Back to Journals » Journal of Inflammation Research » Volume 16
Up-Regulation of ProBDNF/p75NTR Signaling in Spinal Cord Drives Inflammatory Pain in Male Rats
Authors Li H , Liu T, Sun J, Zhao S , Wang X, Luo W, Luo R, Shen W, Luo C, Fu D
Received 23 August 2022
Accepted for publication 20 December 2022
Published 9 January 2023 Volume 2023:16 Pages 95—107
DOI https://doi.org/10.2147/JIR.S387127
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Adam D Bachstetter
Hui Li,1,2,* Tao Liu,1,2,* Jingjing Sun,1,2 Shuai Zhao,1,2 Xin Wang,1,2 Wei Luo,1,2 Ruyi Luo,1,2 Weiyun Shen,1,2 Cong Luo,1,2 Di Fu3
1Department of Anesthesiology, the Second Xiangya Hospital, Central South University, Changsha, People’s Republic of China; 2Hunan Province Center for Clinical Anesthesia and Anesthesiology, Research Institute of Central South University, Changsha, People’s Republic of China; 3Department of Anesthesiology, the XiangYa Hospital, Central South University, ChangSha, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Di Fu, Department of Anesthesiology, the XiangYa Hospital, Central South University, Xiangya Road No. 86, Changsha, Hunan Province, 410011, People’s Republic of China, Tel/Fax +86 85295987, Email [email protected]
Background: The spinal cord expresses brain-derived neurotrophic factor precursor (proBDNF) and its receptor pan neurotrophin receptor 75 (p75NTR). However, the role of spinal proBDNF signaling in the pathogenesis of inflammatory pain remains unknown.
Methods: Rats were locally injected with complete Freund’s adjuvant (CFA) to induce inflammatory pain. The proBDNF signal expression was detected by double-labeled immunofluorescence. ProBDNF protein, p75NTR extracellular domain (p75NTR-ECD), or monoclonal anti-proBDNF (McAb-proB) were administrated by intrathecal injection to investigate their effects on pain behavior. Paw withdrawal thermal latency (PWL) and paw withdrawal mechanical threshold (PWT) were performed to evaluate pain behavior. Immunoblotting, immunohistochemistry, and immunofluorescence were used to assess inflammation-induced biochemical changes.
Results: CFA induced a rapid increase in proBDNF in the ipsilateral spinal cord, and immunofluorescence revealed that CFA-enhanced proBDNF was expressed in NeuN positive neurons and GFAP positive astrocytes. The administration of furin cleavage-resistant proBDNF via intrathecal injection (I.t.) significantly decreased the PWT and PWL, whereas McAb-proB by I.t. alleviated CFA-induced pain-like hypersensitivity in rats. Meanwhile, CFA administration triggered the activation of p75NTR and its downstream signaling extracellular signal-regulated kinase 1/2 (ERK1/2) and nuclear factor (NF)-kappaB p65 in the spinal cord. I.t. administration of p75NTR-ECD suppressed CFA-induced pain and neuroinflammation, including the expression of p-ERK1/2, p-p65, and the gene expression of tumor necrosis factor-α (TNF-α), and interleukin 6 (IL-6).
Conclusion: Our study reveals that the activated proBDNF/p75NTRsignaling in the spinal cord contributes to the development of CFA-induced inflammatory pain. McAb-proB and p75NTR-ECD appear to be promising therapeutic agents for inflammatory pain.
Keywords: brain-derived neurotrophic factor precursor, inflammatory pain, spinal cord, neuron, pan neurotrophin receptor 75
Introduction
Inflammatory pain is one of the most common types of pain that is difficult to treat and can even render patients disabled.1 In the setting of injury, both peripheral and central nervous system constitute the pain transmission pathway involving in enhancing pain signals and hypersensitivity.2 Noxious stimuli to peripheral tissues cause pain hypersensitivity, as a result of peripheral sensitization of primary sensory neurons.2 Although non-steroidal anti-inflammatory drugs and opioids are commonly used to treat inflammatory pain, their side effects and toxicity limit their long-term use and dosage.3–5 Therefore, novel targets to treat inflammatory pain are urgently required.
