Back to Journals » International Medical Case Reports Journal » Volume 12
Unilateral Proptosis As An Initial Sign Of Acute Myeloid Leukemia In A Child: A Case Report
Authors Almalki AMJ, Alotaibi FA, Jabr HF, Mastan AR
Received 24 February 2019
Accepted for publication 5 August 2019
Published 24 October 2019 Volume 2019:12 Pages 319—323
DOI https://doi.org/10.2147/IMCRJ.S206596
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ashwaq Mohammed J Almalki,1 Faisal Ali Alotaibi,1 Hatim Fawzi Jabr,1 Abdul Rehman Mastan2
1Ophthalmology Department, King Abdulaziz Specialist Hospital, Taif, Kingdom of Saudi Arabia; 2ENT Department, King Abdulaziz Specialist Hospital, Taif, Kingdom of Saudi Arabia
Correspondence: Ashwaq Mohammed J Almalki
Ophthalmology Department, King Abdulaziz Specialist Hospital, Taif, Kingdom of Saudi Arabia
Email [email protected]
Abstract: Granulocytic sarcoma (chloroma) is a rare malignant solid tumor representing an extramedullary manifestation of acute myeloid leukemia (AML). Rarely, a chloroma can develop as the sole manifestation and its appearance may precede the systemic manifestations of acute myelocytic leukemia by months to years. We report a rare case of unilateral orbital mass presenting with progressive proptosis involving left globe in an otherwise healthy child, and give a brief overview of the literature about this unusual presentation. Leukemic infiltration should be considered in the differential diagnosis of orbital masses and proptosis even in the absence of systemic manifestations of AML. Early detection and management are crucial to preserve vision and prevent complications.
Keywords: granulocytic sarcoma, ocular leukemia, proptosis, pediatric, acute myeloid leukemia
Background
Orbital myeloid sarcoma (chloroma) as an initial symptom of acute myeloid leukemia (AML), is a rare medical condition. Allen Burns published the first reported case of myeloid sarcoma in 1811, in which he described it as a green tumor involving the orbit.1 These tumors show a characteristic green color, hence the name chloroma (in Greek “chloros” means green).2 The diagnosis of orbital myeloid sarcoma can be challenging, especially when there is an absence of systemic leukemic manifestations. We report this case as AML rarely presents solely as unilateral proptosis in a young child.
Case Report
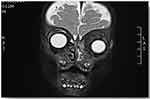
A previously healthy one-year old Saudi boy presented to emergency ophthalmic room accompanied by his family with history of rapidly progressive proptosis of the left globe over the course of two weeks. There was no associated night sweat, fever, or weight loss and the patient had no previous illnesses. Systemic review showed that the patient was born at full term, of SVD, developmentally and vaccination is up to age. The patient had no known allergies, and no surgical history. The patient had stable vital signs, was active and alert. Ophthalmic examination revealed upper and lower lid edema, proptosis with mild downward and outward displacement of the eye without any signs of inflammation or chemosis of the left eye. Vision was central, steady, and maintained. EOM was mildly restricted with normal intraocular pressure. He had normal anterior segment and round, regular, reactive pupil and normal lens and fundus. Right eye examination was within normal limit. A complete physical examination was unremarkable, no signs of acute infection, and no palpable lymph nodes. The investigations ordered for the patient were full blood count (CBC), peripheral blood film, bone marrow aspiration, and CT and MRI imaging of orbits to determine the exact location and size of tumor.
Complete blood count and peripheral blood smears showed elevated, unexpected, and abnormal high level of white blood cell count of 84.01 × 109/L with few atypical and blast cells which pointed toward acute leukemia. Chemistry was normal.
