MRI operations: results from an international survey among 39 active sites. J Nucl Med. 2016;57(12):2016–2021. doi:10.2967/jnumed.116.174169
24. Ratib O, Beyer T. Whole-body hybrid PET/MRI: ready for clinical use? Eur J Nucl Med Mol Imaging. 2011;38(6):992–995. doi:10.1007/s00259-011-1790-4
25. Akgun E, Akgun MY, Selçuk HH, Uzan M, Sayman HB. (68)Ga PSMA PET/MR in the differentiation of low and high grade gliomas: is (68)Ga PSMA PET/MRI useful to detect brain gliomas? Eur J Radiol. 2020;130:109199. doi:10.1016/j.ejrad.2020.109199
26. Anazodo UC, Thiessen JD, Ssali T, et al. Feasibility of simultaneous whole-brain imaging on an integrated PET-MRI system using an enhanced 2-point Dixon attenuation correction method. Front Neurosci. 2015;8. doi:10.3389/fnins.2014.00434
27. Bashir A, Binderup T, Vestergaard MB, et al. In vivo imaging of cell proliferation in meningioma using 3’-deoxy-3’-[(18)F]fluorothymidine PET/MRI. Eur J Nucl Med Mol Imaging. 2020;47(6):1496–1509. doi:10.1007/s00259-020-04704-2
28. Behr SC, Villanueva-Meyer JE, Li Y, et al. Targeting iron metabolism in high-grade glioma with 68Ga-citrate PET/MR. JCI Insight. 2018;3(21). doi:10.1172/jci.insight.93999
29. Celebi F, Cindil E, Sarsenov D, Unalan B, Balcı C. Added value of contrast medium in whole-body hybrid positron emission tomography/magnetic resonance imaging: comparison between contrast-enhanced and non-contrast-enhanced protocols. Med Princ Pract. 2020;29(1):54–60. doi:10.1159/000501497
30. Chen KT, Izquierdo-Garcia D, Poynton CB, Chonde DB, Catana C. On the accuracy and reproducibility of a novel probabilistic atlas-based generation for calculation of head attenuation maps on integrated PET/MR scanners. Eur J Nucl Med Mol Imaging. 2017;44(3):398–407. doi:10.1007/s00259-016-3489-z
31. De Luca F, Bolin M, Blomqvist L, Wassberg C, Martin H, Falk Delgado A. Validation of PET/MRI attenuation correction methodology in the study of brain tumors. BMC Med Imaging. 2020;20(1):126. doi:10.1186/s12880-020-00526-8
32. Deuschl C, Goericke S, Grueneisen J, et al. Simultaneous 11C-methionine positron emission tomography/magnetic resonance imaging of suspected primary brain tumors. PLoS One. 2016;11(12):e0167596. doi:10.1371/journal.pone.0167596
33. Deuschl C, Kirchner J, Poeppel TD, et al. (11)C-METPET/MRI for detection of recurrent glioma. Eur J Nucl Med Mol Imaging. 2018;45(4):593–601. doi:10.1007/s00259-017-3916-9
34. Deuschl C, Nensa F, Grueneisen J, et al. Diagnostic impact of integrated 18F-FDG PET/MRI in cerebral staging of patients with non-small cell lung cancer. Acta Radiol. 2017;58(8):991–996. doi:10.1177/0284185116681041
35. Filss CP, Galldiks N, Stoffels G. Comparison of 18F-FET 18 F-FET PET and perfusion-weighted MR imaging: a PET/MR imaging hybrid study in patients with brain tumors. J Nucl Med. 2014;55(4):540–545. doi:10.2967/jnumed.113.129007
36. Franceschi AM, Matthews R, Bangiyev L, Relan N, Chaudhry A, Franceschi D. Added value of including entire brain on body imaging with FDG PET/MRI. Am J Roentgenol. 2018;211(1):176–184. doi:10.2214/AJR.17.18858
37. Gauvain K, Ponisio MR, Barone A, et al. (18)F-FDOPAPET/MRI for monitoring early response to bevacizumab in children with recurrent brain tumors. Neurooncol Pract. 2018;5(1):28–36. doi:10.1093/nop/npx008