Brain-derived neurotrophic factor precursor (proBDNF) is an intermediate during the synthesis of mature BDNF.6 As a functional protein, proBDNF binds to the high-affinity pan-p75 neurotrophin receptor (p75NTR), which is also a low affinity nerve growth factor (NGF) receptor and BDNF.7,8 The activated p75NTR further elicits the downstream signals, such as nuclear factor kappa B (NF-κB), to induce inflammatory responses.9 We previously reported that proBDNF derived from the immune system played a role in the progression of inflammatory diseases, such as sepsis and aortic dissection.6,9–11 Furthermore, proBDNF is also highly expressed in the injured spinal cord, but the pathological significance and downstream signals of proBDNF from the spinal cord in inflammatory pain are unknown.
The present study demonstrated the pathogenesis of inflammatory pain by focusing on proBDNF in the spinal cord for the first time. Furthermore, we proposed the potential therapeutic strategies for pain relief using McAb-proB and p75NTR inhibitors. These findings reveal a novel mechanism and identify specific therapeutic targets for CFA-induced inflammatory pain.
Materials and Methods
Reagents
CFA was purchased from Sigma-Aldrich (Catalog Number: F5881, St.Louis, MO), and p75NTR-ECD-Fc was gifted by Dr. Zhou as described previously.12 The purity of the material was about 97% as a homodimer in the non-denatured gel. Furin-resistant proBDNF and anti-proBDNF were generated as described in our previous study.13,14
Animal Models
Adult male Sprague-Dawley rats were obtained from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China). Rats weighing 350–450 g were housed in home cages with free access to food and water on a standard 12-hour dark/light cycle at room temperature. The experiments and procedures were compliant with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The study was approved by the Animal Care and Use Committee of Central South University. Extensive efforts were made to minimize suffering and external sources of pain and discomfort.
Rats were placed under sevoflurane anesthesia (3%). 100 μL of CFA was intradermally injected into the left hemi-lateral hindpaw. Control rat were injected with the same volume of saline to the left hindpaw. Rats were sacrificed at 6 h, 1 day (d), 3 d, and 7 d after injection for tests. The procedures are based on a previous study.13
Intrathecal (I.t.) Injection
Rats were anesthetized with 3% sevoflurane (HENGRUI, Jiangsu, China) followed by a single intrathecal injection described previously.15 L5-6 clearance as a puncture point, left thumb, middle finger fixed to the rat sacrum bilaterally, and index finger pressed on the bilateral anterior sacral line midpoint skin to needle using a 10 μL microinjector (GaoGe, Shanghai, China). The puncture direction was 60–70° with the long axis of the spine. The puncture angle decreased about 10° when the bone was touched and continued to advance. The puncture was successful when the tail suddenly shifted laterally or trembled.
ProBDNF, mAb-proB, and p75NTR-ECD-Fc Administration
To demonstrate the crucial role of proBDNF in CFA-induced pain, saline or proBDNF (2 μg, 0.2μg/μL) were administered by I.t. injection. Similarly, to explore the therapeutic strategies, mAb-proB (10 μg) or p75NTR-ECD-Fc (10 μg, 1μg/μL) was administered by I.t. injection. The production of recombinant cleavage resistant proBDNF, mAb-proB and p75NTR-ECD-Fc has been described in our previous studies.4,5,12
Paw Withdrawal Latency (PWL) and Paw Withdrawal Threshold (PWT)
Before measuring the basal threshold, rats were placed in the experimental environment for at least 30 min daily for three days. PWL was determined using a previously described method.16 The transparent plexiglass box was placed on the glass plate, and the rats were adapted to the test for 30 min. The left plantar floor of rats was irradiated vertically by plantar test apparatus (Ugo-Basile, Milan, Italy).17 PWL is the time elapsed between the start of irradiation and foot contraction. Pre-experiment heat stimulation intensity was determined. The normal latency of the foot was 9~11 S. The experiment was performed in a triplicate manner, and the mean value was taken as PWL.