Computed tomography (CT) of the head and orbit disclosed about 2.5 × 1 × 0.6 cm ill-defined focal lesion seen at the left orbital extra-conal region at the superomedial aspect causing inferolateral displacement of the left eye globe with mass effect also on the superior bony orbital roof. The mass showed mild homogenous enhancement after IV contrast (Figures 1–3). Orbital magnetic resonance imaging (MRI) showed left orbital mass of superomedial aspect of left orbit causing inferolateral displacement of left globe and medial orbital wall erosion with periosteal reaction, and opacification of left ethmoid and both maxillary sinuses (Figures 4–6). Based on the clinical, lab, and imaging study results, the differential diagnosis included: leukemia, lymphoma, rhabdomyosarcoma, eosinophilic granuloma, and idiopathic orbital inflammation (inflammatory pseudotumor). The patient was referred to a tertiary center where the patient was cared for by pediatric oncology team for diagnosis and treatment. The patient underwent thorough investigations in which bone marrow biopsy demonstrated leukemic cells and a diagnosis of acute myeloid leukemia (AML) with orbital granulocytic sarcoma was made.
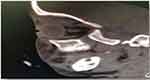 |
Figure 2 Non-enhanced sagittal view CT of head and orbit, showing ill-defined soft tissue mass of superomedial aspect of left orbit causing inferolateral displacement of left eye globe. |
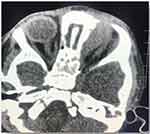 |
Figure 3 Non-enhanced axial view CT of head and orbit, showing ill-defined soft tissue mass at the medial aspect of left orbit causing lateral displacement of left globe. |
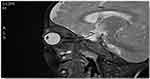 |
Figure 5 T2 weighted image, sagittal view of head and orbit, showing slightly homogenously enhanced lesion seen at superomedial aspect of left globe. |
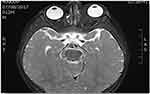 |
Figure 6 T2 weighted image, axial view of head and orbit, showing homogenous isointense lesion, seen at superomedial aspect of left globe causing mild lateral proptosis of left eye globe. |
The patient underwent systemic antileukemic chemotherapy, where the standard regimen for AML was given in the pediatric oncology unit. The regimen was two cycles of highly intensive chemotherapy. The first cycle of chemotherapy consisted of allopurinol, high doses of vitamin A, together with intrathecal methotrexate and dexamethasone, daunorubicin hydrochloride, and intrathecal vidarabine for central nervous system prophylaxis. No side effects or complications of chemotherapy were noted during the follow up period. Two weeks after chemotherapy, remarkable improvement and decrease of proptosis were observed, and the patient was scheduled for further follow up and a second cycle of chemotherapy in the coming weeks.
The parent of the patient provided written informed consent for the case details to be published, and no institutional approval was required to publish the case details.
Discussion
Granulocytic sarcoma occurs in a different locations in the body including the orbit,3,4 even before the diagnosis of leukemia.3,5 It presents during childhood with mean age approximately 7 years old, and can be observed in as early as infancy.3,4 This is consistent with the case we present. Orbital myeloid sarcoma was reported in only 1 of 250 cases in a previous study,5 and orbital infiltration in leukemia was the third most common extramedullary manifestation of acute leukemia.6 The direct infiltration of the orbits in leukemia can present with proptosis, chemosis, lid edema, intra‑retinal macular or sub‑hyaloid hemorrhages, cotton wool spots, blurring of vision, diplopia, palsies of the extra‑ocular muscle, or papilledema due to the increased intracranial pressure.5 Most cases of orbital involvement in AML present with unilateral proptosis,7–11 and few case reports have reported bilateral orbital involvement as an initial manifestation of AML.12,13
Granulocytic sarcoma originates in bone marrow and cells spread through the Haversian canals to gather in the sub-periosteum and form a soft tissue mass. These tumors can affect the orbit,14 and proptosis occurs as a result of the leukemic infiltrates in the orbital tissues, retrobulbar hemorrhage, orbital muscle infiltration or venous blockage.3
In a study done by the USA Armed Forces Institute of Pathology, Washington D.C. presented at the Tenth Pan American Congress of Ophthalmology in 1975, in the majority of cases (88%), orbital or eyelid involvement was the initial clinical manifestation,3 while orbital involvement was reported as 14% in chronic leukemia versus 7.3% in acute cases in the Wilmer institute study.15 Myeloid sarcoma was recorded as the second commonest orbital tumor after Burkitt’s lymphoma that causes proptosis.16 In Arab studies, proptosis was the first manifestation of orbital processes among pediatric population and the first cause of these processes was reported to be secondary to tumors such as chloroma.17,18
The diagnosis of orbital granulocytic sarcoma can be challenging, especially when there is absence of systemic leukemic manifestations, and it is commonly confused with malignant lymphoma, rhabdomyosarcoma, African Burkitt’s lymphoma, idiopathic inflammatory pseudotumor, and neuroblastoma.3 In our case, the development of orbital granulocytic sarcoma occurred before any evidence of systemic leukemia.
