Back to Journals » Biologics: Targets and Therapy » Volume 10
Update on the management of Philadelphia chromosome positive chronic myelogenous leukemia: role of nilotinib
Authors Emole J, Talabi T, Pinilla-Ibarz J
Received 30 August 2015
Accepted for publication 9 November 2015
Published 26 February 2016 Volume 2016:10 Pages 23—31
DOI https://doi.org/10.2147/BTT.S67844
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Doris Benbrook
Josephine Emole,1 Taiwo Talabi,2 Javier Pinilla-Ibarz1
1Department of Malignant Hematology, 2Moffitt Program for Outreach Wellness Education and Resources, H Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA
Abstract: Chronic myelogenous leukemia (CML) is a pluripotent stem cell disease characterized by the presence of the Philadelphia chromosome and the bcr-abl gene. The discovery of tyrosine kinase inhibitors (TKIs) revolutionized therapy for CML, such that durable response, increased overall survival, and increased progression-free survival of patients in chronic phase CML is now possible. Due to resistance and intolerance to imatinib, there was need for development of second- and third-generation TKIs for the treatment of CML. This review examines the role of nilotinib, an oral second-generation TKI, in the treatment of Philadelphia positive CML. The pharmacology, efficacy, and safety of nilotinib are critically evaluated. Patient-related issues, including tolerance, drug interactions, and quality of life issues are also examined.
Keywords: chronic myelogenous leukemia, nilotinib, tyrosine kinase inhibitor
Introduction
Chronic myelogenous leukemia (CML) is a pluripotent stem cell disease and one of the myeloproliferative disorders, accounting for approximately 10%–15% of all leukemias. The worldwide annual incidence of CML is approximately 1–1.5 in 100,000 persons. In 2015, it is estimated that there will be 6,660 new cases of CML, and an estimated 1,140 people will die of this disease. The incidence increases with age, and though the disease can occur at any age, the median age at diagnosis is usually in the sixth decade of life. The disease has a slight male predominance.1–3 As a result of BCR-ABL1 tyrosine kinase inhibitor (TKI) therapy, patients with CML now live longer than before, resulting in increased prevalence of the disease. According to one estimate, the prevalence of CML in the United States will increase to approximately 181,000 patients in 2050.4
CML is characterized in 95% of cases by the fusion of Abelson gene on chromosome 9 with the break point cluster region gene on chromosome 22, resulting in the bcr-abl fusion gene (Philadelphia chromosome, Ph) and protein.5 The 210 kDa bcr-abl protein displays a constitutive tyrosine kinase activity, resulting in the malignant phenotype of affected cells by activation of cellular pathways which lead to increased proliferation, increased resistance to apoptosis, and alteration of adhesion properties.6–8
The clinical course of CML consists of three stages: chronic phase (CP), accelerated phase (AP), and blastic phase/crisis (BP) with approximately 95% of patients being diagnosed in the CP of the disease.9 In CML-CP patients, the leukemic cells are well differentiated and proliferate relatively slowly. The most frequent symptoms in CML-CP are non-specific and include fatigue, anorexia, abdominal discomfort, weight loss, and excessive sweating. Uncommonly, patients may also present with evidence of hypermetabolism (night sweats, weight loss, gout, hyperuricemia, fever). Physical examination of the patient may reveal splenomegaly.
If untreated, CP may progress to AP in which white blood cell counts are poorly controlled, and the numbers of immature blasts in the peripheral blood are increased. AP may also be characterized by clonal evolution in which a patient may acquire new chromosomal abnormalities like a second Ph. If untreated, AP may transit into BP. In BP, there is a rapid expansion of differentiation-arrested blast cells and the disease resembles acute leukemia. Cytopenias, infections, bleeding, organ failure, and death can result from BP CML. AP or BP is referred to as advanced phase CML.
Developments in the treatment of Ph+ CML
Historically, treatment for CML had consisted of irradiation of the spleen and conventional chemotherapy. Busulfan was introduced for treatment of CML in the 1950s, and was shown to reduce white cell counts as well as palliate symptoms. However, it was associated with serious side effects, including myelosuppression and hepatic, cardiac and pulmonary fibrosis. Hydroxyurea had a better side effect profile than busulfan and had a more rapid onset of action,10 but like busulfan, did not affect the biological mechanism of the disease. Neither medications led to achievement of cytogenetic remission, neither did they affect progression to advanced phases. Recombinant interferon was later found to induce durable cytogenetic responses (CyRs) and prolong duration of CML-CP in a limited amount of patients11–13 and in addition to cytarabine, it became standard of therapy for CML. The benefit of interferon was less in patients with advanced disease.14
After several studies established the curative potential of allogeneic stem cell transplant (allo-SCT) for CML in the early 1980s,15–17 allo-SCT became the treatment of choice for this disease. However, many patients were not eligible for allo-SCT due to age restrictions or donor availability. Moreover, allo-SCT has substantial risks of treatment-related morbidity and mortality due to drug toxicities, graft-vs-host disease and infections. Therapy with interferon-alpha alone or in combination with cytarabine remained an alternative first-line treatment for patients ineligible for transplantation.