PWT was determined using the von Frey (Stoelting Wood Dale, Illinois) test, as described previously.18 The rats were placed in the transparent plexiglass box and exposed to the environment for 30 min before becoming quiet. We chose eight von Frey filaments with bending forces ranging from 0.6 to 15 g. For each trial, the 2.0 g filament was applied perpendicular to the plantar surface of the paw. If a positive response (apparent withdrawal, licking, jumping) occurred, an “X” was recorded. Then, a weaker filament was used. We recorded an “O” and used a more robust filament if there was no positive response. Each trial was completed when a six-number sequence of Os and Xs was obtained. The maximum and minimum filament forces were 0.6 and 15 g, respectively. Finally, a modified version of Chaplan’s formula was used to achieve the 50% PWT.19
Immunohistochemistry and Immunofluorescence
At the corresponding time points after CFA injection, the rats were i.p. injected with an excessive overdose of pentobarbital sodium (40 mg/kg, i.p.), then perfused with cold normal saline, followed by cold 4% paraformaldehyde to fix tissues. The enlarged lumbar spinal cords were removed and postfix in 4% paraformaldehyde overnight at 4 °C. The fixed spinal cord tissues were immersed in a 30% sucrose solution for 24 h to dehydrate the tissue. Transverse cryostat spinal cord sections (30 μm) were cut and washed three times with phosphate buffer saline (PBS) for 10 min each. Then, 150 µL blocking solution (5% BSA and 0.1% Triton X-100) was dripped into each pore and incubated at 37 °C for 1 h to block the nonspecific antigen binding site. Without washing, the sections were incubated with the primary antibodies (anti-proBDNF) as described previously,13 anti-p75NTR (catalog: #8238, rabbit monoclonal, 1:1000, Cell Signaling Technology), anti-GFAP (catalog: SAB5201104, mouse monoclonal, 1:1000, Millipore), anti-iba-1 (catalog: ab178846, rabbit monoclonal, 1:1000, Abcam), anti-iba-1 (catalog: ab178846, goat monoclonal, 1:500, Abcam), anti-NeuN (catalog: #94403, rabbit monoclonal, 1:1000, Cell Signaling Technology), anti-p-ERK (catalog: #4370, rabbit monoclonal, 1:1000, Cell Signaling Technology), anti-phospho-p65 (catalog: #3033, 1:1000, Cell Signaling Technology) overnight at 4 °C. For immunohistochemistry, the sections were incubated with the goat anti-rabbit secondary antibody (catalog: #711-547-003, Jackson ImmunoResearch Laboratories, Inc, PA, diluted in 1:200) and ABC kit (Vector Laboratories, Burlingame, CA, diluted in 1:200). Diaminobenzidine tetrahydrochloride (DAB, ZSGB-BIO, Beijing, China) was then used for visualization. For immunofluorescence, the sections were incubated with added fluorescent secondary antibody (catalog: ab150077, 1:1000, Abcam) for 2 h at 37 °C. After drying, the sections were sealed with a fluorescent sealer (Vector Labs, Burlingame, CA), observed, and photographed using a fluorescence microscope (Olympus, Tokyo, Japan) and confocal microscope (Zeiss, Oberkochen, Germany), respectively. Negative control was conducted with the same experimental procedures except the omission of the primary antibody (Supplemental Figure 1). Images were analyzed by using ImageJ software Image-Pro Plus 6.0 (Rockville, MD, US).
Real-Time PCR
Total RNA was extracted using TrizolTM reagent (DP424, Tiangen Biotech, Beijing, CHN). cDNA for each sample was synthesized with the use of a reverse transcription kit (Thermo Fisher Scientific, MA, US). Real-time PCR was performed using SYBR® Green Real-Time PCR Master Mix (Roche, Germany) analyzed by a CFX96 TouchTM Deep Well Real-Time PCR Detection System (Bio-Rad Laboratories, CA, US) and. The primers we used are listed in Supplemental Table 1. Quantitative PCR was performed as described in a previous study and repeated in triplicate.20 GAPDH was used to standardize each gene expression as an internal control. Data were calculated by the 2−ΔΔCt method.
Western Blot
Lumbar enlargements of the rat spinal cord were homogenized in RIPA buffer with proteinase and phosphatase inhibitors (Roche, Mannheim, GER).Homogenates were subsequently centrifuged at 12,000 rpm for 15 min at 4°C. After collecting the supernatants, the protein contents were quantified by using the bicinchoninic acid (BCA) method. Proteins (20 μg) were separated by SDS-PAGE and then transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, MA, US). The membrane was blocked for 1 hour with 5% non-fat dried milk. Then the membranes were probed with primary antibodies overnight at 4 °C, and subsequently incubated with corresponding HRP-conjugated secondary antibody for 1 h at room temperature. The primary antibodies included ERK (catalog: #4695, anti-mouse, 1:500, Cell Signaling Technology), p-ERK (catalog: #4370, anti-mouse, 1:500, Cell Signaling Technology), anti-p65 (catalog: #8242, 1:1000, Cell Signaling Technology), anti-phospho-p65 (catalog: #3033, 1:1000, Cell Signaling Technology).The signals were developed using Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific, USA). Densitometry analysis of the gel images was analyzed with the use of ImageJ software Image-Pro Plus 6.0 (Rockville, MD, US).
