Back to Journals » OncoTargets and Therapy » Volume 13
Up-Regulation of Tiam1 Promotes the Radioresistance of Laryngeal Squamous Cell Carcinoma Through Activation of the JNK/ATF-2 Signaling Pathway
Authors Wang S, Zhu W, Ouyang L, Li J, Li S, Yang X
Received 10 April 2020
Accepted for publication 16 June 2020
Published 21 July 2020 Volume 2020:13 Pages 7065—7074
DOI https://doi.org/10.2147/OTT.S257748
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Shuang Wang,1 Weiyu Zhu,2 Lei Ouyang,1 Jingkun Li,1 Shisheng Li,1 Xinming Yang1
1Department of Otolaryngology-Head and Neck Surgery, The Second Xiangya Hospital, Central South University, Changsha 410011, Hunan, People’s Republic of China; 2Department of Head and Neck Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen 518116, Guangdong, People’s Republic of China
Correspondence: Xinming Yang
Department of Otolaryngology-Head and Neck Surgery, The Second Xiangya Hospital, Central South University, 139 Renmin Road, Changsha 410011, Hunan, People’s Republic of China
Tel +86-731-85296133
Fax +86-731-85533525
Email [email protected]
Purpose: Our previous study has revealed that T-lymphoma invasion and metastasis-inducing factor 1 (Tiam1) overexpression are significantly associated with aggressive behavior and poor prognosis in patients with laryngeal squamous cell carcinoma (LSCC). However, the influence of Tiam1 in the radioresistance of LSCC and its mechanism have never been elucidated.
Materials and Methods: Western blotting was used to confirm the relationship between Tiam1 and the JNK/ATF-2 signaling pathway. To explore the specific functions of Tiam1 and JNK/ATF-2 signaling pathway on the proliferation and apoptosis of LSCC after radiation, cloning formation assay and flow cytometry were conducted in vitro, and the experiments on a xenograft mouse model and TUNEL assay were performed in vivo.
Results: Western blotting indicated that Tiam1 can regulate the JNK/ATF-2 signaling pathway through the influence of the activity of JNK and ATF-2. Up-regulation of Tiam1 could promote proliferation and inhibit apoptosis of LSCC after radiation both in vitro and in vivo. Moreover, the down-regulation of the JNK/ATF-2 signaling pathway reduced the radioresistance of LSCC caused by Tiam1 up-regulation.
Conclusion: These results suggest that the up-regulation of Tiam1 expression can promote the radioresistance of LSCC through activation of the JNK/ATF-2 signaling pathway.
Keywords: JNK/ATF-2 signaling pathway, laryngeal squamous cell carcinoma, radioresistance, Tiam1, up-regulation
Introduction
Laryngeal squamous cell carcinoma (LSCC) is not a rare tumor occurring in the head and neck region, as it amounts to 6% of all cancer cases.1 It comprises over 500,000 new cases and 200,000 deaths annually worldwide.2,3 The overall 5-year accumulative survival rate for patients with LSCC is unsatisfactory (50–70%).4 At present, the comprehensive treatment of LSCC includes surgery, radiotherapy, chemotherapy, and immunotherapy. Radiotherapy, as one of the most important treatments of malignant tumors, can cure some tumors. It plays a vital role in reducing postoperative recurrence and metastasis and improving the survival rate of patients.5 However, the curative effect of radiotherapy for some LSCC patients is currently disappointing. Radiotherapy resistance is one of the major factors compromising the radiotherapy effect on malignant tumors.6 Therefore, the recognition and identification of crucial proteins and the related signaling pathway associated with radiotherapy resistance are of great importance for the development of novel strategies in the prevention and treatment of patients with LSCC.
A gene designated T-lymphoma invasion and metastasis inducing factor 1 (Tiam1) was originally recognized as an invasion-inducing gene by proviral insertion combined with in vitro selection of invasive mouse T-lymphoma variants.7 Tiam1 can specifically activate the Rac signaling pathway, which mediates cellular growth, invasion, and metastasis.8 Tiam1 overexpression has been shown in a large number of tumors, including thyroid,9 nasopharyngeal,10 esophageal,11 and renal carcinoma,12 and colorectal cancer.13 Tiam1 has also been reported to have close correlations with apoptosis,14 migration, and invasion.15,16 Hence, we can conclude that Tiam1 overexpression is related to the malignant progression of tumors.