38. Gerstner ER, Emblem KE, Chang K, et al. Bevacizumab reduces permeability and concurrent temozolomide delivery in a subset of patients with recurrent glioblastoma. Clin Cancer Res. 2020;26(1):206–212. doi:10.1158/1078-0432.CCR-19-1739
39. Haubold J, Demircioglu A, Gratz M, et al. Non-invasive tumor decoding and phenotyping of cerebral gliomas utilizing multiparametric (18) F-FETPET-MRI and MR Fingerprinting. Eur J Nucl Med Mol Imaging. 2020;47(6):1435–1445. doi:10.1007/s00259-019-04602-2
40. Ho KC, Toh CH, Li SH, et al. Prognostic impact of combining whole-body PET/CT and brain PET/MR in patients with lung adenocarcinoma and brain metastases. Eur J Nucl Med Mol Imaging. 2019;46:467–477. doi:10.1007/s00259-018-4210-1
41. Ishii S, Shimao D, Hara T, et al. Comparison of integrated whole-body PET/MR and PET/CT: is PET/MR alternative to PET/CT in routine clinical oncology? Ann Nucl Med. 2016;30(3):225–233. doi:10.1007/s12149-015-1050-y
42. Izquierdo-Garcia D, Hansen AE, Förster S, et al. An SPM8-based approach for attenuation correction combining segmentation and nonrigid template formation: application to simultaneous PET/MR brain imaging. J Nucl Med. 2014;55(11):1825–1830. doi:10.2967/jnumed.113.136341
43. Jena A, Taneja S, Jha A. Simultaneous PET/MRI: impact on cancer management-A comprehensive review of cases. Indian J Radiol Imaging. 2014;24(2):107–116. doi:10.4103/0971-3026.134381
44. Karlberg A, Berntsen EM, Johansen H, et al. Multimodal (18) F-Fluciclovine PET/MRI and ultrasound-guided neurosurgery of an anaplastic oligodendroglioma. World Neurosurg. 2017;108:
45. Kikuchi K, Togao O, Yamashita K, et al. Diagnostic accuracy for the epileptogenic zone detection in focal epilepsy could be higher in FDG-PET/MRI than in FDG-PET/CT. Eur Radiol. 2020;31:2915–2922. doi:10.1007/s00330-020-07389-1
46. Ladefoged CN, Andersen FL, Kjær A, Højgaard L, Law I. RESOLUTE PET/MRI attenuation correction for O-(2-(18) F-fluoroethyl)-L-tyrosine(FET) in brain tumor patients with metal implants. Front Neurosci. 2017;11:453. doi:10.3389/fnins.2017.00453
47. Ladefoged CN, Marner L, Hindsholm A, Law I, Højgaard L, Andersen FL. Deep learning based attenuation correction of PET/MRI in pediatric brain tumor patients: evaluation in a clinical setting. Front Neurosci. 2019;12:1005. doi:10.3389/fnins.2018.01005
48. Lee SM, Goo JM, Park CM, et al. Preoperative staging of non-small cell lung cancer: prospective comparison of PET/MR and PET/CT. Eur Radiol. 2016;26(11):3850–3857. doi:10.1007/s00330-016-4255-0
49. Marner L, Nysom K, Sehested A, et al. Early postoperative (18) F-FETPET/MRI for pediatric brain and spinal cord tumors. J Nucl Med. 2019;60(8):1053–1058. doi:10.2967/jnumed.118.220293
50. Mehranian A, Belzunce MA, Niccolini F. PET image reconstruction using multi-parametric anato-functional priors. Phys Med Biol. 2017;62(15):5975–6007. doi:10.1088/1361-6560/aa7670
51. Melsaether AN, Raad RA, Pujara AC, et al. Comparison of Whole-Body (18)F FDG PET/MR imaging and whole-body (18)F FDG PET/CT in terms of lesion detection and radiation dose in patients with breast cancer. Radiology. 2016;281(1):193–202. doi:10.1148/radiol.2016151155
52. Muehe AM, Yerneni K, Theruvath AJ, et al. Ferumoxytol does not impact standardized uptake values on PET/MR scans. Mol Imaging Biol. 2020;22:722–729. doi:10.1007/s11307-019-01409-3
