Back to Journals » Risk Management and Healthcare Policy » Volume 13
Unattended Pregnancies and Perinatal Mortality in Georgia
Authors Manjavidze T , Rylander C , Skjeldestad FE, Kazakhashvili N , Anda EE
Received 20 December 2019
Accepted for publication 7 March 2020
Published 15 April 2020 Volume 2020:13 Pages 313—321
DOI https://doi.org/10.2147/RMHP.S243207
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kent Rondeau
Tinatin Manjavidze,1 Charlotta Rylander,1 Finn Egil Skjeldestad,1 Nata Kazakhashvili,2 Erik Eik Anda1
1Department of Community Medicine, Faculty of Health Sciences, University of Tromsø – The Arctic University of Norway, Tromsø 9037, Norway; 2Department of Public Health, Faculty of Medicine, Ivane Javakhishvili Tbilisi State University, Tbilisi 0179, Georgia
Correspondence: Tinatin Manjavidze
35a Guramishvili Ave, Tbilisi 0178, Georgia
Tel +995 598292936
Email [email protected]
Introduction: The majority of pregnant women in Georgia attend the free-of-charge, national antenatal care (ANC) programme, but over 5% of pregnancies in the country are unattended. Moreover, Georgia has one of the highest perinatal mortality (PM) rates in Europe (11.7/1000 births).
Purpose: To assess the association between unattended pregnancies and the risk of PM.
Methods: Data were extracted from the Georgian Birth Registry (GBR) and the national vital registration system. All mothers who had singleton births and delivered in medical facilities in Georgia in 2017– 2018 were included in the study and categorised into attended pregnancies (at least one ANC visit during pregnancy) and unattended pregnancies (no ANC visits during pregnancy). After exclusions, the study sample included 101,663 women and their newborns, of which 1186 were either stillborn or died within 7 days. Logistic regression analysis was used to assess the effect of unattended pregnancies on PM.
Results: During the study period, the PM rate was 12.9/1000 births. In total, 5.6% of women had unattended pregnancies. The odds of PM among women with unattended pregnancies were more than double those among women with attended pregnancies (odds ratio=2.21, [95% confidence interval: 1.81– 2.70]). Multiparous women with higher education and who resided/delivered outside of Tbilisi were significantly less likely to experience PM.
Conclusion: The risk of PM doubled among women with unattended pregnancies. Six percent of PM cases were attributable to unattended pregnancies. Targeting women with previous unattended pregnancies will likely reduce the PM rate in Georgia.
Keywords: stillbirth, early neonatal mortality, antenatal care, birth registry
Introduction
The availability of antenatal care (ANC) and subsequent ANC attendance by pregnant women have an influence on pregnancy outcomes. ANC improves maternal and newborn survival because it reduces the risk of preterm birth and perinatal mortality (PM)1–4 through individual risk assessment and monitoring. In both high- and low-income countries, associations between lack of ANC attendance and adverse pregnancy outcomes have been demonstrated.2 Thus, it is important to identify women who never attend ANC in order to prevent severe morbidity and mortality during pregnancy or delivery.
The Auckland Stillbirth Study showed that the odds of stillbirth doubled among women who attended less than half of the recommended ANC visits.5 A study from Saudi Arabia found a 70% increased risk of intra-uterine foetal death in women who did not attend ANC,6 and a systematic literature review from low- and middle-income countries reported that lack of ANC attendance was one of the main factors associated with stillbirth.7 Additionally, a study from Bangladesh showed that women who attended ANC were 18% less likely to experience early neonatal death (END) when compared to those who did not attend ANC.8 Dowswell et al compared the effect of reduced ANC attendance and standard care among women with low-risk pregnancies, and found that women with reduced ANC attendance had a 14% increased risk of PM compared to those in the standard care group. Furthermore, in low- and middle-income countries, the PM rate was significantly higher among women who did not attend the recommended number of ANC visits.9 Previous research has suggested that lack of ANC visits also increases the risk of preterm birth by up to 30%.10 When small for gestational age (GA) newborns were not identified prior to birth, their odds of being stillborn were 9.46 times higher than those of small for GA babies that were identified during the antenatal period.5 Small for GA and preterm birth are recognised as the main contributors to PM.11,12 Although many studies have investigated the associations between recommended ANC visits and PM, very few have assessed the effect of unattended pregnancies.
