Back to Journals » Risk Management and Healthcare Policy » Volume 15
Understanding the Attitudes and Willingness of Adult Chinese Patients with Rheumatic Diseases Towards COVID-19 Vaccination
Authors Zeng H, Liu H, Liu M, Zhou Z, Wang SB, Zhou K, Li W, Dai L, Chen Y, Uy JP, Sun C , Ye Z
Received 30 July 2022
Accepted for publication 17 November 2022
Published 1 December 2022 Volume 2022:15 Pages 2269—2281
DOI https://doi.org/10.2147/RMHP.S384337
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jongwha Chang
Huiqiong Zeng,1,* Hanjiang Liu,2,* Meifen Liu,1 Zhen Zhou,3 Shi-Bin Wang,4 Kaixia Zhou,5 Wengen Li,6 Liping Dai,1 Yashuo Chen,1 John Patrick Uy,7 Chenyu Sun,8 Zhizhong Ye1
1Department of Rheumatology, Shenzhen Futian Hospital for Rheumatic Diseases, Shenzhen, People’s Republic of China; 2Department of Safety Supervision Division, Guangdong Pharmaceutical Association, Guangzhou, People’s Republic of China; 3Department of Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia; 4Department of Mental Health, Guangdong Mental Health Center, Guangdong Provincial People’s Hospital, Guangzhou, People’s Republic of China; 5Department of Clinical Laboratory, Shanghai Cancer Center, Fudan University, Shanghai, People’s Republic of China; 6Department of Rheumatology and Immunology, Meizhou People’s Hospital (Huangtang Hospital), Meizhou, People’s Republic of China; 7Department of Infectious Disease and International Health, Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA; 8Department of AMITA Health Saint Joseph Hospital Chicago, University of Illinois at Chicago, Chicago, IL, USA
*These authors contributed equally to this work
Correspondence: Zhizhong Ye, Department of Rheumatology, Shenzhen Futian Hospital for Rheumatic Diseases, Shenzhen, People’s Republic of China, Tel +852-13902477524, Email [email protected] Chenyu Sun, AMITA Health Saint Joseph Hospital Chicago, University of Illinois at Chicago, 2900 N. Lake Shore Drive, Chicago, Illinois, 60657, USA, Email [email protected]
Background: The SARS-CoV-2 pandemic has imposed substantial health and economic burdens on the societies. COVID-19 vaccination is the most effective method of controlling the epidemic. This study assessed the attitude, willingness, and related factors of adult patients with rheumatic diseases (RDs) in China towards COVID-19 vaccination and identified their reasons for being vaccinated.
Methods: A cross-sectional survey was administered to patients with rheumatic diseases from July 18 to August 18, 2021, using an online questionnaire. Logistic regression analysis was performed to examine the data.
Results: We analyzed data drawn from 464 participants who provided valid responses. A total of 324 (69.83%) RD patients were not willing to be vaccinated, of which 76.97% believed that COVID-19 vaccination might exacerbate the diseases symptoms. Logistic regression analysis showed that a combination of experiencing systemic damage, being in the acute attack stage of the disease, and fear of the adverse impact of vaccination on rheumatism, etc., were the predominant factors affecting the intentional vaccination rate in adult patients with rheumatic diseases (p < 0.05).
Conclusion: The COVID-19 intentional vaccination rate was relatively low in adult Chinese patients with RD. Public health education and the dissemination of government scientific data for patients with RD should be enhanced to increase COVID-19 vaccination rates.
Keywords: China, rheumatic disease, COVID-19, vaccination, attitude, willingness
Introduction
Coronavirus disease (COVID-19) an infectious disease caused by the SARS-CoV-2 virus, has imposed substantial health and economic burdens on the society since its outbreak in 2019.1 Although prevention and control measures for the COVID-19 vary across countries, immunization at the population level-especially for those highly susceptible to the infections-remains the most important preventive strategy. The World Health Organization (WHO) recommends that all individuals who meet the criteria for getting a COVID-19 vaccination and have no relevant contraindications should receive their vaccines and boosters as soon as possible.2 Achieving effective control of the COVID-19 outbreak and ending the spread of the virus will be possible only when there are a sufficient number of people with effective antibodies.3 Achieving an optimal COVID-19 vaccination rate has therefore become a global a primary public health goal.
In contrast to traditional vaccines, COVID-19 vaccines have been rapidly developed and are being approved through Emergency Use Authorization worldwide.
