Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
Ultrasound-Guided Posterior Quadratus Lumborum Block for Acute Postoperative Analgesia in Adult Patients: A Meta-Analysis of Randomized Controlled Trials
Authors Lin C, Wang X, Qin C, Liu J
Received 15 November 2021
Accepted for publication 8 March 2022
Published 29 March 2022 Volume 2022:18 Pages 299—313
DOI https://doi.org/10.2147/TCRM.S349494
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Deyun Wang
Cheng Lin,1,2 Xuemei Wang,1 Chaosheng Qin,2 Jingchen Liu1
1Department of Anesthesiology, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, 530021, People’s Republic of China; 2Department of Anesthesiology, Affiliated Hospital of Guilin Medical University, Guilin, Guangxi, 541001, People’s Republic of China
Correspondence: Jingchen Liu, Department of Anesthesiology, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, 530021, People’s Republic of China, Tel +86 18107830301, Fax +86 7715356250, Email [email protected]
Objective: The quadratus lumborum block provides postoperative analgesia for patients undergoing abdominal surgery, although there are three common approaches to perform this block. The present meta-analysis investigated the effectiveness of posterior quadratus lumborum block (QLB2) after surgery.
Methods: PubMed, Embase, and the Cochrane Central Register were searched from inception to 26 August 2021 for randomized controlled trials that evaluated the analgesic efficacy of QLB2 vs control (placebo or no block). The primary outcomes were pain scores at 6 h, 12 h, and 24 h after surgery. The secondary outcomes were morphine consumption at 24 h after surgery and the postoperative complications.
Results: The present meta-analysis included 14 studies conducted with a total of 1001 patients. In comparison to control group, the QLB2 group presented significantly lower rest pain scores at 6 h (SMD − 0.59; 95% CI: − 1.05, − 0.12; p = 0.01, I2 = 84%; GRADE = moderate), 12 h (SMD: – 0.83; 95% CI: – 1.47, – 0.19; p = 0.01; I2 = 88%; GRADE = low), and 24 h (SMD: – 0.37; 95% CI: – 0.71, – 0.03; p = 0.03; I2 = 80%; GRADE = moderate) after surgery. The dynamic pain scores were significantly reduced, compared to control, in the QLB2 group at 12 h (SMD: – 0.93; 95% CI: – 1.52, – 0.33; p = 0.002; I2 = 83%; GRADE = low) and 24 h (SMD: – 0.52; 95% CI: – 0.93, – 0.11; p = 0.01; I2 = 83%; GRADE = moderate) after surgery. In addition, the QLB2 group presented reduced postoperative opioid consumption at 24 h (SMD: – 0.45; 95% CI: – 0.86, – 0.03; p = 0.03; I2 = 78%; GRADE = moderate). The subgroup analyses revealed that the analgesic benefit of QLB2 did not persist beyond 24 h when the patients were under spinal anesthesia.
Conclusion: Ultrasound-guided QLB2 could provide effective analgesia for patients under general anesthesia by decreasing the intensity of pain and opioid requirement when used within 24 h after abdominal surgery.
Keywords: quadratus lumborum block, postoperative pain, opioid, analgesia
Introduction
The analgesic effectiveness of ultrasound-guided regional anesthesia for abdominal surgeries is supported by an increasing body of evidence.1 Regional blocks prove to be valuable and feasible tools for surgical patients, particularly in cases where neuraxial analgesia techniques cannot be performed due to certain reasons, such as coagulopathy, hypovolemia, and neurological disease. Quadratus lumborum block (QLB) is a novel regional block technique that was first reported in 2007.2 In QLB, ultrasound guidance is utilized to determine the precise anatomical location of local anesthetics. Commonly, there are three approaches to QLB – lateral (termed QLB1), posterior (termed QLB2), and anterior (termed QLB3).3 Each of these approaches has a different mechanism of action, as stated in cadaveric and clinical reports.4 The effectiveness of postoperative analgesia of QLB has been confirmed in certain previous reports.5 However, owing to the high heterogeneity and limited sample size, there was a scarcity of meta-analysis to conduct subgroup analysis of each approach of QLB. Recently, several randomized controlled trials (RCTs) found the analgesia of ultrasound-guided QLB2 remained controversial.6,7 Therefore, the present meta-analysis was aimed to examine the efficacy of only the ultrasound-guided single-injection QLB2 approach in adults.