Prior to 2018 in Georgia, the national ANC programme covered four ANC visits per woman, free of charge.13 On 1 February 2018, this number was increased to eight, as recommended by the World Health Organisation.14 In 2017–2018, the proportion of women attending at least four ANC visits in Georgia increased by 4.5%, thus reaching a total of 80.8% based on the aggregated data from medical facilities in the country.15 However, little is known about maternal and neonatal outcomes among women who do not attend ANC in Georgia.
The aims of this paper are to identify the characteristics of women with unattended pregnancies in Georgia, to assess the association between unattended pregnancies and the risk of PM, and to measure the burden of PM attributable to unattended pregnancies.
Methods
The Georgian Birth Registry
The Georgian Birth Registry (GBR) was established in 2016 as a digital, medical birth registry with national coverage. Doctors or other qualified medical personnel record all pregnancies, related ANC visits, and maternal health conditions arising before, during, and after pregnancy. Moreover, all ANC centres, including those without maternity wards (n=350), are obligated by law to register any ANC visit (state financed or private) in the GBR, and all stillbirths reported by the National Statistics Office of Georgia are also registered.
Study Population
For the present analysis, we extracted maternal and neonatal data (including stillbirths) for all deliveries occurring in 2017–2018. Confirmed END cases were extracted from the vital registration system (VRS), as the GBR does not register neonatal outcomes that occur after hospital discharge or during transfer to other facilities. GBR and VRS data were merged using mothers’ and newborns’ unique 11-digit personal identification number (issued at time of birth). Thirty-eight ENDs without either the mother’s or the newborn’s personal identification number were excluded from the analysis.
During the study period, there were 103,128 mothers and 104,597 newborns registered in the GBR. We excluded multiple births (n=2911) because they have a higher risk of preterm birth, complications during pregnancy and PM than singletons. Biologically implausible values and outliers: parity (>15; n=13); age (>53 years; n=8), and newborns with a GA of >43 weeks (n=2). The final study sample comprised 101,663 mothers and newborns. Among those, we identified 1186 PM cases (658 from 2017 and 528 from 2018) (Figure 1).
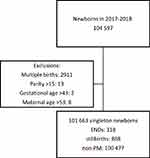 |
Figure 1 Flow chart of the study sample. |
Included Variables
Information on ANC attendance in the GBR was used to categorise mothers into two groups: attended pregnancies (women who attended at least one ANC visit during pregnancy) and unattended pregnancies (women who did not attend any ANC visits during pregnancy). We also included maternal age (≤19, 20–24, 25–29, 30–34, 35–39, ≥40 years), parity (primiparous, multiparous), and education (primary, secondary, and higher). The variable “region of residence and delivery” was combined: resided and delivered in Tbilisi (capital); resided in Tbilisi and delivered outside Tbilisi; resided outside Tbilisi and delivered in Tbilisi; and resided and delivered outside Tbilisi.
Statistical Analysis
Descriptive statistics are presented as means and standard deviations for continuous variables and percentages for categorical variables. We used logistic regression analysis to assess the effect of ANC attendance (attended and unattended pregnancies) on PM. To determine which covariates to include in the regression model, we drew a directed acyclic graph (DAG), including ANC, PM, GA, and the following maternal factors: morbidity, age, parity, education, region of residence and delivery, nationality, marital status, and year of delivery (Figure 2). The DAG assumed a causal effect of ANC on PM, as indicated by the direct arrow from ANC to PM.16–18
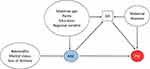 |
Figure 2 Directed acyclic graph presenting causal associations between perinatal mortality (PM), antenatal care (ANC) attendance, and potential confounders. GA: gestational age. |
We assumed that ANC attendance affected GA. Indeed, if a woman has an unattended pregnancy, the risk of early delivery due to medical conditions cannot be recognised, and thus cannot be avoided. If a woman has an attended pregnancy, and for some reason the doctor plans to perform a caesarean section at a particular date, this also affects GA. We further assumed that the maternal factors age, parity, education, and region of residence and delivery affected ANC attendance and increased the risk of PM through GA. Previous research has also highlighted the importance of these variables in ANC attendance.1,8,19,20 Thus, these variables can be considered confounders in the causal pathway between ANC attendance and PM. Maternal morbidity increases the risk of PM and affects GA; however there is no direct effect of maternal morbidity on ANC attendance, or vice-versa. The maternal factors nationality, marital status, and year of delivery have an effect on ANC, but they have no direct effect on PM. As there are three arrows pointing at GA, it becomes a collider; as conditioning on a collider introduces bias,17,21 we did not adjust for GA in our regression model.18,21,22 Thus, based on the DAG, the regression model was adjusted for the following maternal factors: age, parity, education, and region of residence and delivery, to properly assess the effect of ANC attendance on PM. Other studies adjusted for similar variables, with some modifications.1,5,6,8
To estimate the burden of PM attributable to unattended pregnancy, we calculated the population attributable fraction (PAF) using the PUNAFCC Stata package, under the assumption that there is a causal effect of ANC attendance on PM. PAF is defined as the fraction of all cases of a disease or condition in a population which is attributable to the exposure.23 As the GBR contains almost every birth in Georgia and is representative of the whole population, the current study gave us the opportunity to calculate PAF. Statistical analysis was performed using the statistical package STATA (StataCorp, College Station, TX, USA) version 15.0.