Studies have confirmed that the c-Jun N-terminal kinase (JNK)/activating transcription factor-2 (ATF-2) signaling pathway is closely related to invasion, metastasis, epithelial-mesenchymal transition, and apoptosis of malignant tumors.17–19 It has been proved that this pathway is relevant to the radioresistance of lung cancer cells.20 Zhu et al’s research findings revealed that Tiam1 can induce apoptosis by activating the Rac1/JNK signaling pathway.21 The Tiam1/Rac1/JNK signaling pathway plays a significant role in the apoptosis of malignant tumor cells.22,23 Consequently, Tiam1 may be an important regulator in the JNK signaling pathway.
Previous studies from our laboratory based on the analysis of clinical specimens illustrated that the overexpression of Tiam1 is significantly correlated with LSCC aggressive action and a poor outcome. We also confirmed that the up-regulation of Tiam1 could increase LSCC metastatic ability in vitro. Our previous experiment results demonstrated that Tiam1 expression may correlate with the tumorigenesis and development of LSCC and may be a beneficial therapeutic target for LSCC patients.24 Nevertheless, there are relatively few reports on the role of Tiam1 on LSCC radioresistance. In an attempt to gain further insight into the radioresistance of Tiam1 in LSCC and its probable mechanism, here we report that the transfection of a Tiam1/C1199 plasmid to up-regulate Tiam1 expression in LSCC, and we observed changes in the proliferation, apoptosis and the expression of JNK/ATF-2 signaling pathway both in vitro and in vivo after radiation.
Materials and Methods
Cell Culture and Cell Transfection
The human TU686 cell line used in the current study was purchased from the Type Culture Collection of the Chinese Academy of Science (Shanghai, China). All cells were cultured in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS) and 100 IU/mL penicillin/streptomycin. The cells were grown in a 37°C humidified incubator with 5% CO2.
The Tiam1 cDNA C1199 plasmid, a gift provided by Dr. John Collard (The Netherlands Cancer Institute, Amsterdam, Netherlands), which has the Tiam1 cDNA cloned as a BamHI/XhoI fragment in the pcDNA3 plasmid containing a cytomegalovirus promoter and a hemagglutinin tag, was used for the stable transfection of Tiam1 up-regulation. The TU686 cells that transfected with Tiam1/C1199 cDNA and vector control plasmid were named Tiam1+ and Mock, respectively, as established and described in our previous study.24
shRNA targeting JNK (TAGATGCATCTATTACCAG) and the nontargeting control (ATCTCGCTTGGGCGAGAGTAAG) cloned into a pGIPZ lentiviral plasmid were purchased from Dharmacon™. ATF-2 shRNA lentiviral particles (sc-29,205-V) were purchased from Santa Cruz Biotechnology. These shRNAs were transfected into TU686 and Tiam1+ cells, and stably expressed in these cells, which were termed JNK-, Tiam1+/JNK-, ATF-2-, and Tiam1+/ATF-2-. The TU686 cell transfected with the lentiviruses encoding the nontargeting control was termed LV/NC.
Western Blotting
All Western blotting analyses were performed as we described previously.24 Briefly, total protein (40 μg/sample) was separated in SDS-PAGE (8%) and then electroblotted onto polyvinylidene difluoride membranes (Immobilon-P, Millipore, USA). The membranes were separately incubated with anti-Tiam1 mouse monoclonal antibody (sc-393,315, dilution 1:100) (Santa Cruz, CA, USA), rabbit monoclonal antibody against JNK (ab179461, dilution 1:1000) and phosphate (p)-JNK (ab124956, dilution 1:1000) (Abcam, Cambridge, UK), mouse monoclonal antibody against ATF-2 (sc-242, dilution 1:200), and p-ATF-2 (sc-8398, dilution 1:100) (Santa Cruz, CA, USA) at 4°C overnight. After washing three times, the membranes were blocked with HRP-conjugated Goat Anti-mouse IgG and Goat Anti-rabbit IgG for 1 h at room temperature. Then the membranes were incubated with 3, 3-diaminobenzidine (Zhongshan GoldenBridge, Beijing, China) to visualize the bands. The results were obtained using a Kodak film and quantified by software Quantity One (Version 4.6.2, Bio-Rad Technical Service Department, USA). The antibody against GAPDH (BM3874, dilution 1:2000) (Boster, China) was used as control. All assays were performed in triplicate.
