Back to Journals » International Medical Case Reports Journal » Volume 16
Unilateral Episcleritis Following COVID-19 Booster Vaccination of a Crohn’s Disease Patient: A Case Report and Review of the Literature
Authors Veenis A, Haghnegahdar M, Ajlan R
Received 21 November 2022
Accepted for publication 28 December 2022
Published 25 February 2023 Volume 2023:16 Pages 91—96
DOI https://doi.org/10.2147/IMCRJ.S398502
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Aaron Veenis, Megan Haghnegahdar, Radwan Ajlan
Department of Ophthalmology, University of Kansas School of Medicine, Kansas City, KS, USA
Correspondence: Radwan Ajlan, Department of Ophthalmology, University of Kansas School of Medicine, 7400 State Line Road, Prairie Village, Kansas City, KS, 66208, USA, Tel +1 913-588-6600, Fax +1 913-588-6655, Email [email protected]
Purpose: The Coronavirus Disease 2019 (COVID-19) pandemic spurred vaccine development and resulted in the development of the novel mRNA COVID-19 vaccine and with it, a growing public concern of vaccine side effects. There are reports of ocular inflammatory processes such as episcleritis being possible side effects of COVID-19 vaccination. Here we reported the first case of unilateral episcleritis in a Crohn’s disease patient following her third mRNA COVID-19 vaccination booster shot.
Patient and Methods: A 27-year-old female presented with a 1-day history of right eye redness, itching, and burning. Patient reported developing these symptoms within 3– 4 hours after vaccination. Her past medical history was relevant for Crohn’s disease. Ophthalmic examination revealed right 2+ conjunctival injection that blanched with phenylephrine drops. Otherwise, her ophthalmic exam was unremarkable. The patient was started on artificial tears and ibuprofen 200 mg three times daily for one week. After one week all symptoms resolved, and ophthalmic examination was back to baseline.
Conclusion: This is the first case in the literature of ophthalmic side effects in a Crohn’s disease patient after the third mRNA COVID-19 booster. Patients with Crohn’s disease may respond differently to booster vaccination. This case report may help healthcare providers when counselling Crohn’s disease patients about future COVID-19 mRNA vaccine side effects.
Keywords: episcleritis, Crohn’s disease, case report, COVID-19, vaccine
Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic has significantly impacted society. To date, December 2022, approximately 6.6 million lives have been lost to COVID-19 worldwide.1 In response to this toll, a great demand for vaccine development ensued. With the development of novel coronavirus vaccines, vaccine hesitancy and distrust has increased. One often stated reason for vaccine hesitancy is fear of unknown vaccine side effects.2
There are accounts of ocular side effects following COVID-19 vaccination reported in the literature. Ocular manifestations observed include eyelid edema, arteritic anterior ischemic optic neuropathy, central serous chorioretinopathy, Tolosa Hunt syndrome, acute retinal necrosis, retinal vascular occlusion, uveitis, optic neuritis, acute macular neuroretinopathy, Vogt-Koyanagi-Harada syndrome, cranial nerve palsies, choroiditis, thyroid eye disease, episcleritis, scleritis, superior ophthalmic vein thrombosis, corneal graft rejection, multiple evanescent white dot syndrome, acute zonal occult outer retinopathy, and eyelid rash.3–5
The prevalence rate of episcleritis following COVID-19 vaccination is estimated to be 0.3 cases per million doses or less.6 However, there is no consensus on the incidence and prevalence of episcleritis in the general population. One study evaluated a well-defined population in Northern California and found the overall incidence to be 41.0 per 100,000 person-years with an annual prevalence ratio of 52.6 per 100,000.7 Most common cases of episcleritis are idiopathic but episcleritis is also associated with systemic collagen vascular diseases, infections, and autoimmune diseases.8 Demographically, episcleritis is more common in young to middle aged females and in patients with autoimmune disease or systemic collagen vascular disease.8
There are three reported cases of episcleritis following COVID-19 vaccination.9–11 One case involved inactivated vaccine and the two remaining reports did not specify the causative vaccine.9–11 Here we present the first case of unilateral episcleritis following mRNA COVID-19 booster vaccination in a Crohn’s disease patient. This case is unique because the ophthalmic side effects occurred after the third mRNA vaccine shot. Healthcare providers can council Crohn’s disease patients about possible side effects with future vaccine boosters despite unremarkable previous vaccination.