The emergence of TKIs into clinical use approximately 15 years ago revolutionized the management strategy for Ph+ CML. TKIs as a class were developed based on the understanding of the molecular defect underlying the pathogenesis of CML, and they all target the bcr-abl tyrosine kinase. The different TKIs are distinguished by their relative affinities for binding to the bcr-abl1 and other off target kinases. In 2001, the US Food and Drug Administration (FDA) approved imatinib for treatment of CML-CP, based on the results of the International Randomized Study of Interferon and STI571. The study demonstrated imatinib to have significantly better efficacy and tolerability than interferon alpha plus cytarabine in patients with newly diagnosed CML-CP.18
Dasatinib and nilotinib were approved in 2006 and 2007, respectively, for second-line CML treatment and both were approved in 2010 for first-line treatment.19,20 In 2012, bosutinib and ponatinib were approval for second- and subsequent-line treatment of CML.21,22 TKIs, in general, have been found to induce durable response and increase the overall survival and progression-free survival of patients in CML-CP.23 However, advanced phases of CML may still be refractory to available therapies.
Overview of pharmacology of nilotinib
While imatinib, the first bcr-abl TKI, is an effective therapy for CML, and resulted in approximately 83% event-free survival and 93% freedom from progression to accelerated or blast phase at 6 years,23,24 primary and acquired resistance do occur. Moreover, advanced phase CML showed significantly decreased response rates to treatment with imatinib monotherapy, with relapse being common within a year.25,26 Third, some patients develop intolerance to imatinib leading to dose reduction or discontinuation. Several causes of imatinib resistance have been described. Point mutations within the abl kinase domain will reduce the binding affinity of imatinib to the protein and is the most described mechanism of resistance, furthermore, increased expression of bcr-abl kinase through gene amplification, overexpression of the SRC family of kinases as well as other pharmacokinetic and pharmacodynamic factors also have been implicated.27,28 As such, there was need for newer TKIs with more potency against imatinib-resistant CML (so-called second- and third-generation TKIs).
Nilotinib (Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA) is a second-generation phenylaminopyrimidine inhibitor of the bcr-abl. Similar to imatinib, nilotinib binds to the inactive conformation of the abl tyrosine kinase, though with a tighter fit for the abl protein.29 The result is that nilotinib is 10- to 50-fold more potent as an inhibitor of bcr-abl than imatinib and is 10- to 20-fold more active than imatinib in reducing bcr-abl autophosphorylation.29,30 In vitro proliferation assays showed that nilotinib had activity in 32 of 33 imatinib-resistant lines,31 and lacked activity against the T315I mutation.29,30,32 Apart from the bcr-abl kinase, nilotinib also has activity against other kinases such as KIT- and PDGFRα/β.29 Unlike the other second-generation TKIs (dasatinib and bosutinib), nilotinib has minimal effects on Src family kinases.33,34 CML cases with mutations at the Y253H, E255K/V, and F359V/C residues have been described to be less sensitive to nilotinib.35
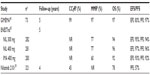
Nilotinib is FDA-approved as front-line treatment of patients with newly diagnosed CP Ph+ CML. It is also approved for treatment of patients with CP or AP Ph+ CML who have resistance or intolerance to prior therapy that included imatinib. The recommended dose of nilotinib is 400 mg twice a day (bid) (in second-line therapy) or 300 mg bid (in front-line therapy) orally to be taken on an empty stomach. The drug is metabolized in the liver mainly by CYP3A4 microsomal enzymes (via oxidation and hydroxylation), though the UDP-glucuronosyltransferase may play a relatively minor role in its metabolism. Its metabolites are not pharmacologically active. Because nilotinib is a competitive inhibitor of cytochrome P450 (CYP) 3A4, CYP2C8, CYP2C9, and CYP2D6, it should not be used concurrently with these medications20 (Table 1). The calculated elimination half-life following multiple daily dosing is approximately 17 hours. Nilotinib is eliminated mainly via the fecal route.
  | Table 1 Important drug interactions with nilotinib |
The most common side effects notable with nilotinib are rash, nausea, fatigue, headache, constipation, diarrhea, and vomiting. Grade 3–4 toxicities (≥10%) include thrombocytopenia, neutropenia, elevation in serum lipase, hyperglycemia, and hypophosphatemia. Nilotinib has been associated with a prolonged QT interval, and sudden death has been reported.
Age, weight, sex, and ethnicity have not been found to affect the pharmacokinetic properties, efficacy, or toxicity of nilotinib.20 No dose adjustment is necessary for renal insufficiency. Recent clinical data in Parkinson’s disease and Lewy body dementia support the penetration of nilotinib into the central nervous system (CNS);36 however, the efficacy of nilotinib in CML affecting the CNS is not well documented.
Nilotinib is classified as a pregnancy category D and is contraindicated in pregnant and/or breastfeeding women. All patients of reproductive age (male or female) should be encouraged to undergo evaluation for fertility preservation prior to starting nilotinib therapy. These patients should remain on contraception during therapy with nilotinib. Nilotinib should be discontinued approximately 3–4 weeks prior to planned conception.
Efficacy of nilotinib for first- and second-line treatment of CML
Second-line therapy
Based on preclinical studies which showed that nilotinib was 20–50 times more potent than imatinib,31 nilotinib was evaluated in a Phase I clinical trial of 119 patients with imatinib-resistant CML or acute lymphoblastic leukemia. The authors reported that nilotinib had a relatively favorable safety profile and was active in imatinib-resistant CML, producing significant hematologic and CyRs in all phases of the disease.37 These encouraging results prompted a Phase II trial to evaluate the efficacy and safety of nilotinib in patients who were resistant or intolerant to imatinib.