Results
The birth rate was 13.7 (95% confidence interval [CI] 13.6–13.8) per 1000 population and the PM rate was 12.9 (95% CI 12.2–13.6) per 1000 births. Stillborn and END rates were 9 (95% CI 8.4–9.6) per 1000 births and 3.9 (95% CI 3.6–4.3) per 1000 livebirths, respectively. Thus, the ratio of stillbirths to ENDs was 2.3. In our study, the proportion of preterm newborns was 8.6%. In total, 5.6% of women had unattended pregnancies. Figure 3 displays the PM rate by GA for attended and unattended pregnancies.
The PM rate among women with attended pregnancies in our study sample was 10.7 per 1000 births (95% CI 10.8–12.1), whereas the PM rate among those with unattended pregnancies was 28.7 per 1000 births (95% CI 25.9–34.7) (Table 1). Women who experienced PM were older, less educated, and resided outside Tbilisi but delivered in Tbilisi compared to women who did not experience PM. The mean birthweight and GA of PM cases were lower than those of non-PM cases (Table 2).
 |
Table 1 Incidence of Early Neonatal Death (END), Stillbirth (SB), and Perinatal Mortality (PM) by Antenatal Care Attendance (Attended and Unattended Pregnanciesa). |
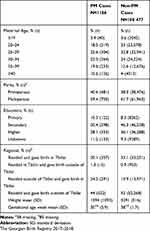 |
Table 2 Maternal and Neonatal Characteristics by Singleton Perinatal Mortality (PM) Cases. |
Most women with unattended pregnancies were 25–29 years old (29%), multiparous (69%), had secondary education (44%), and resided and delivered outside of Tbilisi (52%). Compared to women with attended pregnancies, a higher proportion of women with unattended pregnancies were aged <19 or >35 years and multiparous, whereas the other characteristics were comparable between the two groups.
The mean birthweight (3154 g) and mean GA (38+1 weeks) was lower among women with unattended pregnancies compared to those with attended pregnancies (birthweight: 3278 g, GA: 38+4) (Table 3). Additionally, women from Armenia and Azerbaijan were less likely to seek ANC than Georgian women: 6% of Armenians and 11% of Azerbaijanis had unattended pregnancies, compared to 3.7% of Georgian women. There was a disparity in ANC attendance across regions, with women residing in the regions of Kakheti, Samegrelo and Zemo Svaneti, Mtskheta-Mtianeti, and Abkhazia having a higher than average rate of unattended pregnancies (Figure 4).