Radiotherapy Treatment
TU686 cells were grown to 50% confluence and then divided into six groups. One was the control group, which did not receive any X-ray radiation (termed as TU686 only). The other groups received a total dose 10 Gy (2 Gy each time) X-ray radiation (termed TU686 RT, Mock RT, LV/NC RT, Tiam1+ RT, Tiam1+/JNK- RT, and Tiam1+/ATF-2- RT). The cells and xenograft animal tumors were exposed to X-ray radiation on a Varian Clinac 23 EX medical linear accelerator in a field sized of 35 × 35 cm2 with single energy of 6 MV X-ray and a dose rate of 500 MU/min; the source to skin distance was approximately 100 cm.
Plate Cloning Formation Assay
Cells were digested, and 700 cells/well of each group were inoculated in triplicate in 6-well plates. The medium was changed every other day. Cell culture was performed for ten days and was terminated when macroscopic colonies were found in plates. After washing with phosphate-buffered saline (PBS), fixation with 4% paraformaldehyde for 15 min was carried out. After that, the Giemsa solution was mixed, and staining was performed for 20 min. Clones containing > 50 cells were counted under an inverse microscope. Cloning formation rate (%) = (number of clones/number of inoculated cells) × 100%.
Cell Apoptosis by Flow Cytometry
Cell apoptosis was analyzed by flow cytometry using the Annexin V-FITC Apoptosis Detection kit (Invitrogen, Carlsbad, CA, USA). The test was performed following the manufacturer’s protocol. Cells were washed three times with ice-cold PBS, and the concentration was adjusted to 1×106/mL. Then they were mixed with 5 μL Annexin V-FITC and 5 μL PI. The reaction was carried out for 15 min at room temperature in the dark. After adding another 400 μL of 1×binding buffer, cell apoptosis was detected on a FACS Calibur cytometer (Becton-Dickinson). All experiments were carried out in triplicate.
LSCC Xenograft Mouse Model
The study was approved by the Institutional Animal Care and Use Committee, The Second Xiangya Hospital, Central South University. It was conducted following the Institutional Guidelines and the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85–23, revised 1996). A xenograft model in nude mice of human LSCC was established as described in Zhang et al.25,26 Briefly, 4-week-old athymic BALB/C nude male mice (about 20 g of body weight) were purchased from the Shanghai Laboratory Animal Center, Chinese Academy of Sciences, China, and housed under specific pathogen-free conditions. Each animal was injected with 8.0×106 of TU686 or Tiam1+ cells suspended in 0.4 mL of Hanks-buffered saline into the right armpit. The xenograft tumors were measured every four days, and all nude mice were killed 28 days after cell implantation. The primary tumors of the mice were collected, and tissue serial sections were formalin-fixed, paraffin-embedded, stained with hematoxylin-eosin, and studied on a light microscope.
TUNEL Assay
The TUNEL assay was performed to assess the changes of apoptosis-related factors by using the TUNEL fluorescence kit (Hoffman-La Roche Ltd.) to stain cells. Briefly, the formalin-fixed paraffin-embedded blocks of the xenograft tumors were deparaffinized in xylene and rehydrated in ethanol. The samples were pretreated with proteinase K (20µg/mL) for 15 min. The TUNEL biotin marker (50 µL) was applied, and the sections were then incubated for 60 min. Finally, using a Confocal Laser Scanning Microscope (Leica TCS SP8, Leica Microsystems, Wetzlar, Germany), apoptosis was evaluated by counting the number of cells double-stained by PI and TUNEL.
Statistical Analysis
Statistical analysis was carried out using the SPSS 22.0 software package (SPSS, Inc., Chicago, IL). All quantitative results were expressed as mean ± SD. Statistical differences between groups were compared using one-way analysis of variance (ANOVA). A p-value of < 0.05 was considered to be statistically significant.