So far, COVID-19 vaccines comprise inactivated vaccines, attenuated vaccines, subunit vaccines, nanoparticle vaccines, virus vector vaccines, and nucleic acid vaccines.4 A wealth of data has shown a favorable efficacy profile for COVID-19 vaccines.5–7 However, many studies have revealed that all these vaccines may have multiple adverse effects,8,9 which may trigger concerns among general public regarding the safety of COVID-19 vaccination.10 Faced with a new vaccine that requires rapid development, people may rely more on anxiety, fear and other emotions to make decisions related to COVID-19 vaccines.
Hesitation and unwillingness to receive COVID-19 vaccination may cause the delays in administering vaccines at the population-level.11 This may increase the risk of infection and lead to serious clinical consequences in people who are more susceptible and vulnerable to SARS-CoV-2 virus infection. When infected, people with chronic conditions such as rheumatic diseases (RDs) are more likely to experience severe symptoms and carry a higher risk of death.12,13 Therefore, individuals with chronic diseases who are in a stable condition are strongly recommended to receive the COVID-19 vaccine.14 Rheumatic diseases primarily affect the bones, joints, and soft tissues of the body, and are also known as a heterogeneous set of chronic multi-system inflammatory disorders.15 Interestingly, the literature reveals that COVID-19 is associated with the development of autoimmunity and auto-antibodies. ANA positive Covid-19 patients have been found to have a worse prognosis than that of ANA-negative patients.16
Rheumatoid arthritis (RA) is a common form of RD. Patients with RA are likely to have a greater risk of COVID-19 infection due to immunological abnormalities, the use of anti-rheumatic medicines, and related comorbidities.17 The prevalence of RDs in China was estimated to range from 11.6% to 46.4%.18 Understanding the COVID-19 vaccination status among adult RD patients in China, encouraging RD patients to receive a vaccination, and assisting them in resolving issues that emerge throughout the vaccination process critical for preventing the spread of COVID-19. Numerous studies have investigated the acceptability of the COVID-19 vaccine among the general populations and the factors influencing willingness and acceptance towards COVID-19 vaccination.19 However, no studies have targeted patients with chronic diseases in China.20
A survey on vaccination willingness among healthy people found that 83.5% of those polled were willing to take the COVID-19 vaccine, whereas 64.2% were willing to receive the domestic vaccine. However, the actual number of people who have been vaccinated in China is lower than that of people who are willing to be vaccinated, particularly among patients with chronic diseases.21 Rheumatic disease patients who are in a stable condition can consider receiving the inactivated of subunit COVID-19 vaccine, while most diseases have been removed from the taboo list.14 The primary reasons for the general public’s hesitancy to receive COVID-19 vaccination include the concerns about side effects, expenses, and the severity of the pandemic.22 In this study, we performed a cross-sectional survey to determine the COVID-19 vaccination status of adult RD patients in China. The factors affecting their willingness to be vaccinated were summarized and analyzed to help lay the groundwork for accelerating the process of COVID-19 vaccination, in order to improve and end the COVID-19 epidemic.
Materials and Methods
Participants
The study’s participants were recruited for virtual study. We performed an online survey from July 18 to August 18, 2021, using an anonymous and voluntary questionnaire based on prior research23,24 to evaluate vaccination acceptance. The questionnaire was collected online by the researchers from the Department of Rheumatology, Futian Rheumatology Hospital, Futian District, Guangdong Province, China. The hospital is a specialized for rheumatism, and serves RD patients from all over the country. The diagnosis of each RD patient, conducted according to International Standard ICD-10, was confirmed by a rheumatologist with a senior professional title in the hospital. As of this August 18, 2021, there were rheumatism sub-departments in the hospital, and each sub-department has a corresponding patient management group maintained via WeChat (the largest social media platform in China).
The patients underwent long-term evaluation and follow-up at our hospital, and we used a specialist team for RD patients management support and sustained follow up. This is important because this model of care delivery is likely to improve the health outcomes of RD patients struggling to manage disease progression. Our management model encourages individuals to monitor their condition through regular contact with health provision teams via WeChat. Therefore, the mutual trust between doctors and patients is high. During the data collection in the first month of the study, 3149 patients with RD were recorded from the four sub-departments. This backgrounds ensured that we could complete our work on time.
Participants were recruited through the WenJuan application (https://www.wenjuan.com/), one of China’s most commonly used online survey tools in China. This platform does not allow users to be identified via tracking, and does not use analytical cookies. All patients gave their data anonymously.