Granulocytic sarcoma has a predilection for the orbit and surrounding bone in children, with a male (3:2) and non-Caucasian predominance.19,20 Most cases, in patients without a previous diagnosis, progress to AML within a few months. However, some patients do not develop leukemic hematologic evidence of the disease for as long as 30 months following the presence of an orbital tumor.21 In our case, the orbital mass preceded any hematologic manifestations at the time of presentation. In such cases a high index of suspicion and proper diagnosis of orbital cases by ophthalmologists is helpful in prompt and early detection and referring these patients to the available pediatric oncology units for proper management.
Studies have found that low dose of radiation was recommended for patients with residual orbital disease after chemotherapy. It was found to improve the local disease and quality of life.22,23 One of these studies assessed the effectiveness of various radiotherapy doses for extramedullary leukemic manifestation (chloroma), and low-dose radiotherapy (≤26 Gy) was found to have good local control compared to high-dose regimes.24 Another promising line of treatment with proven survival benefit for poor risk cases of AML is the allogeneic hematopoietic stem cell transplantation (alloSCT). AlloSCT improves survival in patients with unfavorable-risk AML in remission by reducing the risk of relapse, and provides long-term survival in certain patients with advanced AML.25
Conclusion
Acute myeloid leukemia in the form of granulocytic sarcoma (chloroma) should be considered in the differential diagnosis of unilateral proptosis even in the absence of systemic leukemic manifestations.
Consent
Informed consent for publication was obtained.
Acknowledgment
The authors wish to acknowledge King Faisal Specialist Hospital & Research Centre for their cooperation with this research.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Burns A, Granville P. Observations on the Surgical Anatomy of the Head and Neck: Illustrated by Cases and Engravings. Baltimore: F. Lucas … [et al.]; 1823.
2. Uyesugi WY, Watabe J, Petermann G. Orbital and facial granulocytic sarcoma (chloroma): a case report. Pediatr Radiol. 2000;30:276–278. doi:10.1007/s002470050740
3. Zimmerman LE, Font RL. Ophthalmologic manifestations of granulocytic sarcoma (myeloid sarcoma or chloroma): the third Pan American association of ophthalmology and American journal of ophthalmology lecture. Am J Ophthalmol. 1975;80:975–990. doi:10.1016/0002-9394(75)90326-8
4. Cavdar AO, Babacan E, Gozdasoglu S, et al. High risk subgroup of acute myelomonocytic leukemia (AMML) with orbito-ocular granulocytic sarcoma (OOGS) in Turkish children: retrospective analysis of clinical, hematological, ultrastructural and therapeutic findings of thirty-three OOGS. Acta Haemat. 1989;81:80–85. doi:10.1159/000205531
5. Shields JA, Bakewell B, Augsburger JJ, Donoso LA, Bernardino V. Space-occupying orbital masses in children: a review of 250 consecutive biopsies. Ophthalmology. 1986;93:379–384. doi:10.1016/s0161-6420(86)33731-x
6. Koshy J, John MJ, Thomas S, Kaur G, Batra N, Xavier WJ. Ophthalmic manifestations of acute and chronic leukemias presenting to a tertiary care center in India. Indian J Ophthalmol. 2015;63:
7. Balyen L, Deniz Balyen LS, Pasa S. A case of granulocytic sarcoma (chloroma) well responded to treatment. Adv Ophthalmol Vis Syst. 2016;5(1):00139.