Materials and Methods
Search Strategy and Selection Criteria
The present meta-analysis was conducted by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.8 The online databases PubMed, Embase and the Cochrane Central Register were searched by two authors (CL and XMW) independently. The search was limited to RCTs on human subjects published from inception to 26 August 2021. A combination of MeSH with free-text terms was adopted as the search strategy. The search terms used were as follows: (“quadratus lumborum” [All Fields] OR (“abdominal muscles” [Mesh] AND “nerve block” [Mesh])). No language restrictions were applied during the search. In addition, the bibliographies of relevant articles were searched manually for retrieving additional studies. The objective was to search for and retrieve published reports on RCTs that investigated the effects of QLB2 compared to control (placebo or no block) in adults who had undergone abdominal surgery. The exclusion criteria were as follows: a) unavailability of full texts; b) unpublished clinical trials; c) no assessment of the analgesic outcomes; d) use of adjuvants to prolong the duration of nerve block analgesia.
Data Extraction and Quality Assessment
A standardized data collection form was developed by the authors (CL and XMW) to independently extract the following information: type of surgery, anesthesia technique, intervention and comparison, and QLB2 local anesthetic and postoperative analgesia regimen. The primary outcomes were the pain scores at 6 h, 12 h, and 24 h after surgery. The secondary outcomes were opioid consumption at 24 h after surgery and postoperative complications. Studies have demonstrated that Numerical Rating Scale may be used as a substitute for the Visual Analogue Scale.9 The pain score data were adjusted to a 0–10 score scale for analysis. In the case of the data that followed a non-standard distribution, the mean and standard deviation (SD) were approximated using the median and interquartile range as described previously by Wan et al10 and the Cochrane Collaboration.11 Next, all the included studies were evaluated for the risk of bias, independently by the authors (XMW and CSQ), using the Cochrane Collaboration’s Risk of Bias Tool. This tool included the following measurements: adequacy sequence generation, allocation of concealment, blinding throughout the study period, incomplete outcome data, selective outcome reporting, and other biases. The level of certainty was measured for the main results according to the guidelines of the Recommendation Assessment, Development, and Evaluation (GRADE) system. This system included the following measurements: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The disagreements between the two evaluating authors were resolved through discussion with a third author (CL).
Statistical Analysis
Meta-analysis techniques (Revman 5.3, The Cochrane Collaboration 2014) were employed to combine the data wherever possible. In the case of continuous data, the mean difference (MD) was calculated when the outcome in the studies was obtained using the same scale; otherwise, standardized mean difference (SMD) was calculated. Dichotomous data were summarized as the risk ratio (RR) with the associated 95% confidence interval (CI). In the case of significant heterogeneity (I2 > 50%) among the included trials, random-effects modeling was adopted to calculate the pooled effect size; otherwise, fixed effects modeling was selected. When I2 > 50%, one study was omitted sequentially to identify the potential sources of heterogeneity. A p-value of <0.05 was considered statistically significant.
Results
A total of 2065 citations were retrieved in the initial database search, from which only those trials that fulfilled the inclusion criteria were retained (Figure 1). Finally, 14 trials conducted with a total of 1001 patients were included in the present meta-analysis. Table 1 presents the characteristics of the included RCTs. All included RCTs were published between the years 2015 and 2021.
 |
Table 1 Trial Characteristics |
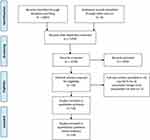 |
Figure 1 Flow diagram. |
Study Characteristics
Six studies12–17 involved patients who underwent elective cesarean section, six studies7,18–22 involved patients who underwent laparoscopic surgery (cholecystectomy, radical gastrectomy, colorectal resection, renal surgery and gynecologic surgery), and the remaining two studies6,23 involved patients who underwent open inguinal hernia repair surgery and full abdominoplasty. Seven RCTs12–17,23 were performed under spinal anesthesia, while the remaining seven RCTs6,7,18–22 employed general anesthesia. In three RCTs,12,15,16 QLB2 and control groups were compared and both groups received intrathecal morphine (ITM). The local anesthetic of QLB2 was ropivacaine in seven studies,6,7,13,16,18,21,22 bupivacaine in five studies,12,14,17,19,23 and levobupivacaine in two studies.15,20 One RCT16 included four groups and involved two comparisons between the QLB2 and control groups, with or without ITM (spinal anesthesia with 100 µg of intrathecal morphine). Each of the two comparisons was considered a separate trial in the present analysis.
