Back to Journals » Adolescent Health, Medicine and Therapeutics » Volume 11
Undernutrition Among HIV-Positive Adolescents on Antiretroviral Therapy in Southern Ethiopia
Authors Shiferaw H, Gebremedhin S
Received 28 May 2020
Accepted for publication 28 July 2020
Published 19 August 2020 Volume 2020:11 Pages 101—111
DOI https://doi.org/10.2147/AHMT.S264311
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Alastair Sutcliffe
Hailegebriel Shiferaw,1 Samson Gebremedhin2
1College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia; 2School of Public Health, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Samson Gebremedhin School of Public Health
Addis Ababa University, P.O. Box: 12485, Addis Ababa, Ethiopia
Tel +251916822815
Email [email protected]
Purpose: Adolescents living with HIV are vulnerable to undernutrition secondary to elevated nutritional needs imposed by growth spurt and HIV-infection. Yet, in low-income countries, evidence on the epidemiology of undernutrition among adolescents living with HIV is scarce. We assessed the prevalence and predictors of stunting and thinness among adolescents receiving anti-retroviral therapy (ART) in Hawassa city, Southern Ethiopia.
Methods: In this facility-based cross-sectional study, we enrolled 260 adolescents (10– 19 years of age) living with HIV on ART in two public hospitals and three health centers. Anthropometric measurements, household food insecurity and dietary diversity were measured following standard approaches. Predictors of stunting and thinness were identified using multivariable logistic regression analyses and interpreted using adjusted odds ratio (AOR) with 95% confidence interval (CI).
Results: One-third of the adolescents were stunted, and 20% were thin. The prevalence of severe stunting (7.7%) and severe thinness (7.3%) was also high. Significant proportions of the adolescents (38.5%) were from food insecure households, and 28.1% had low or medium dietary diversity. Significant predictors of stunting were lack of food or financial support (AOR=2.71; 95% CI: 1.36– 5.39); meal skipping (AOR=2.13; 95% CI: 1.16– 3.91); recent history of opportunistic infections (AOR=2.25; 95% CI: 1.11– 4.55) and disclosure of HIV status to the adolescent (AOR=1.88; 95% CI: 1.12– 4.34). History of opportunistic infection was the only significant predictor of thinness (AOR=3.21; 95% CI: 1.54– 6.73).
Conclusion: The burden of undernutrition among adolescents living with HIV is disturbingly high. Prevention of opportunistic infections promoting social support and discouraging practice of meal skipping may help to reduce the problem.
Keywords: adolescents, HIV, undernutrition, stunting, thinness, opportunistic infections
Introduction
More than 1.5 million adolescents 10–19 years of age are living with HIV worldwide. Each year 200,000 new adolescents are infected and 33,000 die due to AIDS-related causes.1 Around four-fifth of HIV-infected adolescents and young people live in sub-Saharan Africa.2 In Ethiopia, 690,000 adults and children are living with HIV and the prevalence in adults is about 1%.3 However, little is known about the actual prevalence of HIV among adolescents.4
Adolescence is a critical period of rapid physical and psychosocial development.5 Adolescents which represent 16% of the world population are vulnerable to undernutrition, including sub-optimal weight and linear growth and micronutrient deficiencies, due to manifold reasons including increased physiological demand, household food insecurity, lack of access to health and nutrition information, unhealthy meal patterns, unbalanced energy expenditures, early pregnancy in girls and undernutrition accumulated from in-utero life.6,7 Further, HIV infection exacerbates the burden by increasing nutrient requirements, and causing malabsorption and loss of nutrients.8,9 Stress and depression which are commonly observed in HIV-positive individuals can indirectly lead to malnutrition.9 Antiretroviral treatment (ART) may also suppress appetite.10
Undernutrition inflicts long-lasting damages to adolescents including delayed physical and cognitive development, increased future vulnerability to chronic diseases and unfavorable future birth outcomes in girls.11,12 Further, for children entering the adolescence with growth deficits, sub-optimal nutrition undermines the modest window of opportunity offered by the adolescence period for catchup growth.13,14 Specifically, in adolescents living with HIV, undernutrition leads to fast disease progression, increasing susceptibility to opportunistic infections and mortality.8 Wealth of evidence suggest that undernutrition in people living with HIV results in treatment failure.15–17
Despite these serious and multifold consequences, limited information is available on the epidemiology of malnutrition among adolescents living with HIV in low-income countries where the prevalence of undernutrition and HIV are alarmingly high. The available few studies aggregated adolescent and younger children together and thus did not adequately show the actual burden malnutrition among adolescents.18–21 This may have resulted in over or underestimation of the extent of the problem because systematic difference in the prevalence of undernutrition between young children and adolescents likely exists.