Case Presentation
A 27-year-old female patient presented with right eye redness, itching, and burning four hours after receiving her COVID-19 mRNA booster vaccine, third total dose. She used artificial tears, but her symptoms were unchanged on the following day which prompted her to seek an ophthalmological exam. She denied photophobia, blurry vision, double vision, foreign body sensation, epiphora, visual flashes, or visual floaters. There were no prior similar episodes with the same vaccine prior two doses. The left eye was asymptomatic and unaffected. The patient’s past medical history was significant for myopic astigmatism of both eyes, bilateral allergic conjunctivitis, allergic rhinitis, Crohn’s disease, and mild intermittent asthma.
On ophthalmic examination best corrected visual acuity remained 20/20 bilaterally. Intraocular pressures measured at 15 mmHg in the right eye and 16 mmHg in the left eye. The right conjunctiva had 2+ injection that blanched with phenylephrine drops. There was no scleral injection, no scleral tenderness, and no physical irritation adjacent to the episcleritis area (eg, no trichiasis). The anterior chamber of the right eye appeared deep and quiet. The associated episcleritis described can be visualized in Figure 1. The remainder of the anterior and posterior segments clinical exam was unremarkable. The patient continued artificial tears and started ibuprofen 200 mg three times daily for one week. The patient adhered to and tolerated the described regimen and one week later, all ocular symptoms and signs resolved.
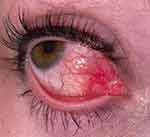 |
Figure 1 Episcleritis of the right eye. Nasal view of the right eye showing 2+ conjunctival injection. |
The patient was satisfied by the care she received. She was relieved that her symptoms resolved, and her final vision was not affected. She plans to double check with her rheumatologist before future COVID-19 booster vaccine doses. Written informed consent was obtained from the patient for publication and for use of data and images.
Discussion
To the best of our knowledge, this is the first report of episcleritis after an mRNA COVID-19 vaccine booster in a Crohn’s disease patient. The patient’s Crohn’s disease was under control with ustekinumab. The presentation did not affect visual acuity and resolved after one week with artificial tears use and oral non-steroidal anti-inflammatory drugs (NSAIDs).
The mechanism as to why ocular inflammatory processes can occur following COVID-19 vaccination has not yet been determined. However, there are three frequently proposed mechanisms. Commonly proposed mechanisms include molecular mimicry between peptides present in the vaccine and ocular structures, antibody-mediated hypersensitivity and antigen-specific cell reactions, and adjunctive or additive induced inflammatory reactions seen in subunit and inactivated vaccines.5,11–14 Another mechanism specific to live attenuated vaccines involves direct infection by the attenuated virus strain.13 It is likely that a combination of these mechanisms leads to the ocular inflammation observed following mRNA and inactivated COVID-19 vaccination. However, direct causality cannot be determined in our case.
The overall prevalence of COVID-19 vaccine associated ocular adverse events are quite rare.6 In a case series of nine patients, one case of episcleritis following inactivated COVID-19 vaccination was described.9 This patient presented five days after vaccination.9 An additional case series reported two cases of episcleritis following COVID-19 vaccination but did not specify the vaccine type.11 Mean time to side effects onset in all patients in the Tetsi et al cohort was five and six days after the first and second doses of vaccine, respectively. In our case, symptoms appeared rapidly, 3–4 hours, after vaccine administration.