Statistical Analysis
All data are presented as the means ± standard deviation. SPSS 25.0 software (SPSS Inc., Chicago, IL) was used for statistical analysis of the data. One-way ANOVA with the Tukey’s test was used to evaluate immunofluorescence results between experimental groups. A repeated-measure analysis of variance (two-way ANOVA) was used to assess the results of PWT and PWL. A P value < 0.05 was considered statistically significant.
Results
Upregulation of ProBDNF in the Spinal Cord after CFA Inflammation
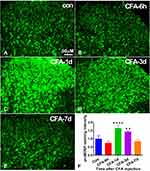
We successfully established the CFA mouse model by intraplantar injection of CFA. To demonstrate the role of spinal proBDNF in CFA-induced inflammatory pain, we first examined the optical densities of proBDNF positive staining in the ipsilateral spinal cord. CFA induced a rapid increase of proBDNF in the ipsilateral spinal cord. The proBDNF expression increased on day 1 and 3, reaching its peak on day 1(P < 0.001) (Figure 1). However, the saline injection did not affect the expression of spinal proBDNF at any time point (P > 0.05).
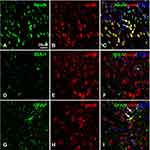
To examine the distribution and cellular localization of proBDNF, we performed double-labeling immunofluorescence on the section of L4-L6 spinal cord. As shown, proBDNF positive staining were abundantly localized with NeuN positive neurons (Figure 2A–C). However, there are few co-localizations between proBDNF positive staining with microglia marker, IBA-1 positive staining in the spinal cord dorsal horn of CFA rats (Figure 2D–F). Interestingly, numerous proBDNF positive staining is also expressed in the GFAP positive astrocytes (Figure 2G–I). These findings suggested that the activated proBDNF staining was primarily expressed in spinal cord neurons and astrocytes.
ProBDNF Mediates CFA-Induced Pain Hypersensitivity
To investigate whether proBDNF is sufficient to induce mechanical allodynia, exogenous proBDNF was administrated into the spinal cord via I.t. injection. In the present study, furin resistant mutant proBDNF was used because the wild type proBDNF is easily decomposed by the proteases in particular furin or plasmin. There was no difference in basal PWT and PWL between rat given IgG and rat given proBDNF.I.t. injection of proBDNF significantly decreased PWT and PWL at 1 and 3 h post-injection compared with the IgG injection group (P < 0.05) (Figure 3A and B). In contrast, spinal treatment with McAb-proB significantly alleviates the CFA-induced decrease in PWT and PWL (P < 0.05) (Figure 3C and D). These findings suggest that CFA-induced mechanical allodynia is mediated by spinal proBDNF.
Activation of Spinal p75NTR/p-ERK1/2 Pathway in Inflammatory Pain
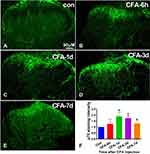
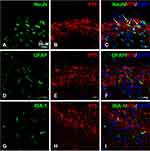
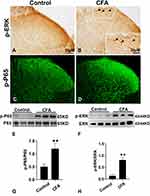
The p75NTR is a high-affinity receptor of proBDNF, and its downstream signals ERK1/2 and P65 have revealed an important role in pain induction.21,22 To better understand the molecular mechanism of CFA-induced inflammatory pain, we further investigated the expression of p75NTR, p-ERK1/2, and p-P65 in the spinal cord after CFA injection. Figure 4 shows that p75NTR positive cells in the dorsal horn significantly increased on day 1, 3, and 7 after CFA injection compared to the control group (P < 0.05). Furthermore, consistent with spinal proBDNF, the expression of p75NTR in the spinal cord also peaked on day 1 after CFA injection (Figure 4). Morphologically, the increased p75NTR expression was found in the ipsilateral dorsal horn. Confocal microscopy showed that p75NTR positive staining was co-localized with fibers and neuronal soma (Figure 5A–C), but not GFAP (Figure 5D–F) or IBA-1 (Figure 5G–I) positive staining.