The survey team investigated RD patients who met the sampling inclusion criteria using a convenient sampling method.The vaccination status of adult Chinese RD patients was explored using a questionnaire that solicited data such as basic information, vaccination awareness, and vaccination willingness status.
Simultaneously, we recruited off-line patients through outpatient or inpatient face-to-face consultations and project introductions. The inclusion criteria were as follows: The patient was diagnosed with RD by a local rheumatologist, was aged between 18 and 59 years, was aware of their diagnosis, had basic reading skills, used online apps, and understood text descriptions. The study excluded patients who refused to cooperate with the investigation, were in a critical condition requiring emergency medical treatment, or did not meet the basic requirements of vaccination according to government vaccination guidelines (such as being ≥ 60 years old). This study was approved by the Ethics Committee of the Futian Rheumatology Hospital. Before participating, all offline and online respondents were informed about the survey’s objective in the questionnaire title (see the Supplementary File for details.), and about our commitment to maintaining the participants’ personal information in confidence. The respondents received no financial compensation for completing the online survey.
Study Design
Baseline assessments were conducted using a standardized questionnaire to collect demographic data (age, gender, education status, marital status, monthly income, work status, payment of medical expenses, and channels of communication with physicians), disease-related characteristics (course of disease, diagnosis, concomitant systemic impairment, medications, and treatment), sources of information, and knowledge and understanding of the COVID-19 vaccine. The questionnaire (see Supplementary File) was completed independently by the participants.
A pre-tested well organized structured questionnaire adapted from a various of published articles was used.23,24 The questionnaire design was discussed and assessed by three specialists (including two professors of public health, and one rheumatologist; and the experts’ agreement rate was ≥80%). Researchers have also conducted a pre-test among 23 patients with RD to validate the feasibility of the questionnaire. We considered invalid samples (defined as respondents who choose the same answer for more than the two-thirds of the questions). The questionnaire received a response rate of 90.00%. Cronbach’s alpha showed moderate reliability (0.701). After pre-testing and optimization, the final version of the questionnaire was adopted.
The questionnaire was divided into four sections. The first section asked about participants’ general characteristics. The second section asked about the participants’ self-reported health conditions. The third section inquired about participants’ knowledge and attitudes concerning COVID-19 vaccination. The fourth section asked about the status of the COVID-19 vaccination. We arranged the order of the questions according to the purpose of this study. These questions were asked to better capture how various factors may have affected their acceptance of the COVID-19 vaccine. We attempted to be objective by avoiding using superfluous terms that could have mislead the participants.
All questions were closed-ended, with check boxes (single choice or multiple-choice questions) provided for responses. Most of the questions were treated as categorical variables. The questions were asked using a similar three- or five-point Likert scale.25 Importance was quantified as “1-very important”, “2-moderately important”, “3-neutral”, “4-less important”, and “5-least important.” Degree of identification was expressed as “1 = strongly agree”, “2 = somewhat agree”, “3 = indifferent”, “4 = somewhat disagree”, and “5 = strongly disagree.” Degree of effectiveness was expressed as “1-effective”, “2-neutral”, and “3-ineffective.” Degree of risk was expressed as “1 = minimal risk”, “2 = small risk”, “3 = moderate risk”, “4 = high risk”, and “5 = significantly high risk.”
Sample Size
We used the following formula for sample size in the cross-sectional study to determine our study group: n = Zα2 × proportion (1−proportion)/precision2. In the formula, α = 0.05, Zα = 1.96, and the precision was 0.05. The proportion was 64.01%, which was found to be the rate of people who were willing to be vaccinated against COVID-19 in a previous study done in China.26 After calculation, the minimum sample size was found to be 354. Ultimately, 464 questionnaires were considered valid.
Statistical Analysis
SPSS17.0 software was used for statistical analysis to establish a database for data entry, collation, statistical processing, and analysis. The chi-squared test was performed for the categorical data. Bivariate and multivariable analyses were used to assess the associations between the predictor clinical variables and intention to obtain the COVID-19 vaccine. The adjusted regression coefficients (B) with 95% confidence intervals (CIs) was assessed to evaluate the relationship between them, with P < 0.05 indicating statistical significance.
Results
RD Patients COVID-19 Vaccination Situation
As of August 15, 2021, a total of 140 (30.17%) patients with RD were willing to receive COVID-19 vaccine, whereas 324 (69.83%) were not.