Results
The Interplay Between Tiam1 and JNK/ATF-2 Signaling Pathway in LSCC
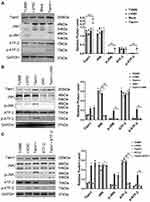
Stable clones overexpressing the Tiam1 were established, as mentioned before24 (Figure 1A). Western blotting revealed that the JNK shRNA (Figure 1B) and ATF-2 shRNA (Figure 1C) were successfully transfected into the TU686 and Tiam1+ cells. We obtained the following stable cell lines: Tiam1+ (TU686 cells transfected with the Tiam1/C1199 plasmid), Mock (TU686 cells transfected with vector control plasmid), LV/NC (TU686 cells transfected with a lentivirus encoding the nontargeting control), JNK- (TU686 cells transfected with JNK shRNA), ATF-2- (TU686 cells transfected with ATF-2 shRNA), Tiam1+/JNK- (Tiam1+ cells transfected with JNK shRNA), and Tiam1+/ATF-2- (Tiam1+ cells transfected with ATF-2 shRNA). Western blotting showed that the levels of p-JNK (p<0.01) and p-ATF-2 (p<0.01) were higher in Tiam1+ cells than those in the TU686 cells; however, the JNK (p=0.874) and ATF-2 (p=0.512) expression had no change. This suggests that Tiam1 may regulate the JNK/ATF-2 signaling pathway by influencing the activity of JNK and ATF-2 (Figure 1A). Furthermore, Western blotting results revealed that the Tiam1 expression (p=0.794) had no change in Tiam1+/JNK- and Tiam1+/ATF-2- cells compared with Tiam1+ cell (Figure 1B and C). In addition, the down-regulation of JNK and ATF-2 can reverse the phosphorylation of the JNK/ATF-2 signaling pathway (p-JNK, p<0.01, and p-ATF-2, p<0.01) caused by the Tiam1 up-regulation (Figure 1B and C). These results further show that Tiam1 is the upstream factor of the JNK/ATF-2 signaling pathway.
Up-Regulated Tiam1 Expression Promotes Proliferation and Inhibits the Apoptosis of LSCC Cells After X-Ray Radiation in vitro
To confirm the role of Tiam1 on the proliferation and apoptosis of LSCC cells after X-ray radiation in vitro, a plate cloning formation assay and a flow cytometry analysis were performed, respectively. As shown in Figure 2, the cloning formation rate was decreased in TU686 RT cells compared with TU686 cells (P<0.01), but increased in Tiam1+ RT cells compared with the TU686 RT cells (P<0.01). This result reveals that the up-regulated Tiam1 can promote the proliferation of LSCC cells after radiation. In addition, the apoptotic rate of TU686 RT cells was increased compared with the TU686 cells (P<0.01), but the apoptotic rate of the Tiam1+ RT cells was significantly decreased compared with TU686 RT cells (P<0.01) (Figure 3). This result demonstrates that up-regulated Tiam1 can inhibit the apoptosis of LSCC cells after radiation. Therefore, we concluded that the up-regulation of Tiam1 can increase the radioresistance of LSCC.
Tiam1 Promotes the Radioresistance of LSCC Through the JNK/ATF-2 Signaling Pathway
To elucidate the mechanism of Tiam1 in the promotion of radioresistance of LSCC, the Tiam1+/JNK-, and Tiam1+/ATF-2- cells were also treated with X-ray radiation. The results demonstrated that the cloning formation rate was decreased in Tiam1+/JNK- RT and Tiam1+/ATF-2- RT cells compared with Tiam1+ RT cells (both P<0.01) (Figure 2), but the apoptotic rate of Tiam1+/JNK- RT and Tiam1+/ATF-2- RT cells were significantly increased (both P<0.01) (Figure 3). These results demonstrate that Tiam1 can regulate the JNK/ATF-2 signaling pathway and further influence the radiation sensitivity of LSCC.