Immunohistochemistry and immunofluorescence revealed that p-ERK1/2 and p-P65 were upregulated in the ipsilateral dorsal horn on day 1 after CFA injection (Figure 6A–D). Higher magnification in the box (Figure 6B) showed that p-ERK was highly expressed in the ipsilateral spinal cord dorsal horn on day 1 after CFA injection. CFA also markedly induced elevated p-ERK/ERK (P < 0.01) and p-P65/P65 (P < 0.01) expression in spinal cord dorsal horn (Figure 6E–H).
p75NTR-ECD Administration Suppressed CFA-Induced Neuroinflammation
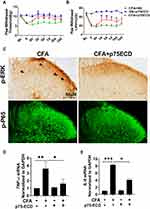
To investigate whether proBDNF-mediated CFA neuroinflammation is p75NTR dependent, we blocked p75NTR function with p75NTR-ECD-Fc. The result showed that I.t.injection of p75NTR-ECD-Fc partially restrained the downtrend of PWT and PWL from day 3 (P < 0.05) (Figure 7A and B). Consistent with behavior tests, after I.t. injection with p75NTR-ECD, p-ERK expression was also reduced, as confirmed by immunohistochemistry (Figure 7C). Furthermore, p75NTR-ECD inhibited p-P65 expression in the spinal cord of rat injected with CFA (Figure 7C). As expected, p75NTR-ECD treatment reversed the increase of TNF-α and IL-6 mRNA in spinal cord after CFA exposure (P < 0.05) (Figure 7D and E). Collectively, p75NTR-ECD inhibited p-ERK and p-P65, possibly suppressing inflammatory responses.
Discussion
The current study revealed several significant findings. We showed that the spinal proBDNF/p75NTR signaling participated in CFA-induced pain through regulating neuroinflammation. Furthermore, we proposed a novel therapeutic strategy for treating CFA-induced inflammatory pain by antagonizing the biological functions of proBDNF and its receptor p75NTR.
ProBDNF is the precursor of BDNF that is synthesized and secreted by neurons, glial cells, and immune cells.6 Through its high-affinity receptor tropomyosin kinase B (TrkB), BDNF critically regulates neuronal function by promoting survival, increasing synaptic plasticity, and promoting long-term potentiation.23 In contrast, proBDNF-p75NTR signaling mediates neuronal apoptosis, dendrites pruning and long-term depression.24 Accumulating evidence has shown that BDNF-TrKB signaling plays a role in the onset and progression of pain.25–29 For example, BDNF-TrkB signaling causes peripheral nerve injury-induced neuropathic pain by activating microglia and subsequently depolarizing the neuronal anion in the spinal dorsal horn30,31 In addition, the activated BDNF-TrkB signaling in dorsal root ganglia (DRG) contributes to the inflammatory pain in response to peripheral stimulus.32 However, the precise role of proBDNF in pain pathophysiology is unknown.6 In the current study, an I.t. injection of proBDNF induced mechanical allodynia and thermal hyperalgesia, suggesting that proBDNF is a potent pain inducer. However, it should be noted that anti-proBDNF antibody or p75-ECD only partially alleviated pain hypersensitivity induced by CFA. Indeed, many other mechanisms are involved in inflammatory pain including TrkB, purinergic receptor P2X3 (P2X3) and so on.3 Thus, the partial analgesic effect of anti-proBDNF antibody or p75-ECD for inflammatory pain is mainly due to other signaling pathways such as TrkB signaling are not blocked.
The p75NTR is a multifunctional membrane receptor that belongs to the tumor necrosis factor receptor superfamily.33 It activates several pathways, some of which have opposing functions. For example, p75NTR can activate the apoptotic signaling through caspase activation or promote cell survival via receptor-interacting protein 2 (rip2), a member of the RIP kinase family.34 In a study, Fukui found that administering a p75NTR antibody to the pinched sciatic nerve suppressed CGRP and p75NTR expression and pain behavior in the mouse sciatic nerve crush model.35 In diabetic neuropathy, the loss of Schwann cell p75NTR aggravates its progression associated with the lysosome, phagosome, and immune pathways.36 There are little data on the role of spinal p75NTR in CFA-induced inflammatory pain. The soluble ectodomain of p75NTR (p75NTR-ECD) has been shown to be beneficial in various diseases, including Alzheimer’s disease and myocardial ischemia.37,38 In the current study, I.t., administration of p75NTR-ECD significantly restored the decreased PWL and PWT. Meanwhile, p75NTR-ECD inhibited the expression of p-P65 and p-ERK and inflammatory cytokines, indicating that CFA-induced neuroinflammation was reduced. Taken together, our results show that spinal proBDNF-p75NTR signaling contributes to neuroinflammation through p-ERK/NF-κB pathways during the process of CFA-induced inflammatory pain. The application of p75NTR-ECD may be a promising therapeutic intervention for inflammatory pain (Figure 8).