53. Ponisio MR, McConathy JE, Dahiya SM, et al. Dynamic 18F-FDOPA-PET/MRI for the preoperative evaluation of gliomas: correlation with stereotactic histopathology. Neurooncol Pract. 2020;7(6):656–667. doi:10.1093/nop/npaa044
54. Preuss M, Werner P, Barthel H, et al. Integrated PET/MRI for planning navigated biopsies in pediatric brain tumors. Childs Nerv Syst. 2014;30(8):1399–1403. doi:10.1007/s00381-014-2412-9
55. Pyatigorskaya N, Sgard B, Bertaux M, Yahia-Cherif L, Kas A. Can FDG-PET/MR help to overcome limitations of sequential MRI and PET-FDG for differential diagnosis between recurrence/progression and radionecrosis of high-grade gliomas? J Neuroradiol. 2020. doi:10.1016/j.neurad.2020.08.003
56. Rausch I, Zitterl A, Berroterán-Infante N, et al. Dynamic [18F]FET-PET/MRI using standard MRI-based attenuation correction methods. Eur Radiol. 2019;29(8):4276–4285. doi:10.1007/s00330-018-5942-9
57. Roytman M, Pisapia DJ, Liechty B, et al. Somatostatin receptor-2 negative meningioma: pathologic correlation and imaging implications. Clin Imaging. 2020;66:18–22. doi:10.1016/j.clinimag.2020.04.026
58. Ruhlmann V, Heusch P, Kühl H, et al. Potential influence of Gadolinium contrast on image segmentation in MR-based attenuation correction with Dixon sequences in whole-body 18F-FDG PET/MR. MAGMA. 2016;29(2):301–308. doi:10.1007/s10334-015-0516-1
59. Sacconi B, Raad RA, Lee J, et al. Concurrent functional and metabolic assessment of brain tumors using hybrid PET/MR imaging. J Neurooncol. 2016;127(2):287–293. doi:10.1007/s11060-015-2032-6
60. Schwenzer NF, Stegger L, Bisdas S, et al. Simultaneous PET/MR imaging in a human brain PET/MR system in 50 patients–current state of image quality. Eur J Radiol. 2012;81(11):3472–3478. doi:10.1016/j.ejrad.2011.12.027
61. Shankar A, Bomanji J, Hyare H. Hybrid PET-MRI imaging in paediatric and TYA brain tumors: clinical applications and challenges. J Pers Med. 2020;10(4):218. doi:10.3390/jpm10040218
62. Slipsager JM, Ellegaard AH, Glimberg SL, et al. Markerless motion tracking and correction for PET, MRI, and simultaneous PET/MRI. PLoS One. 2019;14(4):e0215524. doi:10.1371/journal.pone.0215524
63. Sogani SK, Jena A, Taneja S, et al. Potential for differentiation of glioma recurrence from radionecrosis using integrated (18) F-fluoroethyl-L-tyrosine(FET) positron emission tomography/magnetic resonance imaging: a prospective evaluation. Neurol India. 2017;65(2):293–301. doi:10.4103/neuroindia.NI_101_16
64. Song S, Cheng Y, Ma J, et al. Simultaneous FET-PET and contrast-enhanced MRI based on hybrid PET/MR improves delineation of tumor spatial biodistribution in gliomas: a biopsy validation study. Eur J Nucl Med Mol Imaging. 2020;47(6):1458–1467. doi:10.1007/s00259-019-04656-2
65. Song S, Wang L, Yang H, et al. Static (18) F-FETPET and DSC-PWI based on hybrid PET/MR for the prediction of gliomas defined by IDH and 1p/19q status. Eur Radiol. 2020;31(6):4087–4096. doi:10.1007/s00330-020-07470-9
66. Starzer AM, Berghoff AS, Traub-Weidinger T, et al. Assessment of central nervous system lymphoma based on CXCR4 expression in vivo using 68Ga-Pentixafor PET/MRI. Clin Nucl Med. 2021;46(1):16–20. doi:10.1097/RLU.0000000000003404