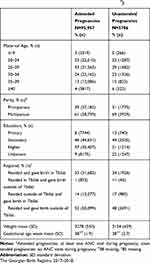 |
Table 3 Maternal and Neonatal Characteristics by ANC Attendance (Attended and Unattended Pregnanciesa). |
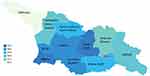 |
Figure 4 Map of Georgia – maternal residential regions by antenatal care attendance rates. |
After adjustments for maternal age, parity, education, and region of residence and delivery, women with unattended pregnancies had more than two times higher odds of experiencing PM, compared to women with attended pregnancies (odds ratio [OR]=2.21, [95% CI 1.81–2.70]). Increased maternal age was strongly associated with PM, with women aged ≥40 years had more than three-fold higher odds of experiencing PM (OR=3.50, [95% CI 2.78–4.42]) compared to women aged 25–29 years. Primiparous women were 43% more likely to experience PM, compared to multiparous women (OR=1.43, [95% CI 1.25–1.63]). Maternal education was inversely associated with PM (higher vs secondary, OR=0.56, [95% CI 0.48–0.65]). Women who resided outside of the capital, Tbilisi, but delivered in Tbilisi had 93% higher odds of experiencing PM compared to women who resided and delivered in Tbilisi (OR=1.93, [95% CI 1.63–2.29]) (Table 4). If women with unattended pregnancies had attended at least one ANC visit, 5.9% (4.9–6.9%) of PM cases could have been avoided, which translates into 71 singleton PM cases in 2017–2018.
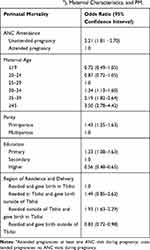 |
Table 4 Odds Ratios and 95% Confidence Intervals for the Association Between Antenatal Care (ANC) Attendance (Attended and Unattended Pregnanciesa), Maternal Characteristics, and PM. |
Discussion
In this register-based study of 101,663 women from Georgia who delivered singleton newborns, we found that women with unattended pregnancies (ie, who did not attend any ANC visits), had more than two times higher odds of experiencing PM when compared to those with an attended pregnancy (ie, those who attended at least one ANC visit). Older maternal age, primiparity, primary education, and residing outside and delivering in Tbilisi increased the odds of PM, whereas higher education, multiparity, and residing and delivering outside of Tbilisi were associated with reduced odds of PM. Assuming a causal effect of ANC non-attendance on PM, we estimated that almost 6% of singleton PM cases in Georgia could have been avoided if the mothers had attended at least one ANC visit.
Our results suggested that unattended pregnancy increases the odds of PM, which is in line with prior studies that have demonstrated the importance of ANC with regard to PM.9,24,25 Earlier research showed that missing attendance or lack of ANC had a strong impact on the risk of stillbirth5–7 and END.8 Moreover, lack of ANC was strongly associated with the risk of preterm birth and small for GA newborns,5,10 both of which are main contributors to PM.11,12 The coverage of at least one ANC visit differed by region of residence, which might be partially explained by the geographical distribution of maternity hospitals and ANC centres in the country. Based on the perinatal regionalisation programme, all level three hospitals, which provide the highest level of care and have neonatal intensive care units, are located in the regions of Tbilisi, Kvemo Kartli, Imereti, Adjara, and Kakheti. Moreover, the majority of all hospitals are situated in Tbilisi, Imereti, Adjara, and Kvemo Kartli. However, all other regions have a minimum of two hospitals, and some have more depending on the population size and the number of births. In this study, we showed that as many as 71 singleton PM cases could have been avoided during the 2-year study period if all women with singleton pregnancies attended ANC at least once. Thus, targeted efforts to increase ANC attendance among non-attending women could potentially save lives. Multiparous women from Azerbaijan or Armenia, women living and delivering outside of larger cities, and those with secondary education should be the primary audience for such interventions. This study should also trigger future research to identify the reasons why women did not seek ANC.
In line with other studies,26–28 the odds of PM increased with increasing maternal age, whereas higher education was negatively associated with PM. Moreover, primiparous women had higher odds of experiencing PM than multiparous women. In accordance with the present results, a meta-analysis of selected maternal and foetal factors for PM demonstrated an increased risk of PM among primiparous women; however, several other studies did not find a statistically significant association between parity and PM.29 Additionally, women residing outside Tbilisi (the most populated city), but who gave birth in Tbilisi, had 93% higher odds of experiencing PM. This is reasonable, as many of these deliveries may have had complications that needed treatment at a level three hospital. These findings may be somewhat limited by internal migration, as people tend to move to larger cities.