Participant Characteristics
This study comprised 464 adult RD patients. Among them, 319 (68.75%) patients were females and 145 were males (31.25%). In total, 198 (42.67%) cases were aged between 18 and 35; 132 (28.45%) patients were aged between 36 and 45; and 134 (28.88%) were aged between 46 and 59. Table 1 summarizes the descriptive statistics, disease characteristics, and chi-squared test results for the sample.
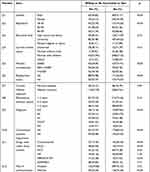 |
Table 1 Demographic Features and Disease Characteristics Toward COVID-19 Vaccination in RD (n = 464) |
The chi-square test revealed that gender, diagnosis, concomitant systemic impairment, use of corticosteroids or hydroxychloroquine (HCQ) within three months, contraindications for vaccination, being in the acute attack stage of acute or chronic diseases, fear of disease progression due to drug discontinuation during vaccination, vaccine shortage, and other vaccines currently administered (such as the HPV vaccine) were all associated with vaccine hesitancy (P < 0.05). Age, personal income, work status, use of febuxostat/corticosteroid/HCQ within three months, use of WeChat as the primary contact approach with Doctors, and pregnancy preparation had a negative impacts on willingness to receive receive vaccination among RD (P < 0.05).
As shown in Table 1, the intentional COVID-19 vaccination rate was significantly higher in male patients (65 subjects, 44.83%) than in female patients (75 subjects, 23.51%); patients aged between 36 and 45 years had the highest intentional vaccination rate (51 subjects, 38.64%); patients with a monthly income of between 5001 and 10,000 yuan had the highest intentional vaccination rate (54 patients, 36.24%); and the intentional vaccination rate of the employed patients (33.98%) was higher than that of unemployed patients (25.37%);
Among vaccine hesitancy (VH) individuals, RD patients who had used corticosteroids within three months had the lowest intentional vaccination rate (84.30%). A total of 143 (79.01%) subjects who used HCQ were not willing to be vaccinated, while 10 (47.62%) subjects who used febuxostat were not willing to be vaccinated; 19 patients (90.48%) who were planning pregnancy and 146 patients (88.48%) who were in the acute disease stage were not willing to be vaccinated; Fear of adverse events and concern that vaccination may affect their RD was also a reason to refuse vaccination. Additional information is provided in Table 1.
Predictors of Attitude Toward COVID-19 Vaccination
Table 2 summarizes the cognition and attitudes of patients with RD regarding vaccination. The findings of the chi-square test indicated that the antithetic answers to questions Q14, Q15, Q16, Q18, Q19, and Q21 had a negative effect on the desire for COVID-19 immunization in RD patients (P < 0.05).
 |
Table 2 Predictors of Attitude and Knowledge Toward the COVID-19 Vaccine in RD Patients (n=464) |
Among the study participants, 234 (50.43%) thought that getting the COVID-19 vaccination was very important, 92 (19.83%) considered it relatively important, 131 (28.23%) rated it as uncertain, six thought it was less important, and only one thought it was of least importance. The patients who deemed the vaccination to be insignificant were not willing to be immunized. The intentional vaccination rate was lower in patients (117 participants, 76.97% versus 105 participants, 33.65%) who had not known about the COVID-19 Vaccination Guideline (first version) issued by National Health and Family Planning Commission of the People’s Republic of China (NHFPC of PRC) in March 2021. Those patients (31 individuals, 88.57%) who did not consider that COVID-19 immunization should be universal had a considerably lower intentional vaccination rate than other patients who confirmed that (136 participants, 31.7%). Regarding Q19, we found that fear (cognitive impairment) may affect vaccination decision. The number of participants who were willing to be vaccinated was significantly lower among those who expressed concerns about the vaccine’s safety and thought that it might aggravate their rheumatoid symptoms than it was among those who did not express such concerns.
Regarding Q21, we found that the intentional vaccination rate of patients who believed COVID-19 posed a greater vaccine risk was significantly lower than that of other patients (one patient, 3.03%), followed by those who believed the risk was modest (27 patients, 20.61%), and those who believed the risk was minimal (the highest intentional vaccination rate; 74 patients, 40.88%).
Independent Predictors Associated with the Acceptance to Vaccinate Against COVID-19
Binary logistic regressions were used to examine the association between personal disease status and the intention to receive the COVID-19 vaccine.