Few studies have so far explored the prevalence and determinants of undernutrition among adolescents living with HIV.22–24 A study in Addis Ababa, Ethiopia found 37% prevalence of stunting and 16% thinness and pertinent risk factors were meal skipping, treatment interruption, household food insecurity and lack of access to nutrition counseling.22 In Uganda, the magnitudes of stunting and thinness among adolescents enrolled in HIV-care program were 36% and 18%, respectively, and the risk factors were rural place of residence and male gender.23 A multicounty retrospective study on adolescents with perinatally acquired HIV infection found 37% prevalence of stunting and significant predictors of linear growth were age, sex and age at ART initiation.24
Accordingly, the purpose of the current study is to assess the prevalence and predictors of stunting and thinness among HIV-positive adolescents on ART in public health institutions in Hawassa city, Southern Ethiopia.
Methods and Materials
Study Setting, Design and Subjects
The study was conducted in Hawassa city, the capital of Southern Region, Ethiopia, having an approximate population of 350,000 population. Excluding private health institutions, Hawassa has two public hospitals and twelve health centers. Among them, the two hospitals (Hawassa University Comprehensive Specialized Hospital, Adare General Hospital) and three health centers (Bushulo, Millennium and Tulla) provide ART services. Private facilities are not involved in the provision of ART service. Though empirical evidences are scarce, Hawassa is regarded as one of the epicenters of HIV infection in Ethiopia.
We underwent an institution-based cross-sectional study in June and July 2018 and enrolled 260 HIV-positive adolescents 10–19 years of age. Adolescents living with HIV on ART follow-ups in the aforementioned hospitals were eligible for the study. Conversely, those who were on their first ART visit or missed their follow-up during the entire data collection period were excluded.
Sample Size Estimation and Sampling Procedure
To assess the prevalence of undernutrition, all adolescents living with HIV in the city who fulfilled the inclusion criteria were enrolled. The adequacy of the sample size for identifying key predictors of stunting and thinness (meal skipping, type of primary caregiver, access to nutrition counseling22) was assessed at 80% power and 95% confidence level using two population proportion formula and found to be sufficient. Study subjects were recruited consecutively.