Quality Assessment of the Selected Studies
According to the Cochrane Collaboration’s tool, most of the trials included in the present meta-analysis presented a low risk of bias (Figure 2). Table 2 presents the level of certainty for the main results.
 |
Table 2 The GRADE Level of Certainty for Main Outcome |
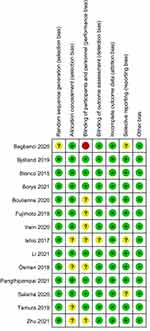 |
Figure 2 Risk-of-bias summary. Abbreviations: “+”, low risk of bias; “?”, unclear risk of bias; “-”, high risk of bias. |
Effect of Interventions
Pain Scores at 6 h, 12 h, and 24 h After Surgery
The pain scores at 6 h, 12 h, and 24 h after surgery were determined. A random effects model was adopted to calculate the pooled effects size (I2 > 50%). In comparison to control, the QLB2 group exhibited significantly lower pain scores at 6 h (SMD −0.59; 95% CI: −1.05, −0.12, p =0.01, I2 = 84%; GRADE = moderate; Figure 3A), 12 h (SMD: –0.83; 95% CI: –1.47, –0.19; p = 0.01; I2 = 88%; GRADE = low; Figure 3B), and 24 h (SMD: –0.37; 95% CI: –0.71, –0.03; p = 0.03; I2 = 80%; GRADE = moderate; Figure 3C) after the surgery and at rest. The dynamic pain scores at 6 h after surgery did not differ significantly between the two groups (SMD: –0.33; 95% CI: –0.79, 0.12; p = 0.15; I2 = 73%; GRADE = low; Figure 4A). However, the dynamic pain scores were significantly reduced in the QLB2 group at 12 h (SMD: –0.93; 95% CI: –1.52, –0.33; p = 0.002; I2 = 83%; GRADE = low; Figure 4B) and 24 h (SMD: –0.52; 95% CI: –0.93, –0.11; p = 0.01; I2 = 83%; GRADE = moderate; Figure 4C) after surgery.
 |
Figure 3 Forest plot of postoperative rest pain score. (A) Rest pain scores at 6 h after surgery. (B) Rest pain scores at 12 h after surgery. (C) Rest pain scores at 24 h after surgery. |
 |
Figure 4 Forest plot of postoperative dynamic pain score. (A) Dynamic pain scores at 6 h after surgery. (B) Dynamic pain scores at 12 h after surgery. (C) Dynamic pain scores at 24 h after surgery. |
Opioid Consumption at 6 h, 12 h, and 24 h After Surgery
The opioid consumption at 6 h (SMD: –0.43; 95% CI: –0.87, 0.01; p = 0.05; I2 = 55%; GRADE = low; Figure 5A) and 12 h (SMD: –0.13; 95% CI: –1.08, 0.82; p = 0.78; I2 = 86%; GRADE = low; Figure 5B) did not differ significantly between the two groups. The opioid consumption at 24 h after the surgery was assessed in eight trials,6,12,15,17,20–23 which included a total of 453 patients (230 patients in the QLB2 group and 223 patients in the control group). In comparison to control group, the QLB2 group presented reduced postoperative opioid consumption at 24 h (SMD: –0.45; 95% CI: –0.86, –0.03; p = 0.03; I2 = 78%; GRADE = moderate; Figure 5C).
 |
Figure 5 Forest plot of opioid consumption. (A) Opioid consumption at 6 h after surgery. (B) Opioid consumption at 12 h after surgery. (C) Opioid consumption at 24 h after surgery. |
Side-Effects
Among the included studies, six trials reported the incidence of postoperative nausea and vomiting (PONV).6,12,19,21–23 A fixed-effects model was adopted as there was no significant heterogeneity among these studies (I2 = 0%). No significant difference in PONV was observed between the two groups (RR: 0.67; 95% CI: 0.45, 1.01; p = 0.06; Figure 6). The differences in the methods used for reporting outcomes in different studies and the lack of relevant data rendered it impossible to conduct the meta-analysis of postoperative pruritus and sedation. The main adverse event associated with QLB2 was lower limb numbness or weakness. With the use of QLB2, Fujimoto et al reported five cases of lower limb numbness in 29 patients who underwent laparoscopic gynecological surgery,20 while Ökmen et al reported 2 cases of lower limb sensory loss and weakness in 30 patients who underwent laparoscopic cholecystectomy.19
 |
Figure 6 Forest plot of postoperative nausea and vomiting. |
Subgroup Analysis
The studies were divided into two subgroups –general anesthesia and spinal anesthesia. The results of the subgroup analysis revealed that the general anesthesia group presented significantly reduced rest pain scores (SMD: –0.87; 95% CI: –1.65, –0.09; p = 0.03; I2 = 88%) and dynamic pain scores (SMD: –1.50; 95% CI: –1.89, –1.10; p < 0.00001; I2 = 0%) at 24 h after the surgery compared to the spinal anesthesia group (rest pain scores: SMD: –0.08; 95% CI: –0.27, 0.01; p = 0.39; I2 = 0%; Figure 7; dynamic pain scores: SMD: –0.24; 95% CI: –0.52, 0.03; p = 0.08; I2 = 52%; Figure 8). The resting pain scores at 6 h after surgery and the opioid consumption at 24 h after surgery did not present any notable differences between the two subgroups (Table 3). The risk of PONV also did not differ significantly between the two groups (Table 3). The subgroup analysis could not be performed for the other primary outcomes as only one trial was available for analysis in each subgroup.