Five factors were associated with the intention to receive the vaccine in RD patients. These included related system damage (OR = 0.42, 95% CI:0.27–0.65, P < 0.05), contraindication forOR = 0.12, 95% CI:0.03–0.51, P < 0.05), acute onset of chronic disease (OR = 0.20, 95% CI:0.15–0.36, P < 0.05), and worry about the negative impact of COVID-19 vaccine on rheumatism (OR = 0.25, 95% CI:0.14–0.44, P < 0.05); for more information, see Table 3.
 |
Table 3 Analysis of the Influence of General Conditions and Attitudes on COVID-19 Vaccination in RD Patients (n=464) |
The differences in the responses to questions Q15 and Q19 regarding the cognitive status of RD patients were statistically significant (P < 0.05). Those who did not pay more attention to the vaccine’s latest news about the vaccine (OR = 0.60, 95% CI:0.38–0.95, P < 0.05) and those who believed that the COVID-19 vaccine would worsen their disease (OR = 0.12, 95% CI:0.26–0.65, P <0.05) were less willing to be vaccinated. (For more details, see Table 4).
 |
Table 4 Analysis of the Influence of Cognitions on COVID-19 Vaccination |
Discussion
SARS-CoV-2 is responsible for the continuing COVID-19 pandemic. As of August 1, 2021, the total number of COVID-19 infections (or cases) caused by SARS-CoV-2 exceeded 200 million globally.27 The COVID-19 Global Rheumatism Alliance Registry, which includes data from 600 Covid-19-infected patients from 40 countries, revealed that patients with systemic lupus erythematosus(SLE) accounted for 17% (48/277) of the RD cohort, while more than half of the RD patients were hospitalized (55%).28 The COVID-19 vaccine is the most effective way to combat the this pandemic.29 China has initiated Phase III clinical trials for five types of COVID-19 vaccines.30 According to statistics issued by the NHFPC of the PRC and Our World in Data, global immunization doses totaled 646.6 million as of April 6, 2021, with China ranking second n number of doses (142.8 million).31 Achieving herd immunity requires an intentional vaccination rate of at least 70% is required. This study aimed to identify vaccination needs in RD patients, resolve vaccination process problems, and enhance the intentional vaccination rate by assessing the factors influencing COVID-19 vaccination acceptance among RD patients. To our knowledge, this study is the first to explore COVID-19 vaccination rates among RD patients in mainland China.
This study found that the intentional COVID-19 vaccination rate among Chinese RD patients was 30.17% (see Table 5). Studies have shown that 60.4% (95% CI:57.4, 63.4) of the adult population in China was willing to receive the COVID-19 vaccine in China.29 The key to promoting vaccination is that people aged 30 to 49 have confidence in the vaccine’s efficacy and pay close attention to vaccine news.32 This demonstrates that the actual rate of COVID-19 immunization in adult patients with RD in China is significantly lower than the willing acceptance rate. Additionally, research has revealed that males and those with higher incomes have a considerably higher explicit intention to vaccinate.33 We found that the intentional vaccination rate at 36–45 years was higher in males than in females. Patients with a monthly income of more than 5000 yuan RMB had a considerably higher vaccination rate than those with a monthly income of less than 5000. Employed patients may have a higher monthly income than unemployed ones. These results are consistent with earlier findings.31
 |
Table 5 Demographic Information of COVID-19 Vaccination Situation Among RD (n = 464) |
We found that a variety of RD-related conditions, such as concomitant systemic damage, use of drugs within the last three months, presence of contraindications to vaccination, being in the acute attack stage of acute or chronic diseases, and concerns about RD progression caused by drug discontinuation during vaccination were the primary barriers to the intention to receive COVID-19 vaccination in RD patients. The intentional vaccination rate could therefore be improved through adequate communication that focuses on the patient’s condition assessment and informs them as to whether they are eligible for vaccination and whether the disease will progress after vaccination. Effective communication between the patient and doctor is critical to the patient’s healing process. When a physician and patient work collaboratively, they have a greater chance of achieving better health outcomes.34
As shown in Table 1, the intentional vaccination rate of RD patients who communicated with their doctors through WeChat was significantly lower (23.64%) than the average intentional vaccination rate of RD patients, who communicated with their physicians by telephone or through face-to-face services (30.17%). Online text communication alone is insufficient, as patients may be unable to comprehend or evaluate their true disease status quickly and correctly, or provide timely input to their doctor. The information offered in the WeChat official accounts of public health agencies is insufficient to meet the needs of all patients. Thus, enhancing physician–patient communication and professional illness assessment will increase patients’ awareness of vaccination. Media opinion, misinformation, and untimely public health information affect the public’s attitude and willingness towards vaccination. We found that 92.46% of RD patients believed that universal immunization was necessary. Participants who had read the Guideline for COVID-19 Vaccination (First Version) had a significantly higher intentional vaccination rate, which indicates that enhancing government advocacy can help increase vaccination willingness.