Data Collection and Measurements
The dependent variables were stunting status (stunted vs non-stunted) and thinness status (thin vs non-thin) as defined based on the standard −2 z-score cutoffs for height-for-age (HFA) and body mass index-for-age (BMI-for-age) indices, respectively. Independent variables include socio-demographic characteristics of the adolescents (age, sex and religion of the adolescents), type of primary caregiver (parents vs others), maternal educational status, household size, monthly household income, household food security, meal skipping, dietary diversity, intentional physical activity, exposure to nutritional counseling, HIV status disclosure (knowledge of the adolescents’ about their own status), substance (alcohol and Cigarette) use, social support (receiving financial or food aid) and clinical characteristics including CD4 count, HIV clinical staging, duration of ART treatment, history of opportunistic infections and receiving Isoniazid (INH) prophylaxis. The independent variables were selected based on hypothesis informed by previous literature.22–24
Data were collected by trained personnel using interviewer-administered pretested questionnaire, directly from the primary caregivers and the adolescents while they visit the health facilities for ART follow-ups. In order to assure privacy, we used health care providers at the ART clinics as data collectors. Dietary diversity was measured by the standard tool of the Food and Agriculture Organization of the United Nations (FAO) as the number of food groups consumed over the preceding day, out of the standard list of 12 groups. Ultimately, dietary diversity score was computed and categorized as low (<4), medium (4–5) or high (>5).25 Household food insecurity was measured and classified into four ordinal categories (secure, mild, moderate and severe insecurity) using the standard Household Food Insecurity Access Scale (HFIAS).26
Body height and weight were measured in duplicates via calibrated tools following standardized procedures. Weight was measured by digital scale to the nearest 0.1 kg and height was measured using portable stadiometer to the nearest 0.1 cm. Stunting and severe stunting were defined as HFA z-score less than −2 and −3, respectively. Similarly, thinness and severe thinness were demarcated based on similar cutoffs for BMI-for-age index. Clinical characteristics (CD4 count, HIV staging, occurrence of opportunistic infections) were extracted from individual medical records.
Data Management and Analysis
Data analysis was made using SPSS version 23 software. Z-scores for HFA and BMI-for-age indices were generated using World Health Organization (WHO) Anthro-plus software. Bivariable and multivariable logistic regression models were fitted for identifying predictors of stunting and thinness and the outputs are presented using crude (COR) and adjusted (AOR) with their respective 95% confidence intervals (CI). Any independent variable whose bivariable test had p-value below 0.25 was considered as a candidate variable for the multivariable model. Once the candidate variables have been identified, two multivariable models (one for stunting and the other for thinness) with all candidate variables were fitted.27 Absence of multicollinearity was determined using variance inflation factor index and model goodness-of-fit assessed using Hosmer-Lemeshow statistic.
Results
Basic Characteristics of the Study Participants
In the five health facilities, 268 adolescents were on ART follow up during the study period. Among them, eight were excluded because they were either having their first ART visit (n=3) or did not come to the health facilities during the entire study period (n=5). Accordingly, the study included the remaining 260 adolescents comprising 127 boys and 133 girls. Their mean (±SD) age of the adolescents was 13.9 (±2.5) years and 55.8% were below 15 years of age. All were in-school adolescents, more than three-fourth (77.7%) were primary school students and the remaining 22.3% secondary school students. Nearly half (52.7%) were living with their biological parents.
Regarding their medical profile, the mean (±SD) duration of ART treatment was 5.7 (± 3.1) years and ranged from 1 month to 14 years. Half of the adolescents have been on ART for at least five years. About a quarter (23.3%) had low CD4 count (≤500 cells/µL) at the time of the survey and 19.6% had history of opportunistic infections. Among them, the most commonly encountered opportunistic infections were pneumocystis pneumonia (33.3%), tuberculosis (27.5%), chronic diarrhea (15.7%), herpes zoster (13.7%) and candidiasis (7.8%). According to the WHO clinical staging of HIV, the majority (78.8%) were at the first clinical stage of the illness.28 At the time of the survey, 5.8% were receiving tuberculosis treatment and 73.8% were on INH therapy (Table 1).
 |
Table 1 Basic Characteristics of Adolescents Living with HIV on ART in Hawassa City, Southern Ethiopia, 2018 |
Household Food Security, Dietary Diversity and Access to Nutrition Counseling
Based on the HFIAS, 38.5% of the adolescents were from food insecure households. Analysis of food groups consumed by the adolescents in the preceding day of the survey suggested that the mean (±SD) dietary diversity score was 6.8 (±2.1) and in the reference period more than three-fourths have taken foods made with oils, fat or butter (98%), condiments (88%), cereals (86%), white roots and tubers (76%) and sweets (75%). Conversely, consumption of nutrient-dense foods including animal source foods and fruits and vegetables was relatively lower. In aggregate, 28.1% of the adolescents had low or medium dietary diversity score.