The present study was designed to determine the effect of unattended pregnancies on PM, and one significant contributor to the outcome is GA at delivery. Thus, we plotted the relationship between GA and PM by ANC attendance, and the graph confirmed that the shape of the curve is comparable to that of other countries that have had systematic birth registration for many years.22,30 The graph shows a PM rate that is similar across attended and unattended pregnancies before a GA of 36 weeks. After a GA of 37 weeks, the PM rate increased among women with unattended pregnancies. It is obvious that GA-specific PM rates differ by ANC attendance, and the PM rates among women with unattended pregnancies remained higher at all GAs. This figure confirms that the decision not to adjust for GA in our study was correct.
GA-specific PM is the focus of the Euro-Peristat project, which showed a wide variety in GA patterns of stillbirth and neonatal mortality in Europe.31 In general, countries with low foetal mortality have a higher prevalence of foetal death at earlier GAs, while countries with high foetal mortality have higher percentages at and near term.31 Georgia fits in the latter category; thus, the country’s main concern is the PM cases delivered at a GA of 37–41 weeks, which comprised 21% of all PM cases in Georgia. The slight difference in mean GA between attended and unattended pregnancies can be explained by the high number of planned caesarean sections among women with attended pregnancies.
According to a study on differences in PM and infant mortality in high-income countries, the stillbirth to livebirth ratio among all newborns at GA 37–41 weeks is 0.1 in Finland, Iceland, and the US; and 0.2 in Denmark, Norway, Sweden, and Canada.32 Our results showed that the same ratio was 0.3 in Georgia. Hence, the proportion of PM cases born at term might indirectly highlight the importance of ANC in the early identification of complications during pregnancy, and how this identification could improve perinatal outcomes33 if proper treatment is provided during pregnancy or childbirth.
This study is the first attempt to determine the effect of unattended pregnancies on PM in Georgia. The main strength of this study is its substantial size, as it included nation-wide data from the GBR. Almost all women (99.8%) that delivered in Georgia during the study period were included in our analyses, which makes our study representative of the Georgian population. Another strength of the study is that the completeness, validity, and consistency of the GBR is ensured by a different registration system: the VRS; the GBR and the VRS represent two independent reporting systems with individual-level data. The number of mothers and newborns were validated by the VRS, which ensures the high coverage of the GBR. We were also able to validate the outcome of each pregnancy by merging the data from the GBR and the VRS.
We deliberately did not adjust our analysis for GA and maternal morbidity, because the aim of the study was to identify the overall effect of unattended pregnancies on PM, and we needed to adjust for maternal age, parity, education, and region of residence and delivery to block all backdoor pathways from PM to ANC. If our assumptions regarding the direction of the relationships between the included variables are wrong, our results may be biased. However, this is highly unlikely, as others have found similar associations between unattended pregnancies and PM, and most of the research adjusted for potential confounders.5,9,24,25
We were not able to validate the main exposure – unattended pregnancies –since the only data source for both public and private ANC attendance is the GBR. Thus, if there are women who had private ANC visits that were not registered, these women would have been misclassified in our analysis as unattended pregnancies. However, since ANC clinics and maternity houses are obligated by law to register ANC information in the GBR, we consider this unlikely, and thus that the proportion of women misclassified as having unattended pregnancies is very low.
Additionally, our findings may be somewhat limited as we did not take into account the causes of PM. In general 32–43% of stillbirths are due to unexplained causes in high- and low-income settings,34 compared to 80% in Georgia. Unfortunately, we did not have the possibility to distinguish between preventable and inevitable causes of death.35 In addition to the missing causes of stillbirth, the GBR contains incomplete information on morbidity during pregnancy. However, this fact does not undermine the importance of our main finding, which clearly identifies the importance of ANC with regard to PM in Georgia and suggests the value of increasing ANC attendance among women with previous unattended pregnancies.
Conclusion
Unattended pregnancy nearly doubled the odds of PM. Advanced maternal age, primiparity, and primary education also increased the risk of PM. The PAF of unattended pregnancies on PM was almost 6%; thus, an estimated maximum of 71 singleton PM cases would have been prevented in Georgia during the 2-year study period if all pregnant women had attended at least one ANC visit.
Policy and Practice Implications
Our study has important implications for ANC program development and future research. The major contribution of the present study is the illustration of the real effects of unattended pregnancies on PM in Georgia, as it provides actual numbers based on registry data. These numbers show that targeting women with previous unattended pregnancies could lead to a lower rate of unattended pregnancies and positively contribute to PM rates. Our results clearly underline the importance of ANC in Georgia for a better pregnancy experience. Strengthening family planning services, informing reproductive-age women about the ANC programme and about services covered by the government would also improve the rate of attended pregnancies. Finally, our study revealed several uninvestigated topics, including reasons for not attending ANC and barriers to pregnancy care, which we suggest should be the subject of future studies.