Daniel et al found that most of their US interviewees expressed no concerns about the safety of the COVID-19 vaccine (69%) and believed that its benefits far outweighed the risks (80%). Moreover, the majority (73%) claimed that the vaccination recommendation of the US Centers for Disease Control was appropriate.35 In our study, 68.91% of VH RD patients considered the COVID-19 vaccination to be effective, 79.39% thought the risk to be average, and 96.97% assessed it to be relatively high. The VH respondents were concerned about the safety of the COVID-19 vaccine (65.18%) and its impact on rheumatism (76.97%), indicating that patients were more concerned about the effect of immunization on illnesses. Additionally, we discovered that 36.73% of RD patients believed that their disease state would worsen after vaccination (95% CI:0.211, 0.818, P < 0.01); this factor, for influenza vaccination, was reported by Gabriel et al at the lower proportion of 20.1%.36 This study’s outcome has not been previously described.
Vaccination rates have been reported for other chronic inflammatory diseases. A Greek study of potential changes in influenza vaccination rates among RD patients during the COVID-19 pandemic revealed an increase in the vaccination rate from 76% to 83%.37 Findings on the knowledge, attitude, and willingness (KAW) of RD patients to be vaccinated against COVID-19 vary from place to place. In two major public hospitals in Hong Kong, the vaccination rate was 30.2% among RD patients,38 similar to our result. An Australian survey found VH in approximately one-third of individuals with RD,39 while an Italian study found that the overall COVID-19 vaccination rate was 17%.40 This discrepancy may be related to factors such as vaccine supply, government policy and regulations, and patient catharsis support.
Misleading media reports regarding adverse reactions to COVID-19 vaccines may have a negative effect on the public vaccination rate.41 The research indicates that a lack of confidence in vaccine safety is the primary reason for VH.42 The results of our study corroborated these findings. Numerous clinical trials conducted in China have demonstrated that the local COVID-19 inactivated vaccine is safe and tolerable.6,11 Media should thus provide more positive coverage of the vaccine.
This study had several limitations. First, because we recruited individuals only from academic clinics and hospitals in China’s metropolitan areas, our sample may not be representative of all patients with RD, particularly those living in regional and remote areas with inadequate medical care. Second, we could not compare COVID-19 vaccination rates between RD and non-RD patients because our study was limited to the RD population. Third, we used self-managed questionnaires to collect the COVID-19 vaccination data; therefore, we could not confirm participants’ real vaccination status based on online results, which may have led to misclassification bias. Fourth, the study’s sample was relatively small. This was a cross-sectional study conducted during a pandemic period, and information about the vaccine and patients’ willingness and knowledge is constantly changing. The results of a similar study performed today might be different.
Conclusion
We found that the intentional COVID-19 vaccination rate was low (30.17%) among adult RD patients; this rate was influenced by a variety of factors. Regression analysis found that the barriers to receiving the COVID-19 vaccine for RD patients included combined system damage, having contraindications, being in the attack stage of disease, concerns about disease development caused by drug discontinuation during the vaccination process, pregnancy preparation, vaccine side effects and cognition. Strategies for improving the vaccination rates should include, but must not be limited to, publicizing COVID-19 vaccination through various channels, increasing vaccination awareness among RD patients, encouraging them to seek help for disease assessment, and enhancing their communication with doctors.
Data Sharing Statement
The datasets analyzed in this manuscript are not publicly available. Requests to access the datasets should be directed to [email protected].
Ethics Approval and Consent to Participate
The research was approved by the Shenzhen Futian Hospital for Rheumatic Diseases’ local ethics council in accordance with the Helsinki Declaration.
Consent for Publication
Written informed consent for publication was obtained from all participants.
Acknowledgments
We sincerely appreciate the families and clinicians for their participation in this project.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was financially supported in part by research grants from the Sanming Project of Medicine in Shenzhen(SZSM201602087), Shenzhen Science and Technology Project (JCYJ20180302145033769). Research on public welfare project in Futian District,Shenzhen(FTWS 2021062).
Disclosure
The authors declared no conflicts of interest.