Up-Regulation of Tiam1 Promotes the Proliferation and Inhibits the Apoptosis of LSCC Cells After X-Ray Radiation in vivo
An established LSCC xenograft mouse model was used to determine whether up-regulated Tiam1 could regulate the radiation sensitivity of LSCC in vivo. The experiment comprised three groups (five mice/group). Subsequently, TU686 (ten mice in total) and stable transfected Tiam1+ (a total of five mice) cells were injected subcutaneously into the right armpit. The TU686 (five mice) and Tiam1+ (five mice) groups received X-ray radiation (a total dose of 10 Gy, 2 Gy each time) 8 days after inoculation (termed TU686 RT and Tiam1+ RT groups, respectively). All the tumor formation was carefully monitored. After 28 days, all of the nude mice were sacrificed, and the tumor weights were measured. We used the TUNEL assay to test cell apoptosis in paraffin-embedded blocks of the xenograft tumors. Moreover, to clarify the mechanism of Tiam1 on radioresistance of LSCC, Western blotting was performed to test the expression of the JNK/ATF-2 signaling pathway in the xenograft tumors.
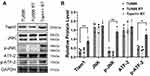
As shown in Figure 4, the average tumor volume and tumor weight of mice in the TU686 RT group were lower than those in the TU686 group (without radiation) (P<0.01). However, the average tumor volume and tumor weight of mice in the Tiam1+ RT group were higher than that those in the TU686 RT group (P<0.01). This result reveals that the up-regulation of Tiam1 can promote the proliferation of LSCC cells after radiation. The TUNEL assay showed that the PI and TUNEL double-stained cells in the TU686 RT group were increased compared to the TU686 group (P<0.01). However, the percentage of PI and TUNEL double-stained cells in the Tiam1+ RT group was lower than that in the TU686 RT group (P<0.01) (Figure 5). This result demonstrates that up-regulated Tiam1 can inhibit the apoptosis of LSCC cells after radiation. Finally, Western blotting revealed that the expression of Tiam1 (p<0.01), p-JNK (p<0.01), and p-ATF-2 (p<0.05) was increased in Tiam1+ RT group compared to the two other groups (Figure 6). Hence, the results of the in vivo and in vitro experiments are consistent. Consequently, we further demonstrated that the up-regulated Tiam1 expression could increase the radiation resistance of LSCC through the JNK/ATF-2 signaling pathway in vivo.
Discussion
Tiam1 was originally identified as an invasion and metastasis gene by Habets et al.7 Like all members of the Ras superfamily, the Rho-like small guanosine triphosphate (GTP) binding proteins function as molecular switches, cycling between an inactive GDP-bound state and an active GTP-bound state. The cycle is catalyzed by Guanine nucleotide exchange factors (GEFs), which positively stimulate GTP binding proteins in response to multiple signaling pathways.8 As the specific GEFs for the Rho GTPase, the oncogenic effects of Tiam1 is a consequence of activation of the Rac signaling pathway.27 Tiam1/Rac signaling is attributed to a stimulation of the c-Jun N-terminal kinase pathway, p38 mitogen-activated protein kinase pathway, and extracellular signal-regulated kinase pathway,28–30 which are thought to correlate with the regulation of gene expression at transcription. In addition, Tiam1 participates in oncogenic transformation31 and modulates changes in the actin cytoskeleton, cell migration,32–34 cell polarity,35 and tumor microenvironment,36 which are considered to be related to the tumor progression.
As we mentioned before, up to now, several studies have investigated the relationship between Tiam1 overexpression and multiple types of cancer.9–13 With respect to LSCC, our previous study focused on clinical tissue specimens had confirmed that overexpression of Tiam1 is significantly correlated with the LSCC aggressive action and poor outcome.24 This suggests that Tiam1 may play a vital role in the progression of LSCC. Moreover, radiotherapy is one of the most important treatment methods for LSCC. Hence, we infer that Tiam1 may have an impact on the radiotherapy sensitivity of LSCC. However, to the best of our knowledge, there have been few published studies on the role of Tiam1 in tumor radioresistance. Consequently, to gain further insight into the radioresistance of Tiam1 in LSCC, in the current study, we up-regulated Tiam1 in cell lines of LSCC, and we observed the changes in cell proliferation and apoptosis after radiation both in vitro and in vivo.