Nearly one-third (30.0%) of the adolescents received nutrition counseling in the last one month. Further, in the preceding one year, 30.8% got social support (food aid or financial supports) from different entities. Assessment of dietary habit indicated, 30.4% skipped their meal at least once in preceding two weeks. Further, only 4.2% were regularly involved in intentional physical activity. Small proportion of adolescents considered themselves as regular users of alcohols (1.2%) and tobacco (0.8%) (Table 2).
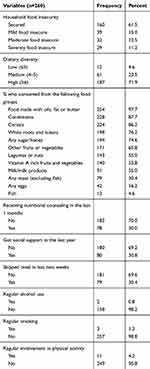 |
Table 2 Dietary Pattern of Adolescents Living with HIV Receiving ART, Hawassa, Southern Ethiopia, 2018 |
Prevalence and Predictors of Stunting and Thinness
The mean (±SD) HFA z-score of the adolescents was −1.5 (±1.0) and 33.1% were stunted; of them, 7.7% were severely stunted. The prevalence of stunting was 30.3% and 36.5% in adolescents 10–14 and 15–19 years, respectively. The prevalence was comparable between boys (32.3%) and girls (33.9%).
Similarly, the mean BMI-for-age z-score was −1.02 (±1.3) and 20% were thin; among them, 7.3% were severely thin. The prevalence of thinness among adolescents 10–14 and 15–19 years was 23.4% and 15.7%, respectively. Gender-wise, magnitude thinness was more or less the same among boys (19.7%) and girls (20.3%). Only 5 (1.9%) of the adolescents had BMI-for-age z-score >2 indicative of overweight or obesity.
Regarding predictors of stunting, in the bivariable analyses, type of primary caregiver, history opportunistic infection, use of INH prophylaxis, receiving social support, skipping meal, access to nutritional counseling and HIV status disclosure fulfilled the candidacy criteria (p<0.25) for the multivariable analysis. In multivariable model, significant predictors of stunting were: lack of social support (AOR=2.71; 95% CI: 1.36, 5.39); meal skipping (AOR=2.13; 95% CI: 1.16, 3.91); recent history of opportunistic infections (AOR=2.25; 95% CI: 1.11, 4.55) and disclosure of HIV status (AOR=1.88; 95% CI: 1.12–4.34) (Table 3).
 |
Table 3 Predictors of Stunting Among Adolescents Receiving ART in Hawassa City, Southern Ethiopia, 2018 |
Regarding predictors of thinness, age, religion, type of primary caregiver, WHO clinical stage and history of opportunistic infections fulfilled the candidacy criteria and entered into the multivariable model. In the ultimate model, history of recent opportunistic infection was the only significant predictor of thinness (AOR=3.21; 95% CI: 1.54, 6.73) (Table 4).
 |
Table 4 Predictors of Thinness Among Adolescents Receiving ART at Governmental Health Facilities, Hawassa City, Southern Ethiopia, 2018 |
Discussion
In this study, 33.1% of HIV-positive adolescents were stunted and 7.7% were severely stunted. A study among HIV-positive adolescents in Addis Ababa also came up with comparable prevalence figures of 37.4% stunting and 8.2% severe stunting.25 Likewise, in Uganda, stunting among adolescents enrolled into an HIV-care program was 36%.23 A multicounty study on adolescents with perinatally acquired HIV found an aggregate 37% prevalence of stunting.24 The findings suggest that stunting is extremely common and may affect more than one-third of the adolescents living with HIV.
Though we have not compared the prevalence of stunting between HIV-positive and -negative adolescents, studies conducted among HIV-negative adolescents in Ethiopia reported much lower prevalence figures.29 A recent meta-analysis that included 22 surveys conducted among HIV-negative adolescents in Ethiopia found 20.7% pooled prevalence of stunting.29 Further, studies from urban areas reported stunting prevalence below 10%.30,31 This may suggest that the burden of stunting among HIV-positive adolescents is substantially higher than the general HIV-negative adolescents in Ethiopia.