Neurotrophins, particularly NGF and BDNF, have emerged as novel pain treatment targets. NGF-TrkA signaling activation in the sensory neurons contributes to the pain; therefore, targeting NGF signaling has been proposed as a pain therapy strategy.39 Many clinical trials have demonstrated that anti-NGF monoclonal antibody (Tanezumab) binding to the local and circulating NGF has slowed the progression of pain associated with osteoarthritis, low back pain, and diabetic peripheral neuropathy.40 The binding of NGF to TrkA may be affected by its co-receptor p75NTR. The analgesic effect of Tanezumab may also be due to the functionally disruption of p75NTR signaling, at least in part.41 However, when combined with non-steroid anti-inflammatory drugs, the potential risk of osteonecrosis may limit its widespread use in chronic pain. On the other hand, BDNF-TrkB signaling regulates various types of pain at the spinal or supra-spinal levels.28,31 But BDNF has pleiotropic physiological functions in the brain. More importantly, BDNF may cause gender-differential modulation in visceral pain. For example, BDNF alleviates visceral pain in female rats but has the opposite effect in male rats.42 These factors hinder BDNF’s suitability as an ideal target to treat pain. In contrast, our findings suggested that proBDNF-p75NTR is a potential candidate for pain treatment.
Conclusion
Our study found that the spinal proBDNF-p75NTR signal is involved in CFA-induced pain-like hypersensitivity in rats. The administration of McAb-proB and p75NTR-ECD may be a promising therapeutic intervention for pain syndrome attenuation.
Abbreviations
proBDNF, brain-derived neurotrophic factor precursor; p75NTR, pan neurotrophin receptor 75; p75NTR-ECD, p75NTR extracellular domain; McAb-proB, monoclonal anti-proBDNF; PWL, paw withdrawal thermal latency; PWT, paw withdrawal mechanical threshold; I.t., intrathecal injection; NS, normal saline; ERK1/2, extracellular signal-regulated kinase 1/2; NF-κB, nuclear factor kappa B; CFA, Complete Freund’s adjuvant; rip2, receptor-interacting protein 2; DRG, Dorsal root ganglia; IL-6, interleukin 6; TNF-α, tumor necrosis factor-α.
Acknowledgments
The authors would like to thank all of the co-investigators and colleagues who made this study possible.
Funding
This research was supported by the National Natural Science Foundation of China (NSFC 81873770 to HL).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ruskin DN, Sturdevant IC, Wyss LS, Masino SA. Ketogenic diet effects on inflammatory allodynia and ongoing pain in rodents. Sci Rep. 2021;11(1):725. doi:10.1038/s41598-020-80727-x
2. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi:10.1016/j.cell.2009.09.028
3. Xiang X, Wang S, Shao F, et al. Electroacupuncture stimulation alleviates CFA-induced inflammatory pain via suppressing P2X3 expression. Int J Mol Sci. 2019;20:13. doi:10.3390/ijms20133248
4. Westin BD, Walker SM, Deumens R, Grafe M, Yaksh TL. Validation of a preclinical spinal safety model: effects of intrathecal morphine in the neonatal rat. Anesthesiology. 2010;113(1):183–199. doi:10.1097/ALN.0b013e3181dcd6ec
5. Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 2013;16(5):821–847. doi:10.18433/J3VW2F
6. Shen WY, Luo C, Hurtado PR, et al. Up-regulation of proBDNF/p75(NTR) signaling in antibody-secreting cells drives systemic lupus erythematosus. Sci Adv. 2022;8(3):eabj2797. doi:10.1126/sciadv.abj2797
7. Johnson D, Lanahan A, Buck CR, et al. Expression and structure of the human NGF receptor. Cell. 1986;47(4):545–554. doi:10.1016/0092-8674(86)90619-7