Ethics and Consent Statements
The NCDC Institutional Review Board revised and approved the study protocol (IRB # 2017-010 31.03.2017). The Regional Committee for Medical and Health Research Ethics, North Norway, approved the use of the data from the GBR for research purposes (2017/404/REK Nord).
Author Contributions
T.M. coordinated data collection, performed statistical analysis, drafted the manuscript and revised it based on other authors’ comments. C.R. conceptualized and designed data collection instruments, created the theoretical framework for the analysis, and critically reviewed the manuscript. F.E.S. conceptualized and oversaw the study, and critically reviewed the manuscript. N.K. critically reviewed the manuscript. E.E.A. conceptualized, designed, and oversaw the study, and critically reviewed the manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
The project was fully funded by the Norwegian Centre for International Cooperation in Higher Education.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ntambue AM, Malonga FK, Dramaix-Wilmet M, Ngatu RN, Donnen P. Better than nothing? maternal, newborn, and child health services and perinatal mortality, Lubumbashi, democratic republic of the Congo: a cohort study. BMC Pregnancy Childbirth. 2016;16:89. doi:10.1186/s12884-016-0879-y
2. Villar J, Bergsjo P. Scientific basis for the content of routine antenatal care. I. Philosophy, recent studies, and power to eliminate or alleviate adverse maternal outcomes. Acta Obstet Gynecol Scand. 1997;76:1–14. doi:10.3109/00016349709047778
3. Pervin J, Moran A, Rahman M, et al. Association of antenatal care with facility delivery and perinatal survival - a population-based study in Bangladesh. BMC Pregnancy Childbirth. 2012;12:111. doi:10.1186/1471-2393-12-111
4. Bhutta ZA, Ali S, Cousens S, et al. Alma-Ata: rebirth and revision 6 interventions to address maternal, newborn, and child survival: what difference can integrated primary health care strategies make? Lancet. 2008;372:972–989. doi:10.1016/S0140-6736(08)61407-5
5. Stacey T, Thompson JM, Mitchell EA, Zuccollo JM, Ekeroma AJ, McCowan LM. Antenatal care, identification of suboptimal fetal growth and risk of late stillbirth: findings from the Auckland Stillbirth Study. Aust N Z J Obstet Gynaecol. 2012;52:242–247. doi:10.1111/j.1479-828X.2011.01406.x
6. Al-Kadri HM, Tamim HM. Factors contributing to intra-uterine fetal death. Arch Gynecol Obstet. 2012;286:1109–1116. doi:10.1007/s00404-012-2426-z
7. Aminu M, Unkels R, Mdegela M, Utz B, Adaji S, van den Broek N. Causes of and factors associated with stillbirth in low- and middle-income countries: a systematic literature review. BJOG. 2014;121 Suppl 4:141–153. doi:10.1111/1471-0528.12995
8. Roy S, Haque MA. Effect of antenatal care and social well-being on early neonatal mortality in Bangladesh. BMC Pregnancy Childbirth. 2018;18:485. doi:10.1186/s12884-018-2129-y
9. Dowswell T, Carroli G, Duley L, et al. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst Rev. 2010:CD000934. doi:10.1002/14651858.CD000934.pub2.
10. Heaman MI, Newburn-Cook CV, Green CG, Elliott LJ, Helewa ME. Inadequate prenatal care and its association with adverse pregnancy outcomes: a comparison of indices. BMC Pregnancy Childbirth. 2008;8:15. doi:10.1186/1471-2393-8-15
11. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi:10.1016/S0140-6736(08)60074-4
12. Nardozza LM, Araujo Junior E, Barbosa MM, Caetano AC, Lee DJ, Moron AF. Fetal growth restriction: current knowledge to the general Obs/Gyn. Arch Gynecol Obstet. 2012;286:1–13. doi:10.1007/s00404-012-2330-6
13. Georgia Go. Ordinance of the Government of Georgia - the State Programmes of Healthcare in Georgia in 2018. In Georgian; 2017.