The nilotinib 2101 was an open label Phase II study in which patients with Ph+ CML-CP with proven imatinib resistance or intolerance were treated with 400 mg nilotinib was administered orally twice daily and followed up for at least 6 months. To be classified as imatinib resistant in this study, the patients must have been treated with at least 600 mg of imatinib for 3 months. Interim analysis of the first 280 patients enrolled in this study showed that at 6 months, the rate of major CyR (MCyR) was 48%, whereas 31% and 16% had complete CyR (CCyR) and partial CyR, respectively. The estimated survival at 12 months was 95%. Nilotinib was found to be effective in patients harboring bcr-abl mutations associated with imatinib resistance (except T315I), and also in patients with a resistance that were independent of bcr-abl mutations. Grades 3–4 neutropenia and thrombocytopenia were observed in 29% of patients. Notable was the fact that there was minimal cross intolerance with imatinib in terms of the adverse events (AEs).38
At the 48-month follow-up of the nilotinib 2101 study,39 59% of patients had achieved MCyR, whereas 45% achieved CCyR. The estimated rate of OS and PFS both at 48 months was 78% (95% CI, 73%–83%) and 57% (95% CI, 51%–64%), respectively. Deeper levels of molecular responses at 3 and 6 months were reported to positively correlate with long-term outcomes, including PFS and OS at 48 months. Earlier response on treatment was also noted to be related to PFS. Approximately 31% (98 patients) of the patients initially enrolled in the study were able to continue the treatment for at least 48 months. For the patients who discontinued therapy, this was due to disease progression in 30% or AEs in 21%. Overall, nilotinib remained well-tolerated even at this 48 months follow-up, and minimal new hematological toxicity was observed since the original analysis at 24 months. The most frequent drug-related non-hematological AEs were rash (31%), pruritus (26%), and nausea (25%). Grade 3–4 non-hematological AEs occurred in less than 2% of patients and included diarrhea (1.9%), rash (1.9%), headache (1.6%), and arthralgia (1.6%).39
First-line therapy
Two separate Phase II studies initially evaluated the efficacy and safety of nilotinib as first-line therapy for CML-CP. Rosti et al treated a cohort of 73 treatment naïve Ph(+) CML patients, with nilotinib at a dose of 400 mg bid in a GIMEMA CML working party study.40 After a median follow-up of 15 months, the CCgR rate at 1 year was 96%, with a major molecular response (MMR) rate of 85%. Responses were rapid, with 78% CCyR and 52% MMR occurring at 3 months. In the second Phase II study, 51 patients in CML-CP were treated with nilotinib 400 mg bid and after a median follow-up of 17 months, 50 patients (98%) achieved a CCyR, whereas 39 (76%) achieved MMR. Again, these responses occurred rapidly, with 96% of patients achieving CCyR by 3 months and 98% achieving CCyR by 6 months.41
The ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials – Newly Diagnosed) trial was a Phase III multicenter study in which 846 patients with chronic-phase Ph+ CML were randomized in a 1:1:1 ratio to receive nilotinib 300 mg bid or nilotinib 400 mg bid or imatinib 400 mg once daily.42 The primary end point was the rate of MMR at 12 months. The rates of MMR at 12 months were found to be 44% for nilotinib (300 mg dose), 43% for the nilotinib 400 mg dose but 22% for imatinib (P<0.001 for both comparisons). The rates of CCyR by 12 months were also significantly higher for nilotinib (80% for the 300 mg dose and 78% for the 400 mg dose) than for imatinib (65%) (P<0.001 for both comparisons). Nilotinib (either the 300 or 400 mg dose) was also found to lead to significant improvement in time to progression to AP or blast crisis compared with imatinib (P=0.01 and P=0.004, respectively). No patient with progression to the AP or blast crisis had an MMR. The most frequent non-hematological AEs reported for the nilotinib groups were rash, headaches, and nausea, occurring in 36%, 21%, 19% of the nilotinib 400 mg cohort, respectively, and 31%, 14%, 11% of the nilotinib 300 mg group, respectively. Hyperbilirubinemia (53%/62% for nilotinib 300 mg/400 mg dosing, respectively), increased alanine aminotransferase (66%/73% for 300 mg/400 mg dosing, respectively) and increased aspartate aminotransferase (40%/48% for 300 mg/400 mg dosing, respectively) were the most common biochemical abnormalities reported for nilotinib in this study. In all three groups, discontinuations due to aminotransferase and bilirubin elevations were low. Grade 3–4 thrombocytopenia was more common in the nilotinib 400 mg group, occurring in 12% of these patients when compared with 10% in nilotinib 300 mg and 9% in the imatinib group. Neutropenia was more common in the imatinib group (20%) than in either of the nilotinib groups (12% for 300 mg dosing and 10% for 400 mg dosing). Based on the results of this study, nilotinib was approved for the first-line treatment of patients with CML-CP.