67. Stegger L, Martirosian P, Schwenzer N, et al. Simultaneous PET/MR imaging of the brain: feasibility of cerebral blood flow measurements with FAIR-TrueFISP arterial spin labeling MRI. Acta Radiol. 2012;53(9):1066–1072. doi:10.1258/ar.2012.120191
68. Theruvath AJ, Ilivitzki A, Muehe A, et al. A PET/MR imaging approach for the integrated assessment of chemotherapy-induced brain, heart, and bone injuries in pediatric cancer survivors: a pilot study. Radiology. 2017;285(3):971–979. doi:10.1148/radiol.2017170073
69. Verger A, Filss CP, Lohmann P, et al. Comparison of (18) F-FETPET and perfusion-weighted MRI for glioma grading: a hybrid PET/MR study. Eur J Nucl Med Mol Imaging. 2017;44(13):2257–2265. doi:10.1007/s00259-017-3812-3
70. Yan J, Lim JC, Townsend DW. MRI-guided brain PET image filtering and partial volume correction. Phys Med Biol. 2015;60(3):961–976. doi:10.1088/0031-9155/60/3/961
71. Young RJ. Preclinical and first-in-human-brain-cancer applications of [(18)F]poly (ADP-ribose) polymerase inhibitor PET/MR. Neurooncol Adv. 2020;2(1). doi:10.1093/noajnl/vdaa119
72. Zhang J, Tian Y, Li D, et al. (68)Ga-NOTA-Aca-BBN(7-14) PET imaging of GRPR in children with optic pathway glioma. Eur J Nucl Med Mol Imaging. 2019;46(10):2152–2162. doi:10.1007/s00259-019-04392-7
73. Ko GB, Yoon HS, Kim KY, et al. Simultaneous multiparametric PET/MRI with silicon photomultiplier PET and ultra-high-field MRI for small-animal imaging. J Nucl Med. 2016;57(8):1309–1315. doi:10.2967/jnumed.115.170019
74. Schröder S, Wenzel B, Deuther-Conrad W, et al. Synthesis, 18F-radiolabelling and biological characterization of novel fluoroalkylated triazine derivatives for in vivo imaging of phosphodiesterase 2A in brain via positron emission tomography. Molecules. 2015;20:9591–9615. doi:10.3390/molecules20069591
75. Bland J, Mehranian A, Belzunce MA, et al. Intercomparison of MR-informed PET image reconstruction methods. Med Phys. 2019;46(11):5055–5074. doi:10.1002/mp.13812
76. Harries J, Jochimsen TH, Scholz T, et al. A realistic phantom of the human head for PET-MRI. EJNMMI Physics. 2020;7(1):52. doi:10.1186/s40658-020-00320-z
77. Wampl S, Rausch I, Traub-Weidinger T, Beyer T, Gröschl M, Cal-González J. Quantification accuracy of neuro-oncology PET data as a function of emission scan duration in PET/MR compared to PET/CT. Eur J Radiol. 2017;95:257–264. doi:10.1016/j.ejrad.2017.08.024
78. Hope TA, Fayad ZA, Fowler KJ, et al. Summary of the first ISMRM–SNMMI workshop on PET/MRI: applications and limitations. J Nucl Med. 2019;60(10):1340–1346. doi:10.2967/jnumed.119.227231
79. Russo M, Ponsiglione AM, Forte E, Netti PA, Torino E. Hydrodenticity to enhance relaxivity of gadolinium-DTPA within crosslinked hyaluronic acid nanoparticles. Nanomedicine. 2017;12(18):2199–2210. doi:10.2217/nnm-2017-0098
80. De Sarno F, Ponsiglione AM, Grimaldi AM, Netti PA, Torino E. Effect of crosslinking agent to design nanostructured hyaluronic acid-based hydrogels with improved relaxometric properties. Carbohydr Polym. 2019;222:114991. doi:10.1016/j.carbpol.2019.114991
81. Smeraldo A, Netti PA, Torino E. New strategies in the design of paramagnetic CAs. Contrast Media Mol Imaging. 2020;2020:4327479. doi:10.1155/2020/4327479
82. Ponsiglione AM, Russo M, Torino E. Glycosaminoglycans and contrast agents: the role of hyaluronic acid as MRI contrast enhancer. Biomolecules. 2020;10(12):1612. doi:10.3390/biom10121612