In the present study, we found that the cloning formation rate was increased in Tiam1+ RT cells compared with TU686 RT cells. This result reveals that the up-regulation of Tiam1 can promote the proliferation of LSCC cells after radiation. In addition, the apoptotic rate of Tiam1+ RT cells was decreased compared with the TU686 RT cells. This result demonstrates that the up-regulation of Tiam1 can inhibit the apoptosis of LSCC cells after radiation. In the in vivo experiment, we also found that the average tumor volume and tumor weight of mice in the Tiam1+ RT group were higher than those in the TU686 RT group. This result further confirms that the up-regulation of Tiam1 can promote the proliferation of LSCC cells after radiation. The TUNEL assay showed that the PI and TUNEL double-stained cells in the Tiam1+ RT group were decreased compared to the TU686 RT group. This result also certifies that the up-regulated Tiam1 can inhibit the apoptosis of LSCC cells after radiation. Consequently, we demonstrated that the up-regulation of Tiam1 expression could increase the radioresistance of LSCC both in vitro and in vivo.
As we mentioned before, the JNK/ATF-2 signaling pathway is participating in the cell apoptosis, invasion, metastasis and radioresistance of malignant tumors.17–20 Concerning the relationship between Tiam1 and the JNK/ATF-2 signaling pathway, as far as we know, there have been several reports confirming that Tiam1 can induce apoptosis through the activation of the Rac1/JNK signaling pathway.21–23 Therefore, we infer that Tiam1 can probably regulate the JNK/ATF-2 signaling pathway and further influence the radioresistance of LSCC. Then, we down-regulated the expression of the JNK/ATF-2 signaling pathway to test our hypothesis and elucidate the mechanism of Tiam1 on the promotion of radioresistance of LSCC.
In the present study, we first explored the interplay between Tiam1 and JNK/ATF-2 signaling pathway in LSCC by using Western blotting. The results showed that the levels of p-JNK and p-ATF-2 expression were increased after Tiam1 up-regulation; however, the JNK and ATF-2 expression had no change. In addition, Western blotting results revealed that the Tiam1 expression had no change after JNK or ATF-2 down-regulation. Hence, these results show that Tiam1 is the upstream factor of the JNK/ATF-2 signaling pathway. Furthermore, Tiam1 may regulate the JNK/ATF-2 signaling pathway by influencing the activity of JNK and ATF-2.
Subsequently, in the in vitro experiment, we found that the cloning formation rate was decreased in Tiam1+/JNK- RT and Tiam1+/ATF-2- RT cells compared with the Tiam1+ RT cells, but the apoptotic rate of Tiam1+/JNK- RT and Tiam1+/ATF-2- RT cells was significantly increased. These results reveal that the down-regulation of the JNK/ATF-2 signaling pathway can reduce the radioresistance of LSCC caused by Tiam1 up-regulation. Similarly, in the in vivo experiment, Western blotting revealed that the expression of Tiam1, p-JNK, and p-ATF-2 was increased in the xenograft tumors of the Tiam1+ RT group compared to the two other groups. Consequently, we further validated that Tiam1 can influence the radioresistance of LSCC through the JNK/ATF-2 signaling pathway.
Conclusion
In summary, our present study indicates that Tiam1 up-regulation can promote the radioresistance of LSCC through the activation of the JNK/ATF-2 signaling pathway, which suggests that Tiam1 and the JNK/ATF-2-mediated signaling are valuable therapeutic targets to improve the efficacy of radiotherapy for patients with LSCC.
Abbreviations
Tiam1, T-lymphoma invasion and metastasis-inducing factor 1; LSCC, laryngeal squamous cell carcinoma; JNK, c-Jun N-terminal kinase; ATF-2, activating transcription factor-2.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author Dr Xinming Yang upon reasonable request.
Ethics Approval and Informed Consent
The study was approved by the Institutional Animal Care and Use Committee, The Second Xiangya Hospital, Central South University. It was conducted following the Institutional Guidelines and the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85-23, revised 1996).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to disclose.