References
1. Jin Y, Yang H, Ji W, et al. Epidemiology, Pathogenesis, and Control of COVID-19. Viruses. 2020;12:372.
2. COVID-19 advice for the public: getting vaccinated. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice.
3. Lytras T, Dellis G, Flountzi A, et al. High prevalence of SARS CoV-2 infection in repatriation flights to Greece from three European countries. J Travel Med. 2020;27:taaa054.
4. Kandimalla R, Chakraborty P, Vallamkondu J, et al. Counting on COVID-19 vaccine: insights into the current strategies, progress and future challenges. Biomedicines. 2021;9:1740. doi:10.3390/biomedicines9111740
5. Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, Phase 2 trial. Lancet. 2020;396::479–8. doi:10.1016/S0140-6736(20)31605-6
6. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a Phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–468. doi:10.1016/S0140-6736(20)31604-4
7. Li YD, Chi WY, Su JH, Ferrall L, Hung CF, Wu TC. Coronavirus vaccine development: fromSARS and MERS to COVID-19. J Biomed Sci. 2020;27:104. doi:10.1186/s12929-020-00695-2
8. Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: aRandomized clinical trial. JAMA. 2021;326:35–45. doi:10.1001/jama.2021.8565
9. Meng FY, Gao F, Jia SY, et al. Safety and immunogenicity of a recombinant COVID-19 vaccine(Sf9 cells)in healthy population aged 18 years or older: two single-center, randomised, double-blind, placebo-controlled, phase 1 and phase 2 trials. Signal Transduct Target Ther. 2021;6:271. doi:10.1038/s41392-021-00692-3
10. Azarpanah H, Farhadloo M, Vahidov R, Pilote L. Vaccine hesitancy: evidence from an adverse events following immunization database, and the role of cognitive biases. BMC Public Health. 2021;21:1686. doi:10.1186/s12889-021-11745-1
11. Razai MS, Chaudhry UAR, Doerholt K, Bauld L, Majeed A. Covid-19 vaccination hesitancy. BMJ. 2021;373:n1138.
12. Brito-Zerón P, Sisó-Almirall A, Flores-Chavez A, Retamozo S, Ramos-Casals M. SARS-CoV-2 infection in patients with systemic autoimmune diseases. Clin Exp Rheumatol. 2021;39:676–677. doi:10.55563/clinexprheumatol/lekp1y
13. Long JD, Strohbehn I, Sawtell R, Bhattacharyya R, Sise ME. COVID-19 survival and its impact on chronic kidney disease. Transl Res. 2022;241:70–72. doi:10.1016/j.trsl.2021.11.003
14. Curtis JR, Johnson SR, Anthony DD, et al. American college of rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis Rheumatol. 2021;73:60–65.
15. Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278:369–375. doi:10.1111/joim.12395
16. Muratori P, Lenzi M, Muratori L, Granito A. Antinuclear antibodies in COVID 19. Clin Transl Sci. 2021;14:1627–1628. doi:10.1111/cts.13026
17. Favalli EG, Agape E, Caporali R. Incidence and Clinical Course of COVID-19 in Patients with Connective Tissue Diseases: aDescriptive Observational Analysis. J Rheumatol. 2020;47:1296. doi:10.3899/jrheum.200507
18. Li R, Sun J, Ren LM, et al. Epidemiology of eight common rheumatic diseases in China: a large-scale cross-sectional survey in Beijing. Rheumatology. 2012;51:721–729. doi:10.1093/rheumatology/ker370
19. Troiano G, Nardi A. Vaccine hesitancy in the era of COVID-19. Public Health. 2021;194:245–251. doi:10.1016/j.puhe.2021.02.025
20. Adhikari B, Cheah PY. Vaccine hesitancy in the COVID-19 era. Lancet Infect Dis. 2021;21(8):1086. doi:10.1016/S1473-3099(21)00390-X
21. Chen M, Li Y, Chen J, et al. An online survey of the attitude and willingness of Chinese adults to receive COVID-19 vaccination. Hum Vaccin Immunother. 2021;17:2279–2288. doi:10.1080/21645515.2020.1853449
22. Boucher VG, Pelaez S, Gemme C, Labbe S, Lavoie KL. Understanding factors associated with vaccine uptake and vaccine hesitancy in patients with rheumatoid arthritis: a scoping literature review. Clin Rheumatol. 2021;40:477–479. doi:10.1007/s10067-020-05059-7
23. Wang K, Wong ELY, Ho KF, et al. Intention of nurses to accept coronavirus disease 2019 vaccination and change of intention to accept seasonal influenza vaccination during the co ronavirus disease 2019 pandemic: a cross-sectional survey. Vaccine. 2020;38:7049–7056. doi:10.1016/j.vaccine.2020.09.021