At the 5-year follow-up of the ENESTnd study, 60%, 62%, and 50% of patients taking nilotinib 300 mg bid, 400 mg bid, and imatinib 400 mg once a day, respectively, remained on core treatment. Patients on either dosing of nilotinib had significantly greater 5-year MMR (77%) vs imatinib (60%; P≤0.0001 for both comparisons). Similarly, more patients in the nilotinib arms achieved 4.5 log-reduction (MR4.5) (54% for nilotinib 300 mg bid, 52% for nilotinib 400 mg bid, and 31% for imatinib 400 mg daily; P<0.0001). The 5-year freedom from progression to AP/BC was 96% for nilotinib 300 mg (P=0.0403 vs imatinib), 98% for nilotinib 400 mg dosing (P=0.0028 vs imatinib), and 92% for imatinib. Although nilotinib 300 mg did not significantly improve OS compared with imatinib (94% vs 92%; P=0.4881), nilotinib 400 mg improved OS (96% vs 92%; P=0.0266) (Table 2). The 5 year cardiovascular events were highest on nilotinib 400 mg bid and lowest on imatinib: ischemic heart disease rates were 9%, 4%, and 2%, ischemic cerebrovascular events were 3%, 1%, and <1%, whereas peripheral artery disease rates were 3%, 3%, and 0% for nilotinib 400 mg, nilotinib 300 mg, and imatinib, respectively.43
The results of the Phase II study by the GIMEMA CML working party were updated in 2012, after a median follow-up of 51 months44 (Table 2). The cumulative incidence of CCyR for the entire cohort of 73 patients at 12 months was 100%. Only one patient had a confirmed loss of CCyR, whereas three patients had a confirmed loss of MMR due to low adherence (all three still on nilotinib). The overall estimated probability of reaching MR 4.0 was 82%. Eleven patients (15%) discontinued permanently nilotinib due to progression to AP/BP, recurrent episodes of amylase, and/or lipase increase but no pancreatitis, peripheral arterial disease, among other reasons. The estimated probability of overall survival, progression-free survival, and failure-free survival was 97% at 5 years; the estimated probability of event-free survival was 83% at 5 years.
Although data from the ENESTnd long-term follow-up indicate superior cytogenetic and molecular response rates, as well as lower rates of progression to advanced phase CML for nilotinib compared with imatinib 400 mg, no clear OS, or PFS has been established for nilotinib over imatinib 400 mg. While nilotinib is known to show superior response rates compared with imatinib 400 mg daily, no studies have compared nilotinib with higher or adjusted doses of imatinib. There has been no head-to-head comparison of nilotinib and other second-generation bcr-abl TKIs such as dasatinib and bosutinib.
Nilotinib for treatment of advanced phase CML
A Phase II trial by le Coutre et al45 evaluated the efficacy of nilotinib in patients with imatinib-resistant or -intolerant accelerated-phase CML. A total of 119 patients were enrolled and treated with 400 mg of nilotinib twice daily. After a median treatment duration of 202 days (range, 2–611 days), hematological response was observed in 56 patients (47%; 95% CI, 38%–56%), whereas MCyR was observed in 35 patients (29%; 95% CI, 21%–39%). The overall survival rate after 12 months was 79% (95% CI, 70%–87%). The most common grade 3 or higher hematologic AEs were thrombocytopenia (35%) and neutropenia (21%). Grade 3 or higher bilirubin and lipase elevations occurred in 9% and 18% of patients, respectively, and these resulted in treatment discontinuation for one patient.45 The results of this study were updated after 24 months follow-up46 (n=137) and noted a hematological response in 55% of patients, complete hematological response in 31% and MCyR (including some CCyR) in 32%. Responses were durable, with 66% of patients maintaining MCyR at 24 months. The estimated OS rate at 24 months was 70%, whereas the PFS rate at 24 months was 33%. Grade 3–4 neutropenia and thrombocytopenia remained the most common hematological AEs and were each observed in 42% of patients. Non-hematological AEs (rash, pruritus, fatigue) were mostly mild to moderate, similar to findings from the initial study. Grade 3–4 non-hematological AEs were reported in less than 1% of the patients.46
Nilotinib has equally shown some efficacy in the treatment of CML in blast phase, though much lower than its efficacy in CML-CP. Nilotinib at 400 mg bid was evaluated for the treatment of 136 CML patients in BP (n=105 for myeloid blast phase [MBP] and n=31 for lymphoid blast phase [LBP]).47 After a minimum follow-up of 24 months, major hematologic responses were noted in 60% and 59% of MBP and LMP, respectively. MCyRs were noted in 38% (MBP) and 52% (LBP) of patients, whereas CCyRs occurred in 30% and 32%, respectively. Median duration of MCyR was 10.8 (MBP) and 3.2 months (LBP). The survival rates at 12 and 24 months were 42% (MBP 44%, LBP 35%) and 27% (MBP 32%, LBP 10%), respectively. Grade 3–4 neutropenia, thrombocytopenia, and anemia in 68%, 63%, and 47% of patients, respectively. Grade 3–4 hypophosphatemia, hyperbilirubinemia, and lipase elevation were observed in 15%, 11%, and 11% of patients, respectively.47
Post allo-SCT nilotinib has been evaluated in early phase trials and reported to show some efficacy in preventing CML relapse and progression after allo-SCT, but these findings are yet to be confirmed in larger studies.48
Safety and tolerability of nilotinib
Bcr-abl1 TKIs are generally well tolerated. However, longer experience with nilotinib (like other TKIs) has continued to uncover certain AEs of which the clinician and the patients should be aware. AEs of bcr-abl1 are similar but occur at different rates and severities with each of the agents. Cardiovascular AEs, laboratory abnormalities, dermatological side effects, and myelosuppressive effects of nilotinib have been reported in clinical studies as well as in regular clinical practice.