8. Radeke MJ, Misko TP, Hsu C, Herzenberg LA, Shooter EM. Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature. 1987;325(6105):593–597. doi:10.1038/325593a0
9. Hu ZL, Luo C, Hurtado PR, et al. Brain-derived neurotrophic factor precursor in the immune system is a novel target for treating multiple sclerosis. Theranostics. 2021;11(2):715–730. doi:10.7150/thno.51390
10. Luo RY, Luo C, Zhong F, et al. ProBDNF promotes sepsis-associated encephalopathy in mice by dampening the immune activity of meningeal CD4(+) T cells. J Neuroinflammation. 2020;17(1):169. doi:10.1186/s12974-020-01850-0
11. Shen WY, Luo C, Reinaldo Hurtado P, et al. The regulatory role of ProBDNF in monocyte function: implications in Stanford type-A aortic dissection disease. FASEB j. 2020;34(2):2541–2553. doi:10.1096/fj.201901905RR
12. Kelliny S, Lam HY, Parikh A, et al. Preclinical study of the pharmacokinetics of p75ECD-Fc, a novel human recombinant protein for treatment of alzheimer’s disease, in Sprague Dawley rats. Curr Drug Metab. 2020;21(3):235–244. doi:10.2174/1389200221666200502015203
13. Luo C, Zhong XL, Zhou FH, et al. Peripheral brain derived neurotrophic factor precursor regulates pain as an inflammatory mediator. Sci Rep. 2016;6:27171. doi:10.1038/srep27171
14. Bai YY, Ruan CS, Yang CR, et al. ProBDNF signaling regulates depression-like behaviors in rodents under chronic stress. Neuropsychopharmacology. 2016;41:12. doi:10.1038/npp.2016.100
15. Le BD, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53(4):597–652.
16. Xu J, Chen XM, Zheng BJ, Wang XR. Electroacupuncture relieves nerve injury-induced pain hypersensitivity via the inhibition of spinal P2X7 receptor-positive microglia. Anesth Analg. 2016;122(3):882–892. doi:10.1213/ANE.0000000000001097
17. Taliyan R, Sharma PL. Possible mechanism of protective effect of thalidomide in STZ-induced-neuropathic pain behavior in rats. Inflammopharmacology. 2012;20(2):89–97. doi:10.1007/s10787-011-0106-4
18. Chen XM, Xu J, Song JG, Zheng BJ, Wang XR. Electroacupuncture inhibits excessive interferon-gamma evoked up-regulation of P2X4 receptor in spinal microglia in a CCI rat model for neuropathic pain. Br J Anaesth. 2015;114(1):150–157. doi:10.1093/bja/aeu199
19. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi:10.1016/0165-0270(94)90144-9
20. Wang Z, Wu JL, Zhong F, et al. Upregulation of proBDNF in the mesenteric lymph nodes in septic mice. Neurotox Res. 2019;36(3):540–550. doi:10.1007/s12640-019-00081-3
21. Sakurai J, Obata K, Ozaki N, et al. Activation of extracellular signal-regulated protein kinase in sensory neurons after noxious gastric distention and its involvement in acute visceral pain in rats. Gastroenterology. 2008;134(4):1094–1103. doi:10.1053/j.gastro.2008.01.031
22. Luo JG, Zhao XL, Xu WC, et al. Activation of spinal NF-kappaB/p65 contributes to peripheral inflammation and hyperalgesia in rat adjuvant-induced arthritis. Arthritis Rheumatol. 2014;66(4):896–906. doi:10.1002/art.38328
23. Palasz E, Wysocka A, Gasiorowska A, Chalimoniuk M, Niewiadomski W, Niewiadomska G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int J Mol Sci. 2020;21:3. doi:10.3390/ijms21031170
24. Cui YH, Zhou SF, Liu Y, et al. Injection of anti-probdnf attenuates hippocampal-dependent learning and memory dysfunction in mice with sepsis-associated encephalopathy. Front Neurosci. 2021;15:665757. doi:10.3389/fnins.2021.665757
25. Ferrini F, Trang T, Mattioli TA, et al. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl(-) homeostasis. Nat Neurosci. 2013;16(2):183–192. doi:10.1038/nn.3295