14. WHO. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience; 2016.
15. Gamkrelidze AK, Gambashidze M, Kandelaki K, et al. Health Care Statistical Yearbook. Georgia: NCDC; 2018.
16. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. doi:10.1097/00001648-199901000-00008
17. Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi:10.1097/01.ede.0000135174.63482.43
18. Robins JM. Data, design, and background knowledge in etiologic inference. Epidemiology. 2001;12:313–320. doi:10.1097/00001648-200105000-00011
19. Hijazi HH, Alyahya MS, Sindiani AM, Saqan RS, Okour AM. Determinants of antenatal care attendance among women residing in highly disadvantaged communities in northern Jordan: a cross-sectional study. Reprod Health. 2018;15:106. doi:10.1186/s12978-018-0542-3
20. Low P, Paterson J, Wouldes T, Carter S, Williams M, Percival T. Factors affecting antenatal care attendance by mothers of Pacific infants living in New Zealand. N Z Med J. 2005;118:U1489.
21. Elwert F, Winship C. Endogenous selection bias: the problem of conditioning on a collider variable. Annu Rev Sociol. 2014;40:31–53. doi:10.1146/annurev-soc-071913-043455
22. Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol. 2011;174:1062–1068. doi:10.1093/aje/kwr230
23. Mansournia MA, Altman DG. Population attributable fraction. BMJ. 2018;360:k757. doi:10.1136/bmj.k757
24. Darmstadt GL, Bhutta ZA, Cousens S, et al. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. 2005;365:977–988. doi:10.1016/S0140-6736(05)71088-6
25. Raatikainen K, Heiskanen N, Heinonen S. Under-attending free antenatal care is associated with adverse pregnancy outcomes. BMC Public Health. 2007;7:268. doi:10.1186/1471-2458-7-268
26. Laopaiboon M, Lumbiganon P, Intarut N, et al. Advanced maternal age and pregnancy outcomes: a multicountry assessment. BJOG. 2014;121(Suppl 1):49–56. doi:10.1111/1471-0528.12659
27. Usynina AA, Grjibovski AM, Krettek A, Odland JO, Kudryavtsev AV, Anda EE. Risk factors for perinatal mortality in Murmansk County, Russia: a registry-based study. Glob Health Action. 2017;10:1270536. doi:10.1080/16549716.2017.1270536
28. Wang Y, Tanbo T, Abyholm T, Henriksen T. The impact of advanced maternal age and parity on obstetric and perinatal outcomes in singleton gestations. Arch Gynecol Obstet. 2011;284:31–37. doi:10.1007/s00404-010-1587-x
29. Berhan Y, Berhan A. A meta-analysis of selected maternal and fetal factors for perinatal mortality. Ethiop J Health Sci. 2014;24 Suppl:55–68. doi:10.4314/ejhs.v24i0.6s
30. Ananth CV, VanderWeele TJ. Placental abruption and perinatal mortality with preterm delivery as a mediator: disentangling direct and indirect effects. Am J Epidemiol. 2011;174:99–108. doi:10.1093/aje/kwr045
31. Mohangoo AD, Buitendijk SE, Szamotulska K, et al. Gestational age patterns of fetal and neonatal mortality in Europe: results from the Euro-Peristat project. PLoS One. 2011;6:e24727. doi:10.1371/journal.pone.0024727
32. Deb-Rinker P, Leon JA, Gilbert NL, et al. Differences in perinatal and infant mortality in high-income countries: artifacts of birth registration or evidence of true differences? BMC Pediatr. 2015;15:112. doi:10.1186/s12887-015-0430-8
33. Vogel JP, Souza JP, Mori R, et al. Maternal complications and perinatal mortality: findings of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121(Suppl 1):76–88. doi:10.1111/1471-0528.12633
34. Reinebrant HE, Leisher SH, Coory M, et al. Making stillbirths visible: a systematic review of globally reported causes of stillbirth. BJOG. 2018;125:212–224. doi:10.1111/1471-0528.14971
35. Manjavidze T, Rylander C, Skjeldestad FE, Kazakhashvili N, Anda EE. Incidence and causes of perinatal mortality in Georgia. J Epidemiol Glob Health. 2019;9:163–168. doi:10.2991/jegh.k.190818.001
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