References
1. Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91(3):386–396. doi:10.1016/j.mayocp.2015.12.017
2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
4. Obid R, Redlich M, Tomeh C. The treatment of laryngeal cancer. Oral Maxillofac Surg Clin North Am. 2019;31(1):1–11. doi:10.1016/j.coms.2018.09.001
5. Ghanem AI, Schymick M, Bachiri S, et al. The effect of treatment package time in head and neck cancer patients treated with adjuvant radiotherapy and concurrent systemic therapy. World J Otorhinolaryngol Head Neck Surg. 2019;5(3):160–167. doi:10.1016/j.wjorl.2018.09.005
6. Perri F, Pacelli R, Scarpati GD, et al. Radioresistance in head and neck squamous cell carcinoma: biological bases and therapeutic implications. Head Neck. 2015;37(5):763–770. doi:10.1002/hed.23837
7. Habets GG, Scholtes EH, Zuydgeest D, et al. Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell. 1994;77(4):537–549. doi:10.1016/0092-8674(94)90216-X
8. Mertens AE, Roovers RC, Collard JG. Regulation of Tiam1-Rac signalling. FEBS Lett. 2003;546(1):11–16. doi:10.1016/S0014-5793(03)00435-6
9. Liu L, Wu B, Cai HD, et al. Tiam1 promotes thyroid carcinoma metastasis by modulating EMT via Wnt/β-catenin signaling. Exp Cell Res. 2018;362(2):532–540. doi:10.1016/j.yexcr.2017.12.019
10. Ding Y, Chen B, Huang J, et al. Overexpression of Tiam1 is associated with malignant phenotypes of nasopharyngeal carcinoma. Oncol Rep. 2014;32(2):607–618. doi:10.3892/or.2014.3241
11. Wu QY, Wang Y, Tong JC, Zhang M, Zhang K. Expression and clinical significance of Tiam1 gene in esophgeal carcinoma. Int J Clin Exp Med. 2015;8(11):21229–21234.
12. Shan G, Tang T, Qian H, Xia Y. Expression of Tiam1 and Rac1 proteins in renal cell carcinoma and its clinical-pathological features. Int J Clin Exp Pathol. 2017;10(11):11114–11121.
13. Lzumi D, Toden S, Ureta E, Ishimoto T, Baba H, Goel A. TIAM1 promotes chemoresistance and tumor invasiveness in colorectal cancer. Cell Death Dis. 2019;10(4):267. doi:10.1038/s41419-019-1493-5
14. Boissier P, Huynh-Do U. The guanine nucleotide exchange factor Tiam1: a janus-faced molecule in cellular signaling. Cell Signal. 2014;26(3):483–491. doi:10.1016/j.cellsig.2013.11.034
15. Ding M, Li Y, Yang Y, et al. Elevated expression of Tiam1 is associated with poor prognosis and promotes tumor progression in pancreatic cancer. Onco Targets Ther. 2018;11:4367–4375. doi:10.2147/OTT.S171425
16. Kou WX, Xu X, Ji S, et al. The inhibition of the effect and mechanism of vascular intimal hyperplasia in Tiam1 knockout mice. Biochem Biophys Res Commun. 2018;497(1):248–255. doi:10.1016/j.bbrc.2018.02.065
17. Chen LC, Chueh TC, Tuan YF, et al. Activation of MAPK pathways and downstream transcription factors in 2-aminobiphenyl-induced apoptosis. Environ Toxicol. 2015;30(2):205–211. doi:10.1002/tox.21886
18. El Btaouri H, Morjani H, Greffe Y, Charpentier E, Martiny L. Role of JNK/ATF-2 pathway in inhibition of thrombospondin-1 (TSP-1) expression and apoptosis mediated by doxorubicin and camptothecin in FTC-133 cells. Biochim Biophys Acta. 2011;1813(5):695–703. doi:10.1016/j.bbamcr.2011.02.004
19. Desai S, Laskar S, Pandey BN. Autocrine IL-8 and VEGF mediate epithelial-mesenchymal transition and invasiveness via p38/JNK-ATF-2signalling in A549 lung cancer cells. Cell Signal. 2013;25(9):1780–1791. doi:10.1016/j.cellsig.2013.05.025
20. Desai S, Kumar A, Laskar S, Pandey BN. Differential roles of ATF-2 in survival and DNA repair contributing to radioresistance induced by autocrine soluble factors in A549 lung cancer cells. Cell Signal. 2014;26(11):2424–2435. doi:10.1016/j.cellsig.2014.07.021
21. Zhu G, Fan Z, Ding M, et al. DNA damage induces the accumulation of Tiam1 by blocking β-TrCP-dependent degradation. J Biol Chem. 2014;289(22):15482–15494. doi:10.1074/jbc.M114.553388
22. Kawazoe N, Watabe M, Masuda Y, Nakajo S, Nakaya K. Tiam1 is involved in the regulation of bufalin-induced apoptosis in human leukemia cells. Oncogene. 1999;18(15):2413–2421. doi:10.1038/sj.onc.1202555
23. Cao-Hong S-IT, Masuda Y, Shinki T, Nakajo S, Nakaya K. Involvement of Tiam1 in apoptosis induced by bufalin in HeLa cells. Anticancer Res. 2007;27(1A):245–249.