While a clear explanation for the temporal difference in symptom onset observed cannot be identified, the type of vaccine and quantity of doses previously administered could be possible explanations. The case described by Pichit et al developed five days after the first dose of inactivated COVID-19 vaccination. It is possible that our patient developed a quicker presentation due to an overall stronger immune response constituted by the previous two doses. Another possible explanation is that the immune response created by the mRNA vaccines is more robust than the inactivated vaccines, increasing the risk of self-recognition. One study published preliminary data comparing severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) antibody titers of healthcare workers in Hong Kong who had received two doses of a mRNA vaccine compared to two doses of an inactivated vaccine.15 The results demonstrated higher antibody titers after the first and second dose in participants receiving the mRNA vaccination compared to the first and second dose of the inactivated vaccine.15 This data supports the hypothesis of stronger immune response with mRNA vaccination, further increasing risk of immune dysfunction and ocular side effects. The two cases described by Tetsi et al did not specify the specific vaccine administered, nor if prior doses had been received, so a potential explanation cannot be posed.
Episcleritis is a common manifestation in patients with inflammatory bowel disease such as Crohn’s disease.16,17 Approximately 2–5% of inflammatory bowel disease patients develop episcleritis with many being idiopathic in nature.16,18 It is possible this case of episcleritis is idiopathic in nature and not vaccine related. However, the association with vaccination in our patient and rapid resolution suspects this case being vaccine related.
Allergic conjunctivitis is typically bilateral, diffusely affects the ocular surface, and is associated with rhinitis. Patients most commonly suffer from pruritis, and can suffer from foreign body sensation, blurred vision, photophobia, and eyelids swelling.19 Despite our patient’s past medical history of allergic conjunctivitis, she did not have clinical findings to suggest allergic conjunctivitis (ie, no rhinitis, no eyelids itching, no photophobia, no corneal erosions, and no eyelids swelling).
The immune response to vaccination in Crohn’s disease patients is further explored here. Crohn’s disease patients currently on immunosuppression have been shown to have an overall blunted immune response to vaccination, correlating with the level of immunosuppression.20–23 This is thought to be related to overall dampening of the immune system by immunosuppression. Additionally, the immune response to live vaccines does appear to be higher, with increased antibody production, compared to inactivated vaccines.20 The response to mRNA vaccines in Crohn’s disease patients is still being explored as this is a new area of vaccine development, but the immune response does seem to be related to the specific immunosuppressive medication currently prescribed.24 One study found that Crohn’s disease patients have a comparable rate of adverse events following mRNA COVID-19 vaccination compared to the general population.25 Further, Crohn’s disease patients with well controlled disease on biologic therapy may be at lower risk of adverse effects from mRNA COVID-19 vaccination when compared to Crohn’s disease patients not on biologic therapy.25 Thus, Crohn’s disease patients can be educated on their risk of vaccine adverse effects according to their level of disease control with biologic therapy.
Rarely, episcleritis and other inflammatory ocular processes such as scleritis and uveitis can be seen following vaccination of other diseases.16,26 The specific vaccines associated with these side effects include the bacille Calmette-Guerin (BCG) vaccine, intranasal influenza vaccine, measles mumps and rubella (MMR) vaccine, hepatitis B vaccine, human papilloma virus (HPV) vaccine, and varicella vaccine.11,26 In one meta analysis, 289 cases of uveitis were identified following vaccination, with approximately 40% occurring following hepatitis B vaccination, a subunit vaccine.27 Furthermore, with the three previously reported cases of episcleritis following COVID-19 vaccination discussed above, there remains a significant lack of reports describing this complication in the literature. It could be hypothesized that the prevalence of episcleritis following COVID-19 vaccination is much higher than reported in the literature due to the lack of visual impairment and ocular pain associated with episcleritis, causing patients to not seek out medical care.