Peripheral arterial occlusive disease (PAOD) has been described in case reports and many clinical studies of nilotinib.49–57 The 5-year follow-up of ENESTnd indicated that PAOD/ischemic heart disease/ischemic cerebrovascular events occurred in 2.5%/3.9%/1.4% of patients on nilotinib 300 mg, 2.5%/8.5%/3.2% of patients on nilotinib 400 mg, and 0%/1.8%/0.4% of patients on imatinib, respectively.43 PAOD has also been reported with bosutinib and dasatinib at much lower incidences,58,59 and ponatinib carries a black-box warning for arterial thrombosis. Because many patients who experience vascular events on nilotinib had at least one cardiovascular risk factor at baseline,49–55 it is prudent to evaluate each patient’s cardiovascular risk prior to initiating nilotinib therapy. While on therapy, patients should continue to be monitored regularly for cardiovascular events.
The ENESTnd 3-year follow-up revealed that none of the patients treated with nilotinib had QTc ≥500 ms, whereas one (0.2%) patient had QTc ≥480 ms, and four (0.7%) patients had ≥60 ms change in QTc from baseline.57 In the 4-year follow-up of the 2101 study of second-line nilotinib after imatinib failure, there was no reported QTc prolongation of >500 ms. There were three new cases of >60 ms increase in QTc from baseline, but no patient discontinued nilotinib therapy because of QTc prolongation.39 A black-box warning has been issued for nilotinib regarding the risk of QTc prolongation and sudden death. Patients with known history of long QT syndrome or taking other medications which are known to prolong QT interval should not be started on nilotinib.20 Electrolyte imbalance (hypokalemia, hypomagnesemia) should be corrected prior to administration of nilotinib and these electrolytes should be monitored periodically during nilotinib therapy. Electrocardiography should be obtained at baseline, 7 days after initiation, as well as periodically thereafter and following any dose adjustments.
Hyperglycemia is a notable AE of nilotinib. The incidence of grade 3–4 elevated glucose was 12.2% with second-line nilotinib and 5.8% with first-line nilotinib (both doses).57,60 A study of the effect of nilotinib on patients with type 2 diabetes at entry to ENESTnd showed minimal changes in glucose parameters, body weight, and HbA1c in any arm at 12 months’ follow-up, suggesting that nilotinib is well tolerated in this patient group.61 In a single-center study of patients treated with nilotinib (first and second line), nilotinib-induced hyperglycemia was manageable (even in those with diabetes) and mostly related to increased body mass index.62
Pancreatic enzyme abnormalities have been reported with nilotinib use. Grade 3–4 elevated lipase occurred frequently in clinical studies with nilotinib.57,60 The clinical implication of such enzyme elevation is unclear, because the incidence of pancreatitis was low with nilotinib (≤2%).57,60,63 The nilotinib prescribing information recommends monthly monitoring (or as clinically indicated), and withholding therapy and adjusting dosage in severe cases of elevated lipase or amylase.20 More frequent monitoring may be warranted in patients with a history of alcohol abuse or pancreatitis. If lipase elevation occurs with abdominal symptoms, then treatment should be interrupted and patients should undergo evaluation for pancreatitis.
With a 5 year follow-up of the ENESTnd study, newly occurring or worsening total cholesterol elevations were reported in 27.6%, 26.7%, and 3.9% of patients in the nilotinib 300 mg bid, nilotinib 400 mg bid, and imatinib arms, respectively.43 A small single center study (n=27) noted that treatment with nilotinib significantly increased total, low- and high-density lipoprotein cholesterol within 3 months, while leading to decrease in triglycerides.64 It is therefore recommended that lipid profiles should be checked prior to initiating nilotinib therapy, and should be assessed at 3 and 6 months after initiating therapy and at least yearly during chronic therapy.20
Other laboratory abnormalities seen with nilotinib therapy include hypophosphatemia, increase in liver enzymes, and hyperbilirubinemia. Grade 3–4 hypophosphatemia has been reported in 16.9% of patients on second-line nilotinib.60 Hypophosphatemia may lead to disorders of bone mineralization in the long term.65 Grade 3–4 elevated alanine and AST reported in the ENESTnd study for instance (3%–4% ALT, 1%–3% AST).20 Grade 3–4 hyperbilirubinemia was reported in 4%–8% of patients being treated with nilotinib compared with <1% of patients receiving imatinib in the ENESTnd study.42
Rash is an AE of nilotinib which is common with bcr-abl TKIs. Unlike the rash seen in EGFR inhibitors in which the severity of the rash is a marker for efficacy of the medications, the rash seen following nilotinib has not been linked to efficacy or response.66 As noted in the ENESTnd trial, rash was reported more frequently with nilotinib than with imatinib.