24. Wang S, Li S, Tang QL, et al. Overexpression of Tiam1 promotes the progression of laryngeal squamous cell carcinoma. Oncol Rep. 2015;33(4):1807–1814. doi:10.3892/or.2015.3785
25. Zhang X, Liu Y, Gilcrease MZ, et al. A lymph node metastatic mouse model reveals alterations of metastasis-related gene expression in metastatic human oral carcinoma sublines selected from a poorly metastatic parental cell line. Cancer. 2002;95(8):1663–1672. doi:10.1002/cncr.10837
26. Zhang X, Su L, Pirani AA, et al. Understanding metastatic SCCHN cells from unique genotypes to phenotypes with the aid of an animal model and DNA microarray analysis. Clin Exp Metastasis. 2006;23(3–4):209–222. doi:10.1007/s10585-006-9031-0
27. Van Leeuwen FN, van der Kammen RA, Habets GG, Collard JG. Oncogenic activity of Tiam1 and Rac1 in NIH3T3 cells. Oncogene. 1995;11(11):2215–2221.
28. Coso OA, Chiariello M, Yu JC, et al. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81(7):1137–1146. doi:10.1016/S0092-8674(05)80018-2
29. Buchsbaum RJ, Connolly BA, Feig LA, et al. Interaction of Rac exchange factors Tiam1 and Ras-GRF1 with a scaffold for the p38 mitogen-activated protein kinase cascade. Mol Cell Biol. 2002;22(12):4073–4085. doi:10.1128/MCB.22.12.4073-4085.2002
30. Engers R, Springer E, Kehren V, et al. Rac upregulates tissue inhibitor of metalloproteinase-1 expression by redox-dependent activation of extracellular signal-regulated kinase signaling. FEBS J. 2006;273(20):4754–4769. doi:10.1111/j.1742-4658.2006.05476.x
31. Michiels F, Habets GG, Stam JC, van der Kammen RA, Collard JG. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature. 1995;375(6529):338–340. doi:10.1038/375338a0
32. Subramanian N, Navaneethakrishnan S, Biswas J, Kanwar RK, Kanwar JR, Krishnakumar S. RNAi mediated Tiam1 gene knockdown inhibits invasion of retinoblastoma. PLoS One. 2013;8(8):e70422. doi:10.1371/journal.pone.0070422
33. Cordo-Russo RI, Alaniz LD, Saccodossi N, et al. Hyaluronan induces migration of multidrug-resistant lymphoma cell lines in vitro through Tiam1 activation by a PI3K-dependent mechanism. Leuk Res. 2010;34(11):1525–1532. doi:10.1016/j.leukres.2010.02.020
34. Chandra R, Lenz JD, Gancarz AM, et al. Optogenetic inhibition of D1R containing nucleus accumbens neurons alters cocaine-mediated regulation of Tiam1. Front Mol Neurosci. 2013;6:13. doi:10.3389/fnmol.2013.00013
35. Wang SJ, Watanabe T, Matsuzawa K, et al. Tiam1 interaction with the PAR complex promotes Talin-mediated Rac1 activation during polarized cell migration. J Cell Biol. 2012;199(2):331–345. doi:10.1083/jcb.201202041
36. Xu K, Rajagopal S, Klebba I, et al. The role of fibroblast Tiam1 in tumor cell invasion and metastasis. Oncogene. 2010;29(50):6533–6542. doi:10.1038/onc.2010.385
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.