Most cases of episcleritis resolve without treatment in 2 to 21 days.8 Supportive care typically involves artificial tears, mild topical corticosteroids, topical NSAIDs, and oral NSAIDs.8 Our patient received 200mg ibuprofen three times daily for one week with complete resolution of symptoms. The prognosis of patients with episcleritis is generally good, but approximately 30% of patients will develop recurrent episcleritis.28–32
Conclusions
In conclusion, episcleritis following COVID-19 vaccination is a rare ocular side effect that is self-limiting in nature and generally lacks long term health complications. Here we described the first reported case of unilateral right episcleritis in a patient with Crohn’s disease, developing within hours following Moderna mRNA COVID-19 booster vaccination. Healthcare providers should be aware of this rare ocular side effect in Crohn’s disease patients and the treatment options available.
Abbreviations
COVID-19, Coronavirus disease 2019; SARS-CoV2, severe acute respiratory syndrome coronavirus 2; BCG, bacille Calmette-Guerin; MMR, measles mumps and rubella; HPV, human papilloma virus; NSAIDs, non-steroidal anti-inflammatory drugs.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
University Institutional Review Board approval was not obtained, as this is a single case report.
Consent for Publication
Written informed consent was obtained from the patient for publication and for use of data and images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors received no specific funding for this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Who coronavirus (COVID-19) dashboard. World Health Organization. Available from: https://covid19.who.int/.
2. Solís Arce JS, Warren SS, Meriggi NF, et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat Med. 2021;27(8):1385–1394. doi:10.1038/s41591-021-01454-y
3. Sen M, Honavar SG. After the storm: ophthalmic manifestations of COVID-19 vaccines. Indian J Ophthalmol. 2021;69(12):3398–3420. doi:10.4103/ijo.IJO_2824_21
4. Bolletta E, Iannetta D, Mastrofilippo V, et al. Uveitis and other ocular complications following COVID-19 vaccination. J Clin Med. 2021;10(24):5960. doi:10.3390/jcm10245960
5. Ng XL, Betzler BK, Testi I, et al. Ocular adverse events after COVID-19 vaccination. Ocul Immunol Inflamm. 2021;29(6):1216–1224. doi:10.1080/09273948.2021.1976221
6. Wang MTM, Niederer RL, McGhee CNJ, Danesh-Meyer HV. COVID-19 vaccination and the eye. Am J Ophthalmol. 2022;240:79–98. doi:10.1016/j.ajo.2022.02.011
7. Honik G, Wong IG, Gritz DC. Incidence and prevalence of episcleritis and scleritis in Northern California. Cornea. 2013;32(12):1562–1566. doi:10.1097/ICO.0b013e3182a407c3
8. Schonberg S, Stokkermans TJ. Episcleritis. StatPearls; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534796/.
9. Pichi F, Aljneibi S, Neri P, et al. Association of ocular adverse events with inactivated COVID-19 vaccination in patients in Abu Dhabi. JAMA Ophthalmol. 2021;139(10):1131. doi:10.1001/jamaophthalmol.2021.3477
10. Pang K, Pan L, Guo H, Wu X. Case report: associated ocular adverse reactions with inactivated COVID-19 vaccine in China case report. Front Med. 2022;2022:8.