 |
Table 3 The Result of Subgroup Analysis |
 |
Figure 7 Forest plot of rest pain scores at 24 h after surgery by subgroup. Abbreviations: GA, general anesthesia; SA, spinal anesthesia. |
Publication Bias
According to the funnel plots (Figures 8–10), no evident publication bias was observed in the present meta-analysis.
Discussion
The main findings of the present meta-analysis, which had a moderate-to-low level of certainty, are presented ahead. In comparison to control (placebo or no block), the ultrasound-guided QLB2 group presented significant reductions in the rest pain scores at 6 h,12 h, and 24 h after surgery, dynamic pain scores at 12 h and 24 h after surgery, and opioid consumption in the first 24 h after abdominal surgery in adults. However, the subgroup analysis showed that the spinal anesthesia group did not reduce the postoperative rest and dynamic pain scores at 24 h in the presence of QLB2. The patients who were under general anesthesia could get better 24 h pain-related outcomes compared to those under spinal anesthesia. This could be attributed to the anesthetic effect of spinal anesthesia or the intrathecal opioid use, which covering up the effect of QLB2. In addition, perioperative QLB2 could not reduce the risk of PONV and presented no major complications.
Recently, several systematic reviews on QLB have indicated the analgesic efficacy of QLB, although such findings should be interpreted cautiously depending on different approaches of QLB. Two meta-analyses reported that the opioid consumption at 24 h after surgery was reduced in the QLB (using different QLB approaches) group.5,24 Nevertheless, the subgroup analyses reported by Korgvee et al5 demonstrated that QLB2 did not reduce opioid consumption at 24 h after surgery, compared with placebo, no block or other peripheral block. Their results are not consistent with those observed in the present meta-analysis. This difference could be attributed to the lack of sample size and the differences in the selection method used for control groups between the two analyses. Another reason could be related to the observation of the subgroup analysis conducted in the present study, which revealed that QLB2 did not reduce the rest pain scores and dynamic pain scores at 24 h after surgery when the patients were under spinal anesthesia.
The quadratus lumborum muscle is surrounded by thoracolumbar fascia, which encases the dorsal muscles extending from the thoracic region to the lumbar spine. It is generally accepted that local anesthetics spread to the paravertebral space through the thoracolumbar fascia to exert their analgesic effects. Moreover, it is reported that the blockade of the sympathetic fibers in the thoracolumbar fascia and visceral analgesia could contribute to the analgesic efficacy of QLB.4,17 According to the cadaveric reports, each approach of QLB has a different mechanism of action. In QLB1 or QLB2, the injected local anesthetics are reportedly confined to the thoracolumbar fascia or transversus abdominis plane (TAP). In QLB3, the injected local anesthetics may spread to the mid to lower thoracic paravertebral space and the lumbar nerve roots.25,26 The three-dimensional computed tomography images of patients have revealed that QLB1 spread in the TAP, QLB2 spread in the TAP and posterior region of the quadratus lumborum muscle, and transverse oblique paramedian QLB3 spread to the lumbar and thoracic paravertebral regions. Tamura et al27 reported that in magnetic resonance imaging, QLB2 appeared to have a wider dye spread compared to QLB1. Furthermore, QLB3 is a deep approach, which is reported to result in a greater motor blockade and the risk of needle trauma of the pleura and kidney.26,28 Consequently, in clinical practice, a higher number of people select QLB2. Since there is a lack of studies comparing the different approaches of QLB, whether the other approaches are better than QLB2 remains unclear so far. One small-sample-size study reported that the analgesic effect of QLB3 was a superior to that of QLB2 in cesarean delivery.29 However, Brixel, et al30 recently reported that 30 mL of the QLB2 solution could reach multiple locations around the quadratus lumborum muscle when sonographic localization was used. Therefore, future studies should consider investigating the effects of the different approaches of QLB using varying dosages of the local anesthetic.