Patients on nilotinib may develop treatment-related cytopenias, thereby necessitating dose interruption, dose reduction, hematopoietic growth factors, or transfusion of blood products. Neutropenia and thrombocytopenia were the most common grade 3–4 hematologic abnormalities associated with nilotinib treatment.38,42,45 For patients with absolute neutrophil count <1×109 cells/L or thrombocytopenia <50×109 cells/L (not considered to be due to underlying leukemia), nilotinib should be withheld until count recovery. If neutrophil or platelet counts remain low for more than 2 weeks, nilotinib should be reinitiated at 400 mg daily.20
Nilotinib therapy: patient-focused perspectives
Although the possibility of stopping TKI treatment in patients with prolonged molecular response to TKI therapy is under investigation, TKI use for treatment of CML is a lifelong need. Because TKI therapy may span many years, clinicians must proactively watch out for treatment-related AEs throughout the course of therapy. Persistent low-grade AEs of TKIs (including nilotinib) can negatively affect the patient’s quality of life and treatment adherence over prolonged periods and so should not be overlooked.67,68 Patients should be regularly queried about their quality of life.68 Disease- and symptom-specific assessment tools including the Fact-Leu scale,69 the MDASI-CML,70 and the EORTC-QLQ-CML2471 are helpful for assessment of quality of life issues in the patients. Timely symptom management will serve to help patients to stay on treatment and adhere to treatment schedule.
The GIMEMA and EORTC Quality of Life Group noted that fatigue, muscle cramps, swelling, worries, and uncertainty about health condition in the future, as well as importance of social support in coping with the disease were the most important health-related quality of life concerns for patients on CML TKI therapy (which included nilotinib).72 The physician should have these issues in mind during each clinical encounter with a patient on long-term TKI therapy.
Nilotinib has several notable interactions with other drugs. Concurrent use of CYP3A4 inhibitors such as ketoconazole, itraconazole, erythromycin, and clarithromycin may decrease the rate of metabolism of nilotinib, thereby resulting in increased drug levels and potentially increased toxicity of nilotinib. If possible, patients on nilotinib should avoid these medications; but if a patient requires administration of a CYP3A4 inhibitor, then the nilotinib dose should be reduced to 400 mg once a day.20 The QT interval should be closely monitored in patients who are taking nilotinib and a CYP3A4 inhibitor concurrently.
CYP3A4 inducers (dexamethasone, rifampin, dilantin, phenobarbital, carbamazepine, St John’s wort) may increase the rate of metabolism of nilotinib and have been associated with decreased plasma nilotinib concentrations. Depending on a patient’s tolerability, dose increases should be considered in patients who need any of the CYP3A4 inducers. Patients being treated with nilotinib should be strongly counselled against use of St John’s wort.
Conclusion
Nilotinib is an oral, second-generation bcr-abl TKI indicated for newly diagnosed CML-CP or patients resistant to or intolerant of imatinib. Frequently reported AEs of nilotinib use are myelosuppression, rash, pruritus, nausea, constipation, headache, and fatigue. Some cardiovascular effects and laboratory abnormalities have also been observed with nilotinib. The medication has several drug-to-drug interactions which may limit its concurrent use with some other medications. Despite these side effects and drug interactions, nilotinib remains a well-tolerated and efficacious option in the treatment of CML. Side effects should be actively monitored and managed by the treating physician.
Disclosure
JPI has received research funding and fees from participation in advisory boards from Novartis. The other authors report no conflicts of interest in this work.
References
Garcia-Manero G, Faderl S, O’Brien S, Cortes J, Talpaz M, Kantarjian HM. Chronic myelogenous leukemia: a review and update of therapeutic strategies. Cancer. 2003;98(3):437–457. | |
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. | |
National Cancer Institute. SEER Stat Fact Sheet: Chronic Myeloid Leukemia. Bethesda, MD: National Cancer Institute. 2015. | |
Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118(12):3123–3127. | |
Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243(5405):290–293. | |
Bazzoni G, Carlesso N, Griffin JD, Hemler ME. Bcr/Abl expression stimulates integrin function in hematopoietic cell lines. J Clin Invest. 1996;98(2):521–528. | |
Cortez D, Stoica G, Pierce JH, Pendergast AM. The BCR-ABL tyrosine kinase inhibits apoptosis by activating a Ras-dependent signaling pathway. Oncogene. 1996;13(12):2589–2594. | |
Cambier N, Chopra R, Strasser A, Metcalf D, Elefanty AG. BCR-ABL activates pathways mediating cytokine independence and protection against apoptosis in murine hematopoietic cells in a dose-dependent manner. Oncogene. 1998;16(3):335–348. | |
Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131(3):207–219. | |
Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of busulfan and hydroxyurea in chronic myelogenous leukemia: prolongation of survival by hydroxyurea. The German CML Study Group. Blood. 1993;82(2):398–407. | |