11. Testi I, Brandão-de-resende C, Agrawal R, Pavesio C. Ocular inflammatory events following COVID-19 vaccination: a multinational case series. J Ophthalmic Inflamm Infect. 2022;12(1). doi:10.1186/s12348-021-00275-x
12. Agarwal M, Dutta Majumder P, Babu K, et al. Drug-induced uveitis: a review. Indian J Ophthalmol. 2020;68(9):1799–1807. doi:10.4103/ijo.IJO_816_20
13. Cunningham ET, Moorthy RS, Fraunfelder FW, Zierhut M. Vaccine-Associated Uveitis. Ocul Immunol Inflamm. 2019;27(4):517–520. doi:10.1080/09273948.2019.1626188
14. Ng XL, Betzler BK, Ng S, et al. The eye of the storm: COVID-19 vaccination and the eye. Ophthalmol Ther. 2021;11(1):81–100. doi:10.1007/s40123-021-00415-5
15. Lim WW, Mak L, Leung GM, et al. Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19. Lancet Microbe. 2021;2185 (9):e423. doi:10.1016/S2666-5247(21)00177-4
16. Yilmaz S, Aydemir E, Maden A, Unsal B. The prevalence of ocular involvement in patients with inflammatory bowel disease. Int J Colorectal Dis. 2007;22(9):1027–1030. doi:10.1007/s00384-007-0275-1
17. Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(8):1982–1992. doi:10.1097/MIB.0000000000000392
18. Troncosco LL, Biancardi AL, de Moraes HV, Zalrman C. Ophthalmic manifestations in patients with inflammatory bowel disease: a review. World 175 J Gastroenterol. 2017;23(32):5836–5848. doi:10.3748/wjg.v23.i32.5836
19. Dupuis P, Prokopich CL, Hynes A, Kim H. A contemporary look at allergic conjunctivitis. Allergy Asthma Clin Immunol. 2020;16:5. doi:10.1186/s13223-020-0403-9
20. Reich J, Wasan S, Farraye FA. Vaccinating patients with inflammatory bowel disease. Gastroenterol Hepatol. 2016;12(9):540–546.
21. Banaszkiewicz A, Targońska B, Kowalska-Duplaga K, et al. Immunogenicity of 13-valent pneumococcal conjugate vaccine in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(7):1607–1614. doi:10.1097/MIB.0000000000000406
22. deBruyn J, Fonseca K, Ghosh S, et al. Immunogenicity of influenza vaccine for patients with inflammatory bowel disease on maintenance infliximab therapy: a randomized trial. Inflamm Bowel Dis. 2016;22(3):638–647. doi:10.1097/MIB.0000000000000615
23. Wasan SK, Zullow S, Berg A, et al. Herpes zoster vaccine response in inflammatory bowel disease patients on low-dose immunosuppression. Inflamm Bowel Dis. 2016;22(6):1391–1396. doi:10.1097/MIB.0000000000000743
24. Alexander JL, Kennedy NA, Ibraheim H, et al. COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): a multicentre, prospective, case-control study. Lancet Gastroenterol Hepatol. 2022;7(4):342–352. doi:10.1016/S2468-1253(22)00005-X
25. Botwin GJ, Li D, Figueiredo J, et al. Adverse events after SARS-CoV-2 mRNA vaccination among patients with inflammatory bowel disease. Am J Gastroenterol. 2021;116(8):1746–1751. doi:10.14309/ajg.0000000000001342
26. Moorthy RS, Moorthy MS, Cunningham ET. Drug-induced uveitis. Curr Opin Ophthalmol. 2018;29(6):588–603. doi:10.1097/ICU.0000000000000530
27. Benage M, Fraunfelder FW. Vaccine-Associated Uveitis. Mo Med. 2016;113(1):48–52.
28. Akpek EK, Uy HS, Christen W, et al. Severity of episcleritis and systemic disease association. Ophthalmology. 1999;106(4):729–731. doi:10.1016/S0161-6420(99)90157-4
29. de la Maza M S, Molina N, Gonzalez-Gonzalez LA, et al. Clinical characteristics of a large cohort of patients with scleritis and episcleritis. Ophthalmology. 2012;119(1):43–50. doi:10.1016/j.ophtha.2011.07.013
30. Daniel Diaz J, Sobol EK, Gritz DC. Treatment and management of scleral disorders. Surv Ophthalmol. 2016;61(6):702–717. doi:10.1016/j.survophthal.2016.06.002
31. Watson PG, Hayreh SS. Scleritis and episcleritis. Br J Ophthalmol. 1976;60(3):163–191. doi:10.1136/bjo.60.3.163
32. Sainz de la Maza M, Jabbur NS, Foster CS. Severity of scleritis and episcleritis. Ophthalmology. 1994;101(2):389–396. doi:10.1016/S0161-6420(94)31325-X
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