24. Eastwood K, Durrheim DN, Jones A, Butler M. Acceptance of pandemic (H1N1) 2009 influenza vaccination by the Australian public. Med J Aust. 2010;192(1):33–36. doi:10.5694/j.1326-5377.2010.tb03399.x
25. Flaskerud JH. Cultural bias and Likert-type scales revisited. Issues Ment Health Nurs. 2012;33:130–132. doi:10.3109/01612840.2011.600510
26. Jiang Y, Zhang X, Lv Q, et al. Knowledge, attitude, and practice regarding infection and vaccination in patients with rheumatic diseases in China. Hum Vaccin Immunother. 2019;15:1100–1105. doi:10.1080/21645515.2019.1568160
27. World Health Organization. Coronavirus disease(COVID-19)pandemic[EB/OL]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
28. Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis. 2020;79:859. doi:10.1136/annrheumdis-2020-217871
29. Gan L, Chen Y, Hu P, et al. Willingness to Receive SARS-CoV-2 vaccination and associated factors among Chinese adults: a cross sectional survey. Int J Environ Res. 2021;18:1993. doi:10.3390/ijerph18041993
30. World Health Organization. DRAFT landscape of COVID-19 candidate vaccine. Available from: https://www.who.int/Publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
31. Covid-19 vaccination trackerLatest news, statistics, daily rates and updates. Available from: https://www.pharmaceutical-technology.com/covid-19-vaccination-tracker/.
32. Ishimaru T, Okawara M, Ando H, et al. Gender differences in the determinants of willingness to get the COVID-19 vaccine among the working-age population in Japan. Hum Vaccine Immunother. 2021;17:3975–3981. doi:10.1080/21645515.2021.1947098
33. Alobaidi S. Predictors of Intent to Receive the COVID-19 vaccination among the population in the Kingdom of Saudi Arabia: aSurvey Study. J Multidiscip Healthc. 2021;18:1119–1128. doi:10.2147/JMDH.S306654
34. Faerron Guzmán CA, Montero-Zamora P, Bolaños-Palmieri C, Araya-Amador J, Benavides-Rawson J, Ávila-agüero ML. Willingness to get a COVID-19 vaccine and its potential predictors in Costa Rica: a cross-sectional study. Cureus. 2021;13:18798.
35. Yu Q, Xu L, Li L, et al. Internet and WeChat used by patients with Crohn’s disease in China: a multi-center questionnaire survey. BMC Gastroenterol. 2019;19:97. doi:10.1186/s12876-019-1011-3
36. Figueroa-Parra G, Esquivel-Valerio JA, Santoyo-Fexas L, et al. Knowledge and attitudes about influenza vaccination in rheumatic diseases patients. Hum Vaccin Immunother. 2021;17:1420–1425. doi:10.1080/21645515.2020.1816108
37. Fragoulis GE, Grigoropoulos I, Mavrea E, et al. Increased influenza vaccination rates in patients with autoimmune rheumatic diseases during the Covid-19 pandemic: a cross-sectional study. Rheumatol Int. 2021;41:895–902.
38. Li YK, Lui MPK, Yam LL, et al. COVID-19 vaccination in patients with rheumatic diseases: vaccination rates, patient perspectives, and side effects. Immun Inflamm Dis. 2022;10:e589. doi:10.1002/iid3.589
39. Dodd RH, Cvejic E, Bonner C, Pickles K, McCaffery KJ. Sydney Health Literacy Lab COVID-19 group. Willingness to vaccinate against COVID-19 in Australia. Lancet Infect Dis. 2021;21:318–319. doi:10.1016/S1473-3099(20)30559-4
40. Campochiaro C, Trignani G, Tomelleri A, Cascinu S, Dagna L. COVID-19 vaccine study group. Potential acceptance of COVID-19 vaccine in rheumatological patients: a monocentric comparative survey. Ann Rheum Dis. 2021;80:816–817. doi:10.1136/annrheumdis-2020-219811
41. Paul E, Steptoe A, Fancourt D. Attitudes towards vaccines and intention to vaccinate against COVID-19: implications for public health communications. Lancet Reg Health Eur. 2021;2:12.
42. Harapan H, Wagner AL, Yufika A, et al. Acceptance of a COVID-19 vaccine in Southeast Asia: a cross - sectional study in Indonesia. Public Health Front. 2020;8:381. doi:10.3389/fpubh.2020.00381
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