Talpaz M, Kantarjian HM, McCredie K, Trujillo JM, Keating MJ, Gutterman JU. Hematologic remission and cytogenetic improvement induced by recombinant human interferon alpha A in chronic myelogenous leukemia. N Engl J Med. 1986;314(17):1065–1069. | |
Talpaz M, Kantarjian H, Kurzrock R, Trujillo JM, Gutterman JU. Interferon-alpha produces sustained cytogenetic responses in chronic myelogenous leukemia. Philadelphia chromosome-positive patients. Ann Intern Med. 1991;114(7):532–538. | |
Guilhot F, Chastang C, Michallet M, et al. Interferon alfa-2b combined with cytarabine versus interferon alone in chronic myelogenous leukemia. French Chronic Myeloid Leukemia Study Group. N Engl J Med. 1997;337(4):223–229. | |
Kantarjian HM, Giles FJ, O’Brien SM, Talpaz M. Clinical course and therapy of chronic myelogenous leukemia with interferon-alpha and chemotherapy. Hematol Oncol Clin North Am. 1998;12(1):31–80. | |
Clift RA, Buckner CD, Thomas ED, et al. Treatment of chronic granulocytic leukaemia in chronic phase by allogeneic marrow transplantation. Lancet. 1982;2(8299):621–623. | |
Speck B, Bortin MM, Champlin R, et al. Allogeneic bone-marrow transplantation for chronic myelogenous leukaemia. Lancet. 1984;1(8378):665–668. | |
Goldman JM, Baughan AS, McCarthy DM, et al. Marrow transplantation for patients in the chronic phase of chronic granulocytic leukaemia. Lancet. 1982;2(8299):623–625. | |
O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. | |
Sprycel®. (Dasatinib) [prescribing information] (US). Princeton, NJ: Bristol-Meyers Squibb Company; 2014. | |
Tasigna®. (Nilotinib) [prescribing information] (US). East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2014. | |
Bosulif®. (Bosutinib) [prescribing information]. New York, NY: Pfizer Labs; 2013. | |
Iclusig®. (Ponatinib) [prescribing information]. Cambridge, MA: ARIAD Pharmaceuticals, Inc.; 2014. | |
Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. | |
Hughes TP, Hochhaus A, Branford S, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS). Blood. 2010; 116(19):3758–3765. | |
Sawyers CL, Hochhaus A, Feldman E, et al. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood. 2002;99(10):3530–3539. | |
Talpaz M, Silver RT, Druker BJ, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood. 2002; 99(6):1928–1937. | |
Quintas-Cardama A, Kantarjian HM, Cortes JE. Mechanisms of primary and secondary resistance to imatinib in chronic myeloid leukemia. Cancer Control. 2009;16(2):122–131. | |
Nardi V, Azam M, Daley GQ. Mechanisms and implications of imatinib resistance mutations in BCR-ABL. Curr Opin Hematol. 2004;11(1):35–43. | |
Weisberg E, Manley PW, Breitenstein W, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7(2):129–141. | |
O’Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65(11):4500–4505. | |
Weisberg E, Manley P, Mestan J, Cowan-Jacob S, Ray A, Griffin JD. AMN107 (nilotinib): a novel and selective inhibitor of BCR-ABL. Br J Cancer. 2006;94(12):1765–1769. | |
von Bubnoff N, Manley PW, Mestan J, Sanger J, Peschel C, Duyster J. Bcr-Abl resistance screening predicts a limited spectrum of point mutations to be associated with clinical resistance to the Abl kinase inhibitor nilotinib (AMN107). Blood. 2006;108(4):1328–1333. | |
Manley PW, Drueckes P, Fendrich G, et al. Extended kinase profile and properties of the protein kinase inhibitor nilotinib. Biochim Biophys Acta. 2010;1804(3):445–453. | |
Quintas-Cardama A, Kantarjian H, Cortes J. Flying under the radar: the new wave of BCR-ABL inhibitors. Nat Rev Drug Discov. 2007;6(10):834–848. | |
Hughes T, Saglio G, Branford S, et al. Impact of baseline BCR-ABL mutations on response to nilotinib in patients with chronic myeloid leukemia in chronic phase. J Clin Oncol. 2009;27(25):4204–4210. | |
Pagan F. Nilotinib significantly alters blood and CSF a-Synuclein and p-Tau levels, inhibits dopamine breakdown and increases neuro-restorative markers in an open-labelled Parkinson’s disease with dementia and Lewy body dementia trial. Abstract presented at: Society for Neuroscience 2015 Annual Meeting; October 18; 2015; Chicago, IL. | |
Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354(24):2542–2551. | |
Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110(10):3540–3546. | |
Giles FJ, le Coutre PD, Pinilla-Ibarz J, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013;27(1):107–112. | |
Rosti G, Palandri F, Castagnetti F, et al. Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood. 2009; 114(24):4933–4938. | |
Cortes JE, Jones D, O’Brien S, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol. 2010;28(3):392–397. | |
Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010; 362(24):2251–2259. | |
Larson RA, Kim DW, Jootar S, et al. ENESTnd 5-year update: long-term outcomes of patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib vs imatinib. Poster presented at: The 50th ASCO Annual Meeting; May 30–June 3, 2014; Chicago, IL. | |
Rosti G, Gugliotta G, Castagnetti F, et al. Five-year results of nilotinib 400 mg BID in early chronic phase chronic myeloid leukemia (CML): high rate of deep molecular response – update of the Gimema CML WP Trial CML0307. Blood (ASH Annual Meeting Abstracts). 2012;120(21):3784. | |
le Coutre P, Ottmann OG, Giles F, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is active in patients with imatinib-resistant or -intolerant accelerated-phase chronic myelogenous leukemia. Blood. 2008;111(4):1834–1839. | |