In the current study, the prevalence of thinness was 20% with 7.3% severely thin. In 2016, in Addis Ababa, the corresponding figure was marginally lower – 15.6% thinness and 2.9% severe thinness.25 In Uganda, 18% and 8% of HIV-positive adolescents had thinness and severe thinness.23 A study in South-West Nigeria among ART receiving orphaned children 6–18 years of age, thinness was even more common (27%).32 The high prevalence of thinness among HIV-positive subjects is concerning because several studies have documented that underweight status is an important predictor of treatment failure and mortality.15–17 A retrospective study in Southern Ethiopia among HIV-positive children younger than 18 years found that underweight children were four times more likely to end up in treatment failure.33
HIV-positive individuals should consume nutrient-rich diversified foods because macro- and micronutrient deficiencies may accelerate HIV progression.34 In the current study, 28.1% of the adolescents had low or medium dietary diversity and their diets were dominated by nutrient-poor staples or empty calorie foods. A systematic review that included studies from low-income countries concluded that the intake of adolescents was limited in diversity, mainly comprising plant-based staple foods poor in nutrient-rich fruits and vegetables.35 This finding may suggest that nutrition interventions among adolescents living with HIV need to work towards promoting and advancing access to nutrient-dense foods.
We found that meal skipping was common among HIV-positive adolescents and the practice was a significant predictor of stunting. Meal skipping can occur in adolescents for multiple reasons including household food insecurity, lack of understanding of the harms of the practice and wrongly considering it as a strategy for weight loss. Parallel to our findings, a study in Addis Ababa concluded that meal skipping raised the risk of stunting in HIV-positive adolescents by almost two-folds.22 A study in Nigeria among healthy adolescents reported that 10% of adolescents regularly skipped meal and such adolescents were three times more likely to be stunted.36 A study among HIV-positive Tanzanian children younger than 15 years of age also identified lower feeding frequency as an important predictor of stunting.37 This can be due to skipping of meals leading to inadequate dietary intake. On the other hand, the observed association can also be the indirect reflection of the underlying food insecurity in the population.
The study also witnessed a significant association between stunting and social support status. Regular food or financial support may improve consumption of essential macro and micronutrients to improve linear growth. Further food and nutrition assistance may increase adherence and uptake of HIV care38 and contribute to better treatment outcome and nutrition.
We observed that adolescents who knew their HIV status had almost two times increased odds of stunting. To the best of our knowledge, no study has investigated the impact of HIV status disclosure on the nutritional status of children and adolescents. However, a study conducted in Kenya suggested, adolescents knowing their status were more likely to develop anger, hopelessness, depression and have lower adherence to ART.39 Such psychological factors may affect appetite and dietary intake and lead to undernutrition. The finding implies that disclosure of HIV status to adolescents need to be carefully planned and has to be adapted according to their age. We recommend future studies to investigate the mechanisms by which disclosure affects nutritional status of children living with HIV.
We also found that the presence of at least one opportunistic infection was significantly associated with two times increased odds of stunting and three times increased odds of thinness. Multiple studies have so far identified opportunist infections as important predictors of nutritional status of HIV-positive children.37,40 The study conducted in Northwest Ethiopia found that diarrhea, oral ulcer and other co-morbidities were significant predictors of nutritional status of children 2–15 years living with HIV.40 The observed association is likely to be the reflection of the vicious relationship between opportunistic infections and undernutrition.
The findings of the study should be interpreted in consideration of its multiple limitations. Given the study was cross-sectional, it was not possible to prove causation and in some instances, reverse causations cannot be excluded. This study did not assess some important predictors of nutritional status including adherence to ART and actual intake of nutrients. Further, we could not separately present the prevalence of malnutrition according to the mode of HIV transmission (perinatally or sexually acquired) as such information was not collected in the study. There might be also a recall bias while assessing some independent variables including occurrence of opportunistic infection, household food insecurity and dietary diversity retrospectively based on cross-sectional data. The fact that substance use was assessed in the presence of caregivers could have caused reporting bias and underestimated the extent of the problem.