83. Russo M, Bevilacqua P, Netti PA, Torino E, Microfluidic A. Platform to design crosslinked Hyaluronic Acid Nanoparticles (cHANPs) for enhanced MRI. Sci Rep. 2016;6:37906. doi:10.1038/srep37906
84. Costagliola Di Polidoro A, Zambito G, Haeck J, et al. Theranostic design of angiopep-2 conjugated hyaluronic acid nanoparticles (Thera-ANG-cHANPs) for dual targeting and boosted imaging of glioma cells. Cancers. 2021;13(3):503. doi:10.1155/2021/666447110.3390/cancers13030503
85. De Sarno F, Ponsiglione AM, Torino E. Emerging use of nanoparticles in diagnosis of atherosclerosis disease: a review. AIP Conf Proc. 2018;1990(1):020021. doi:10.1063/1.5047775
86. Costagliola Di Polidoro A, Grassia A, De Sarno F, et al. Targeting nanostrategies for imaging of atherosclerosis. Contrast Media Mol Imaging. 2021;2021:6664471. doi:10.1155/2021/6664471
87. Patil-Sen Y, Torino E, De Sarno F, et al. Biocompatible superparamagnetic core-shell nanoparticles for potential use in hyperthermia-enabled drug release and as an enhanced contrast agent. Nanotechnology. 2020;31(37):375102. doi:10.1088/1361-6528/ab91f6
88. Abakumov MA, Nukolova NV, Sokolsky-Papkov M, et al. VEGF-targeted magnetic nanoparticles for MRI visualization of brain tumor. Nanomedicine. 2015;11(4):825–833. doi:10.1016/j.nano.2014.12.011
89. Shang L, Wang Q, Chen K, et al. SPIONs/DOX loaded polymer nanoparticles for MRI detection and efficient cell targeting drug delivery. RSC Adv. 2017;7(75):47715–47725. doi:10.1039/C7RA08348C
90. Lahooti A, Sarkar S, Saligheh Rad H, et al. PEGylated superparamagnetic iron oxide nanoparticles labeled with 68Ga as a PET/MRI contrast agent: a biodistribution study. J Radioanal Nucl Chem. 2017;311(1):769–774. doi:10.1007/s10967-016-5058-0
91. Vecchione D, Aiello M, Cavaliere C, Nicolai E, Netti PA, Torino E. Hybrid core shell nanoparticles entrapping Gd-DTPA and 18F-FDG for simultaneous PET/MRI acquisitions. Nanomedicine. 2017;12(18):2223–2231. doi:10.2217/nnm-2017-0110
92. Vecchione D, Grimaldi AM, Forte E, Bevilacqua P, Netti PA, Torino E. Hybrid core-shell (HyCoS) nanoparticles produced by complex coacervation for multimodal applications. Sci Rep. 2017;7:45121. doi:10.1038/srep45121
93. Mishra SK, Kannan S. Doxorubicin-conjugated bimetallic silver–gadolinium nanoalloy for multimodal MRI-CT-optical imaging and pH-responsive drug release. ACS Biomater Sci Eng. 2017;3(12):3607–3619. doi:10.1021/acsbiomaterials.7b00498
94. Mastrogiacomo S, Kownacka AE, Dou W, et al. Bisphosphonate functionalized gadolinium oxide nanoparticles allow long-term MRI/CT multimodal imaging of calcium phosphate bone cement. Adv Healthc Mater. 2018;7(19):1800202. doi:10.1002/adhm.201800202
95. Tammaro O, Costagliola Di Polidoro A, Romano E, Netti PA, Torino E. A microfluidic platform to design multimodal PEG - crosslinked Hyaluronic Acid Nanoparticles (PEG-cHANPs) for diagnostic applications. Sci Rep. 2020;10(1):6028. doi:10.1038/s41598-020-63234-x
96. Lee D, Koo H, Sun I, Ryu JH, Kim K, Kwon IC. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem Soc Rev. 2012;41(7):2656–2672. doi:10.1039/C2CS15261D
97. Ma Y, Huang J, Song S, Chen H, Zhang Z. Cancer-targeted nanotheranostics: recent advances and perspectives. Small. 2016;12(36):4936–4954. doi:10.1002/smll.201600635
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