le Coutre PD, Giles FJ, Hochhaus A, et al. Nilotinib in patients with Ph+ chronic myeloid leukemia in accelerated phase following imatinib resistance or intolerance: 24-month follow-up results. Leukemia. 2012;26(6):1189–1194. | |
Giles FJ, Kantarjian HM, le Coutre PD, et al. Nilotinib is effective in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blastic phase. Leukemia. 2012;26(5):959–962. | |
Shimoni A, Volchek Y, Koren-Michowitz M, et al. Phase 1/2 study of nilotinib prophylaxis after allogeneic stem cell transplantation in patients with advanced chronic myeloid leukemia or Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. 2015; 121(6):863–871. | |
Aichberger KJ, Herndlhofer S, Schernthaner GH, et al. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol. 2011;86(7):533–539. | |
Hadzijusufovic E, Herndlhofer S, Aichberger KJ, et al. Nilotinib exerts direct effects on vascular endothelial cells and may act as a co-trigger of atherosclerosis in patients with Ph+ CML. Blood (ASH Annual Meeting Abstracts). 2011;118(21):2753. | |
Kim TD, Rea D, Schwarz M, et al. Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or imatinib. Leukemia. 2013;27(6):1316–1321. | |
Le Coutre P, Rea D, Abruzzese E, et al. Severe peripheral arterial disease during nilotinib therapy. J Natl Cancer Inst. 2011;103(17):1347–1348. | |
Levato L, Cantaffa R, Kropp MG, Magro D, Piro E, Molica S. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in chronic myeloid leukemia: a single institution study. Eur J Haematol. 2013;90(6):531–532. | |
Quintas-Cardama A, Kantarjian H, Cortes J. Nilotinib-associated vascular events. Clin Lymphoma Myeloma Leuk. 2012;12(5):337–340. | |
Tefferi A, Letendre L. Nilotinib treatment-associated peripheral artery disease and sudden death: yet another reason to stick to imatinib as front-line therapy for chronic myelogenous leukemia. Am J Hematol. 2011;86(7):610–611. | |
Giles FJ, Mauro MJ, Hong F, et al. Rates of peripheral arterial occlusive disease in patients with chronic myeloid leukemia in the chronic phase treated with imatinib, nilotinib, or non-tyrosine kinase therapy: a retrospective cohort analysis. Leukemia. 2013;27(6):1310–1315. | |
Larson RA, Hochhaus A, Hughes TP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26(10):2197–2203. | |
Le Coutre P, Hughes TP, Mahon F-X, et al. Peripheral arterial occlusive disease (PAOD) in patients (pts) receiving dasatinib: experience across multiple clinical trials. Blood (ASH Annual Meeting Abstracts). 2013; 122(21):1489. | |
Cortes JE, Kantarjian H, Khoury HJ, et al. Long-term evaluation of vascular toxicity in patients with Ph+ leukemias treated with bosutinib. J Clin Oncol (ASCO Meeting Abstracts). 2014;32(5 Suppl):7060. | |
Kantarjian HM, Giles FJ, Bhalla KN, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood. 2011;117(4):1141–1145. | |
Saglio G, Larson RA, Hughes TP, et al. Efficacy and safety of nilotinib in chronic phase (CP) chronic myeloid leukemia (CML) patients (Pts) with type 2 diabetes in the ENESTnd Trial. Blood (ASH Annual Meeting Abstracts). 2010;116(21):3430. | |
Breccia M, Loglisci G, Salaroli A, Serrao A, Alimena G. Nilotinib-mediated increase in fasting glucose level is reversible, does not convert to type 2 diabetes and is likely correlated with increased body mass index. Leuk Res. 2012;36(4):e66–e67. | |
Palandri F, Castagnetti F, Soverini S, et al. Pancreatic enzyme elevation in chronic myeloid leukemia patients treated with nilotinib after imatinib failure. Haematologica. 2009;94(12):1758–1761. | |
Rea D, Mirault T, Cluzeau T, et al. Early onset hypercholesterolemia induced by the 2nd-generation tyrosine kinase inhibitor nilotinib in patients with chronic phase-chronic myeloid leukemia. Haematologica. 2014;99(7):1197–1203. | |
Berman E, Nicolaides M, Maki RG, et al. Altered bone and mineral metabolism in patients receiving imatinib mesylate. N Engl J Med. 2006;354(19):2006–2013. | |
Irvine E, Williams C. Treatment-, patient-, and disease-related factors and the emergence of adverse events with tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia. Pharmacotherapy. 2013; 33(8):868–881. | |
Jabbour EJ, Kantarjian H, Eliasson L, Megan Cornelison A, Marin D. Patient adherence to tyrosine kinase inhibitor therapy in chronic myeloid leukemia. Am J Hematol. 2012;87(7):687–691. | |
Pinilla-Ibarz J, Cortes J, Mauro MJ. Intolerance to tyrosine kinase inhibitors in chronic myeloid leukemia: definitions and clinical implications. Cancer. 2011;117(4):688–697. | |
Cella D, Jensen SE, Webster K, et al. Measuring health-related quality of life in leukemia: the functional assessment of cancer therapy-leukemia (FACT-Leu) questionnaire. Value Health. 2012;15(8):1051–1058. | |
Williams LA, Garcia Gonzalez AG, Ault P, et al. Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood. 2013;122(5):641–647. | |
Efficace F, Breccia M, Saussele S, et al. International development of an EORTC measure to assess patient-reported quality of life (QoL) and symptoms in chronic myeloid leukemia (CML). Blood (ASH Annual Meeting Abstracts). 2011;118(21):3132. | |
Efficace F, Breccia M, Saussele S, et al. Which health-related quality of life aspects are important to patients with chronic myeloid leukemia receiving targeted therapies and to health care professionals? GIMEMA and EORTC Quality of Life Group. Ann Hematol. 2012; 91(9):1371–1381. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.