Furthermore, as the study did not compare the magnitude of undernutrition among HIV-positive and -negative adolescents, it was not possible to compare the burden between the two groups. As study subjects presented at health facilities during the study period were included in the study, the generalizability of the findings to all HIV-positive adolescents in the city could theoretically be affected. Finally, due to sample size concern, we could not be able to do stratified analysis based on age and sex. Regardless of the above limitations, the study adds up to the existing body of literature on the nutritional status of adolescents living with HIV in low-income settings.
Conclusion
We assessed the magnitude and predictors of stunting and thinness among adolescents receiving ART in Southern Ethiopia and found that one-third were stunted and one-fifth had low BMI-for-age suggestive of thinness. After controlling for potential confounders, lack social support, meal skipping, history of opportunistic infection and HIV-disclosure status turned-out as significant predictors of undernutrition among HIV-positive adolescents.
Prevention and timely treatment of opportunistic infections, promoting social support and discouraging practice of meal skipping may help to reduce the burden of undernutrition. Nutrition counseling provided to adolescents living with HIV should target at discouraging the practice of meal skipping. The relationship between HIV status disclosure and nutritional status of adolescents should be explored further through longitudinal studies.
Abbreviations
AIDS, acquired immunodeficiency syndrome; AOR, adjusted odds ratio; ART, anti-retroviral therapy; BMI, body mass index; CD4, cluster of differentiation 4; CI, confidence interval; COR, crude odds ratio; FAO, Food and Agriculture Organization of the United Nations; HFA, height-for-age; HFIAS, Household Food Insecurity Access Scale; HIV, human immunodeficiency virus; INH, isoniazid; SD, standard deviation; WHO, World Health Organization.
Data Sharing Statement
The analyzed dataset is available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was conducted in accordance with the Declaration of Helsinki. The protocol was cleared by the Institutional Review Board of College of Medicine and Health Sciences, Hawassa University. Permission was secured from all health institutions involved in the study. Data were collected after taking informed written consent from the caretakers of the adolescents. Assent was also sought from the adolescents. In HIV-positive adolescents who were not aware of their HIV status, data were primarily collected from the caregivers.
Consent for Publication
NA
Acknowledgment
We acknowledge Mr. Demelash Mulualem from Hawassa University, Ethiopia for reviewing the study proposal and for providing technical support at different stages of the research.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors do not have any potential, perceived, or real financial or non-financial conflict of interest in this study.
References
1. UNICEF. Adolescent HIV prevention. 2019. Available from: https://data.unicef.org/topic/hivaids/adolescents-young-people/.
2. Kharsany AB, Karim QA. HIV infection and AIDS in Sub-Saharan Africa: current status, challenges and opportunities. Open AIDS J. 2016;10:34–48. doi:10.2174/1874613601610010034
3. UNAIDS. Country factsheet Ethiopia: HIV and AIDS estimates. 2018. Available from: https://www.unaids.org/en/regionscountries/countries/ethiopia.
4. UNICEF. For Every Child AIDS: Seventh Stocktaking Report. New York: UNICEF; 2016.
5. World Health Organization. Programming for Adolescent Health and Development. Geneva: WHO; 1999.
6. UNICEF. Monitoring the situation of children and women. 2019. Available from: https://data.unicef.org/topic/adolescents/demographics/.
7. Christian P, Smith ER. Adolescent undernutrition: global burden, physiology, and nutritional risks. Ann Nutr Metab. 2018;72(4):316–328. doi:10.1159/000488865
8. World Health Organization. Nutrient Requirements for People Living with HIV/AIDS: Report of a Technical Consultation. Geneva: WHO; 2003.