Our meta-analysis has several limitations. Firstly, when sensitivity analysis was performed by omitting one study sequentially, high heterogeneity was still observed. This heterogeneity could be attributed to the different types of surgery, different types of anesthesia, and different durations of the QLB2 procedure adopted in the studies. Moreover, the differences in the study designs, the dosages and types of the local anesthetic, and the postoperative analgesia regimen could also have contributed to the increased heterogeneity. Second, as non-opioid drugs were used for postoperative pain management in certain studies, it was not possible to extract relevant data from all the included trials. Third, the success rate of sensory blocking could not be evaluated adequately as a few patients were under general anesthesia.
Conclusion
Ultrasound-guided QLB2 could provide effective analgesia for adult patients by decreasing the intensity of pain and opioid requirements within 24 h after abdominal surgery. However, when used in conjunction with spinal anesthesia, QLB2 appeared to lose its superior efficacy. Nonetheless, the use of QLB2 is recommended in the setting of general anesthesia.
Abbreviations
QL block, quadratus lumborum block; QLB2, posterior quadratus lumborum block; RCT, randomized controlled trial; SMD, standardized mean difference; CI, confidence interval; RR, relative risk; PONV, postoperative nausea and vomiting; SD, standard deviation; IQR, interquartile range; ITM, intrathecal morphine; TAP, transversus abdominis plane.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Fernandes HDS, Azevedo AS, Ferreira TC, et al. Ultrasound-guided peripheral abdominal wall blocks. Clinics. 2021;76:e2170. doi:10.6061/clinics/2021/e2170
2. Blanco R. TAP block under ultrasound guidance: the description of a ‘no pops technique. Reg Anesth Pain Med. 2007;271. doi:10.1136/rapm-00115550-200709001-00249
3. Dhanjal S, Tonder S. Quadratus lumborum block. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
4. Elsharkawy H, El-Boghdadly K, Barrington M. Quadratus lumborum block: anatomical concepts, mechanisms, and techniques. Anesthesiology. 2019;130(2):322–335. doi:10.1097/ALN.0000000000002524
5. Korgvee A, Junttila E, Koskinen H, et al. Ultrasound-guided quadratus lumborum block for postoperative analgesia: a systematic review and meta-analysis. Eur J Anaesthesiol. 2021;38(2):115–129. doi:10.1097/EJA.0000000000001368
6. Bjelland TW, Yates TGR, Fagerland MW, et al. Quadratus lumborum block for postoperative analgesia after full abdominoplasty: a randomized controlled trial. Scand J Pain. 2019;19(4):671–678. doi:10.1515/sjpain-2019-0013
7. Boulianne M, Paquet P, Veilleux R, et al. Effects of quadratus lumborum block regional anesthesia on postoperative pain after colorectal resection: a randomized controlled trial. Surg Endosc. 2020;34(9):4157–4165. doi:10.1007/s00464-019-07184-0
8. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi:10.1136/bmj.b2535
9. Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10(4):390–392. doi:10.1111/j.1553-2712.2003.tb01355.x
10. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. doi:10.1186/1471-2288-14-135
11. Higgins J, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions, version 6.0, Cochrane; 2019. Available from: http://www.training.cochrane.org/handbook.