26. Thompson SW, Bennett DL, Kerr BJ, Bradbury EJ, McMahon SB. Brain-derived neurotrophic factor is an endogenous modulator of nociceptive responses in the spinal cord. Proc Natl Acad Sci U S A. 1999;96(14):7714–7718. doi:10.1073/pnas.96.14.7714
27. Ha SO, Kim JK, Hong HS, Kim DS, Cho HJ. Expression of brain-derived neurotrophic factor in rat dorsal root ganglia, spinal cord and gracile nuclei in experimental models of neuropathic pain. Neuroscience. 2001;107(2):301–309. doi:10.1016/S0306-4522(01)00353-0
28. Li CQ, Xu JM, Dan L, Zhang JY, Dai RP. Brain derived neurotrophic factor (BDNF) contributes to the pain hypersensitivity following surgical incision in the rats. Mol Pain. 2008;4(1):27. doi:10.1186/1744-8069-4-27
29. Zhou LJ, Peng J, Xu YN, et al. Microglia are indispensable for synaptic plasticity in the spinal dorsal horn and chronic pain. Cell Rep. 2019;27(13):3844–3859 e3846. doi:10.1016/j.celrep.2019.05.087
30. Wang H, Wei Y, Pu Y, et al. Brain-derived neurotrophic factor stimulation of T-type Ca(2+) channels in sensory neurons contributes to increased peripheral pain sensitivity. Sci Signal. 2019;12:600. doi:10.1126/scisignal.aaw2300
31. Coull JA, Beggs S, Boudreau D, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–1021. doi:10.1038/nature04223
32. Lin YT, Ro LS, Wang HL, Chen JC. Up-regulation of dorsal root ganglia BDNF and trkB receptor in inflammatory pain: an in vivo and in vitro study. J Neuroinflammation. 2011;8:126. doi:10.1186/1742-2094-8-126
33. Fujii T, Kunugi H. p75NTR as a therapeutic target for neuropsychiatric diseases. Curr Mol Pharmacol. 2009;2(1):70–76. doi:10.2174/1874467210902010070
34. Kisiswa L, Fernández-Suárez D, Sergaki MC, Ibáñez CF. RIP2 Gates TRAF6 interaction with death receptor p75(NTR) to regulate cerebellar granule neuron survival. Cell Rep. 2018;24(4):1013–1024. doi:10.1016/j.celrep.2018.06.098
35. Fukui Y, Ohtori S, Yamashita M, et al. Low affinity NGF receptor (p75 neurotrophin receptor) inhibitory antibody reduces pain behavior and CGRP expression in DRG in the mouse sciatic nerve crush model. J Orthop Res. 2010;28(3):279–283. doi:10.1002/jor.20986
36. Gonçalves NP, Jager SE, Richner M, et al. Schwann cell p75 neurotrophin receptor modulates small fiber degeneration in diabetic neuropathy. Glia. 2020;68(12):2725–2743. doi:10.1002/glia.23881
37. Fang J, Wei Z, Zheng D, et al. Recombinant extracellular domain (p75ECD) of the neurotrophin receptor p75 attenuates myocardial ischemia-reperfusion injury by inhibiting the p-JNK/Caspase-3 signaling pathway in rat microvascular pericytes. J Am Heart Assoc. 2020;9(13):e016047. doi:10.1161/JAHA.119.016047
38. Yao XQ, Jiao SS, Saadipour K, et al. p75NTR ectodomain is a physiological neuroprotective molecule against amyloid-beta toxicity in the brain of Alzheimer’s disease. Mol Psychiatry. 2015;20(11):1301–1310. doi:10.1038/mp.2015.49
39. Hirose M, Kuroda Y, Murata E. NGF/TrkA Signaling as a Therapeutic Target for Pain. Pain Practice. 2016;16(2):175–182. doi:10.1111/papr.12342
40. Patel MK, Kaye AD, Urman RD. Tanezumab: therapy targeting nerve growth factor in pain pathogenesis. J Anaesthesiol Clin Pharmacol. 2018;34(1):111–116. doi:10.4103/joacp.JOACP_389_15
41. Norman BH, McDermott JS. Targeting the Nerve Growth Factor (NGF) pathway in drug discovery. potential applications to new therapies for chronic pain. J Med Chem. 2017;60(1):66–88. doi:10.1021/acs.jmedchem.6b00964
42. Li F, Zhang JW, Wei R, et al. Sex-differential modulation of visceral pain by brain derived neurotrophic factor (BDNF) in rats. Neurosci Lett. 2010;478(3):184–187. doi:10.1016/j.neulet.2010.05.013
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.