9. Food and Nutrition Technical Assistance. HIV/AIDS: A Guide for Nutrition, Care and Support. Washington DC: Academy for Educational Development; 2001.
10. Arpadi SM. Growth Failure in HIV-Infected Children Infected Children: Consultation on Nutrition and HIV/AIDS in Africa: Evidence, Lessons and Recommendations for Action. Durban: World Health Organization; 2005.
11. Jamshidi Y, Gibson P, Ray KK. Undernutrition in adolescence and risk of cardiovascular disease. Eur Heart J. 2012;33(4):433–435. doi:10.1093/eurheartj/ehr270
12. Bundy DA, de Silva N, Horton S, Jamison DT, Patton GC. Optimizing Education Outcomes: High-Return Investments in School Health for Increased Participation and Learning. Washington, DC: World Bank; 2018.
13. Largo RH. Catch-up growth during adolescence. Horm Res. 1993;39(3):S41–8. doi:10.1159/000182783
14. Martorell R, Khan LK, Schroeder DG. Reversibility of stunting: epidemiological findings in children from developing countries. Eur J Clin Nutr. 1994;48:S45–57.
15. Hussen S, Belachew T, Hussien N. Nutritional status and its effect on treatment outcome among HIV infected clients receiving HAART in Ethiopia: a cohort study. AIDS Res Ther. 2016;13:32.
16. Ebissa G, Deyessa N, Biadgilign S. Predictors of early mortality in a cohort of HIV-infected children receiving high active antiretroviral treatment in public hospitals in Ethiopia. AIDS Care. 2015;27(6):723–730. doi:10.1080/09540121.2014.997180
17. Gebremedhin A, Gebremariam S, Haile F, Weldearegawi B, Decotelli C. Predictors of mortality among HIV infected children on anti-retroviral therapy in Mekelle Hospital, Northern Ethiopia: a retrospective cohort study. BMC Public Health. 2013;13:1047. doi:10.1186/1471-2458-13-1047
18. Mwiru RS, Spiegelman D, Duggan C, et al. Nutritional status and other baseline predictors of mortality among HIV-infected children initiating antiretroviral therapy in Tanzania. J Int Assoc Provid AIDS Care. 2015;14(2):172–179. doi:10.1177/2325957413500852
19. Swetha GK, Hemalatha R, Prasad UV, Murali V, Damayanti K, Bhaskar V. Health & nutritional status of HIV infected children in Hyderabad, India. Indian J Med Res. 2015;141(1):46–54. doi:10.4103/0971-5916.154494
20. Braitstein P, Ayaya S, Nyandiko WM, et al. Nutritional status of orphaned and separated children and adolescents living in community and institutional environments in Uasin Gishu county, Kenya. PLoS One. 2013;8(7):e70054. doi:10.1371/journal.pone.0070054
21. Jesson J, Masson D, Adonon A, et al. Prevalence of malnutrition among HIV-infected children in Central and West-African HIV-care programmes supported by the growing up programme in 2011: a cross-sectional study. BMC Infect Dis. 2015;15:216. doi:10.1186/s12879-015-0952-6
22. Birra A Assessment of nutritional status of adolescents living with HIV receiving care at public hospitals in Addis Ababa, Ethiopia. Avaiable from: http://etd.aau.edu.et/handle/123456789/7304.
23. Lwanga F, Wanyenze RK, Matovu JK, Chimulwa T, Orach CG. Nutritional status of HIV-infected adolescents enrolled into an HIV-care program in urban and rural Uganda: a cross-sectional study. World J Nutr Health. 2015;3(2):35–40.
24. Jesson J, Schomaker M, Malasteste K, et al. Stunting and growth velocity of adolescents with perinatally acquired HIV: differential evolution for males and females. A multiregional analysis from the IeDEA global paediatric collaboration. J Int AIDS Soc. 2019;22(11):e25412. doi:10.1002/jia2.25412
25. Food and Agriculture Organization of the United Nations (FAO). Guidelines for Measuring Household and Individual Dietary Diversity. Rome: FAO; 2007.
26. Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access: Indicator Guide. Washington, DC: Food and Nutrition Technical Assistance Project, Academy for Educational Development; 2007.
27. Hosmer DW, Lemeshow S. Applied Logistic Regression.
28. World Health Organization. WHO Clinical Staging of HIV Disease in Adults, Adolescents and Children. Geneva: WHO; 2007.
29. Berhe K, Kidanemariam A, Gebremariam G, Gebremariam A. Prevalence and associated factors of adolescent undernutrition in Ethiopia: a systematic review and meta-analysis. BMC Nutr. 2019;5:49. doi:10.1186/s40795-019-0309-4
30. Gebreyohannes Y, Shiferaw S, Demtsu B, Bugssa G. Nutritional status of adolescents in selected government and private secondary schools of Addis Ababa, Ethiopia. Int J Food Sci Nutr. 2014;3(6):504–514. doi:10.11648/j.ijnfs.20140306.13
31. Teferi DY, Atomssa GE, Mekonnen TC. Overweight and undernutrition in the cases of school-going adolescents in Wolaita Sodo town, Southern Ethiopia: cross-sectional study. Nutr Metab. 2018;2018:1–10. doi:10.1155/2018/8678561
32. Fagbamigbe AF, Adebowale AS, Ajayi IO. An assessment of the nutritional status of ART receiving HIV-orphaned and vulnerable children in South-West Nigeria. Heliyon. 2019;5(12):e02925. doi:10.1016/j.heliyon.2019.e02925
33. Sidemo NB, Hebo SH. Nutritional status and its effect on treatment outcome among HIV-infected children receiving first-line antiretroviral therapy in Arba Minch General Hospital and Arba Minch Health Center, Gamo Zone, Southern Ethiopia: retrospective Cohort Study. IntechOpen. 2019. doi:10.5772/intechopen.85851
34. Musakwa N, Feeley A, Magwete M, et al. Dietary intake among paediatric HIV-positive patients initiating antiretroviral therapy in Johannesburg, South Africa. Vulnerable Child Youth Stud. 2019. doi:10.1080/17450128.2019.1668581
35. Ochola S, Masibo PK. Dietary intake of schoolchildren and adolescents in developing countries. Ann Nutr Metab. 2014;64(suppl 2):S24–S40. doi:10.1159/000365125
36. Otuneye AT, Ahmed PA, Abdulkarim AA, Aluko OO, Shatima DR. Relationship between dietary habits and nutritional status among adolescents in Abuja municipal area council of Nigeria. Niger J Paediatr. 2017;44(3):128–135. doi:10.4314/njp.v44i3.1
37. Sunguya BF, Poudel KC, Mlunde LB, Urassa DP, Yasuoka J, Jimba M. Poor nutrition status and associated feeding practices among HIV-positive children in a food secure region in Tanzania: a call for tailored nutrition training. PLoS One. 2014;9(5):e98308. doi:10.1371/journal.pone.0098308
38. UNAIDS. Food and nutrition. Geneva; 2014. Available from: https://www.unaids.org/sites/default/files/media_asset/food-nutrition_en.pdf.
39. Vreeman RC, Scanlon ML, Mwangi A, et al. A cross-sectional study of disclosure of HIV status to children and adolescents in western Kenya. PLoS One. 2014;9:e86616. doi:10.1371/journal.pone.0086616
40. Sewale Y, Hailu G, Sintayehu M, Moges NA, Alebel A. Magnitude of malnutrition and associated factors among HIV infected children attending HIV-care in three public hospitals in East and West Gojjam Zones, Amhara, Northwest, Ethiopia, 2017: a cross-sectional study. BMC Res Notes. 2018;11:788. doi:10.1186/s13104-018-3882-8
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