12. Pangthipampai P, Dejarkom S, Poolsuppasit S, et al. Bilateral posterior quadratus lumborum block for pain relief after cesarean delivery: a randomized controlled trial. BMC Anesthesiol. 2021;21(1). doi:10.1186/s12871-021-01309-6
13. Borys M, Zamaro A, Horeczy B, et al. Quadratus lumborum and transversus abdominis plane blocks and their impact on acute and chronic pain in patients after cesarean section: a randomized controlled study. Int J Environ Res Public Health. 2021;18(7). doi:10.3390/ijerph18073500
14. Salama ER. Ultrasound-guided bilateral quadratus lumborum block vs. intrathecal morphine for postoperative analgesia after cesarean section: a randomized controlled trial. Korean J Anesthesiol. 2020;73(2):121–128. doi:10.4097/kja.d.18.00269
15. Irwin R, Stanescu S, Buzaianu C, et al. Quadratus lumborum block for analgesia after caesarean section: a randomised controlled trial. Anaesthesia. 2020;75(1):89–95. doi:10.1111/anae.14852
16. Tamura T, Yokota S, Ando M, et al. A triple-blinded randomized trial comparing spinal morphine with posterior quadratus lumborum block after cesarean section. Int J Obstet Anesth. 2019;40:32–38. doi:10.1016/j.ijoa.2019.06.008
17. Blanco R, Ansari T, Girgis E. Quadratus lumborum block for postoperative pain after caesarean section: a randomised controlled trial. Eur J Anaesthesiol. 2015;32(11):812–818. doi:10.1097/eja.0000000000000299
18. Ishio J, Komasawa N, Kido H, et al. Evaluation of ultrasound-guided posterior quadratus lumborum block for postoperative analgesia after laparoscopic gynecologic surgery. J Clin Anesth. 2017;41:1–4. doi:10.1016/j.jclinane.2017.05.015
19. Ökmen K, Metin ökmen B, Topal S. Ultrasound-guided posterior quadratus lumborum block for postoperative pain after laparoscopic cholecystectomy: a randomized controlled double blind study. J Clin Anesth. 2018;49:112–117. doi:10.1016/j.jclinane.2018.06.027
20. Fujimoto H, Irie T, Mihara T, et al. Effect of posterior quadratus lumborum blockade on the quality of recovery after major gynaecological laparoscopic surgery: a randomized controlled trial. Anaesth Intensive Care. 2019;47(2):146–151. doi:10.1177/0310057x19838765
21. Li X, Xu Z-Z, Li Y-T, et al. Analgesic efficacy of two approaches of ultrasound-guided quadratus lumborum block for laparoscopic renal surgery: a randomised controlled trial. Eur J Anaesthesiol. 2021;38(3):265–274. doi:10.1097/eja.0000000000001433
22. Zhu M, Qi Y, He H, et al. Effect of quadratus lumborum block on postoperative cognitive function in elderly patients undergoing laparoscopic radical gastrectomy: a randomized controlled trial. BMC Geriatr. 2021;21(1):238. doi:10.1186/s12877-021-02179-w
23. Bagbanci O, Kursad H, Yayik AM, et al. Comparison of types 2 and 3 quadratus lumborum muscle blocks: open inguinal hernia surgery in patients with spinal anesthesia. Anaesthesist. 2020;69(6):397–403. doi:10.1007/s00101-020-00766-x
24. Uppal V, Retter S, Kehoe E, et al. Quadratus lumborum block for postoperative analgesia: a systematic review and meta-analysis. Can J Anaesth. 2020;67(11):1557–1575. doi:10.1007/s12630-020-01793-3
25. Carline L, McLeod GA, Lamb C. A cadaver study comparing spread of dye and nerve involvement after three different quadratus lumborum blocks. Br J Anaesth. 2016;117(3):387–394. doi:10.1093/bja/aew224
26. Elsharkawy H, El-Boghdadly K, Kolli S, et al. Injectate spread following anterior sub-costal and posterior approaches to the quadratus lumborum block: a comparative cadaveric study. Eur J Anaesthesiol. 2017;34(9):587–595. doi:10.1097/EJA.0000000000000680
27. Tamura T, Yokota S, Ito S, et al. Local anesthetic spread into the paravertebral space with two types of quadratus lumborum blocks: a crossover volunteer study. J Anesth. 2019;33(1):26–32. doi:10.1007/s00540-018-2578-5
28. Ueshima H, Hiroshi O. Incidence of lower-extremity muscle weakness after quadratus lumborum block. J Clin Anesth. 2018;44:104. doi:10.1016/j.jclinane.2017.11.020
29. Koksal E, Aygun H, Genc C, et al. Comparison of the analgesic effects of two quadratus lumborum blocks (QLBs), QLB type II vs QLB type III, in caesarean delivery: a randomised study. Int J Clin Pract. 2021;75(10):e14513. doi:10.1111/ijcp.14513
30. Brixel SM, Biboulet P, Swisser F, et al. Posterior quadratus lumborum block in total hip arthroplasty: a randomized controlled trial. Anesthesiology. 2021;134(5):722–733. doi:10.1097/aln.0000000000003745
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