8. Qian X, Gigantelli JW, Abromowitch M, Morgan LA, Suh DW. Myeloid Sarcoma in the Orbit. J Pediatr Ophthalmol Strabismus. 2016;53:e64–e68. doi:10.3928/01913913-20161102-01
9. Arif SH, Akhtar K, Ahmad M, Ahmad M. Granulocytic sarcoma presenting with unilateral proptosis: a rare disease entity and presentation. J Clin Immunol Res. 2017;1(1):1–3. doi:10.33425/2639-8494.1004
10. Kesamneni R, Nagarajan P, Johnson T, Scott J. Proptosis, a rare presentation of acute myeloid lukemia – AML M6 in a child. J Applied Haematol. 2018;7(3):111–113.
11. Wang JC, Jimenez Perez JC, Friedman AM, et al. Myeloid sarcoma involving the greater wing of the sphenoid and additional skeletal sites presenting with unilateral proptosis and fevers. Orbit. 2018;20:1–4.
12. Rajput D, Naval R, Yadav K, Tungaria A, Behari S. Bilateral proptosis and bitemporal swelling: a rare manifestation of acute myeloid leukemia. J Pediatr Neurosci. 2010;5:68–71. doi:10.4103/1817-1745.66687
13. Maka E, Lukáts O, Tóth J, Fekete S. Orbital tumour as initial manifestation of acute myeloid leukemia: granulocytic sarcoma: case report. Pathol Oncol Res. 2008;14:209–211. doi:10.1007/s12253-008-9028-x
14. Stein-Wexler R, Wootton-Gorges SL, West DC. Orbital granulocytic sarcoma: an unusual presentation of acute myelocytic leukemia. Pediatr Radiol. 2003;33(2):136–139. doi:10.1007/s00247-002-0834-0
15. Kincaid MC, Green RW. Ocular and orbital involvement in leukemia. Surv Ophthalmol. 1983;27:211–232. doi:10.1016/0039-6257(83)90123-6
16. Templeton AC. Orbital tumours in African children. Brit J Ophthal. 1971;55:234–261. doi:10.1136/bjo.55.4.254
17. Malou M, Baghriche Y, Hamladji RM, Colonna P, Messerschmitt J. Comparison between acute myeloblastic leukemia in children and adults. Sem Hop. 1977;53(16):911–914.
18. Belmekki M, El Bakkali M, Abdellah H, Benchrifa F, Berraho A. Epidemiology of orbital proceses in children. 54 cases. J Fr Ophthalmol. 1999;22(3):394–398.
19. Meis JM, Butler JJ, Osborne BM, Manning JT. Granulocytic sarcoma in nonleukemic patients. Cancer. 1986;58:2697–2709. doi:10.1002/1097-0142(19861215)58:12<2697::aid-cncr2820581225>3.0.co;2-r
20. Portol KM, Schwabe D, Zanella FE, Lanfermann H. Granulocytic sarcoma in children. Neuroradiol. 2004;46:374–377. doi:10.1007/s00234-003-1127-5
21. Da Fonseca Junior NL, Paves L, Nakanami DM, Seivas MT, Manso PG. Sarcoma granulocitico em orbita: relatode caso. Arq Bras Ofthalmol. 2005;68(4):557–560. doi:10.1590/S0004-27492005000400026
22. Pathy S, Venkatesulu BP, Mallik S, Chander S. Radiation therapy in paediatric orbital granulocytic sarcomas: experience from a tertiary cancer centre. J Clin Diag Res. 2016;10(10):XC01–5.
23. Bakst RL, Dabaja BS, Specht LK, Yahalom J. Use of radiation in extramedullary leukemia/chloroma: guidelines from the international lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2018;102(2):314–319. doi:10.1016/j.ijrobp.2018.05.045
24. Oertel M, Elsayad K, Haverkamp U, Stelljes M, Eich HT. Radiotherapy for extramedullary leukaemic manifestation (Chloroma). Strahlenther Onkol. 2018;194(2):164–173. doi:10.1007/s00066-017-1236-4
25. Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–2361. doi:10.1001/jama.2009.813
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.