Back to Journals » Journal of Pain Research » Volume 10
Ultrasound-guided alcohol neurolysis and radiofrequency ablation of painful stump neuroma: effective treatments for post-amputation pain
Authors Zhang X , Xu Y, Zhou J, Pu S, Lv YY, Chen Y, Du D
Received 9 November 2016
Accepted for publication 15 December 2016
Published 3 February 2017 Volume 2017:10 Pages 295—302
DOI https://doi.org/10.2147/JPR.S127157
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Xin Zhang, Yongming Xu, Jin Zhou, Shaofeng Pu, Yingying Lv, Yueping Chen, Dongping Du
Pain Management Center, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, People’s Republic of China
Background: Post-amputation pain (PAP) is highly prevalent after limb amputation, and stump neuromas play a key role in the generation of the pain. Presently, PAP refractory to medical management is frequently treated with minimally invasive procedures guided by ultrasound, such as alcohol neurolysis and radiofrequency ablation (RFA).
Objective: To record the immediate and long-term efficacy of alcohol neurolysis and RFA. We first used alcohol neurolysis and then, when necessary, we performed RFA on PAP patients.
Study design: Prospective case series.
Setting: Pain management center.
Methods: Thirteen subjects were treated with ultrasound-guided procedures.
Results: All patients were treated with neurolysis using alcohol solutions guided by ultrasound. Seven (54%) of 13 subjects achieved pain relief after 1–3 alcohol injection treatments. The remaining 6 subjects obtained pain relief after receiving 2 administrations of ultrasound-guided RFA. After a 6-month follow-up evaluation period, pain quantities were also assessed. Both stump pain (including intermittent sharp pain and continuous burning pain) and phantom pain were relieved. The frequency of intermittent sharp pain was decreased, and no complications were noted during the observation.
Conclusion: The use of ultrasound guidance for alcohol injection and RFA of painful stump neuromas is a simple, radiation-free, safe, and effective procedure that provides sustained pain relief in PAP patients. In this case series, RFA was found to be an effective alternative to alcohol injection.
Keywords: post-amputation pain, neuroma, ultrasound-guided, alcohol neurolysis, radiofrequency ablation
Introduction
Post-amputation pain (PAP) is highly prevalent after limb amputation but remains as an extremely challenging condition to treat.1 The loss of a body part can lead to 3 distinct descriptive sensory categories, phantom sensation, stump pain, and phantom pain.1,2 Phantom sensation means that patients can still feel the existence of the missing limb after amputation. Usually, this type of sensation is not painful and not a clinical problem. Stump pain occurs immediately after amputation and usually is relieved after a few weeks as the wound heals. However, in some cases, persistent stump pain can occur and can be difficult to treat. Phantom pain means abnormal pain localized in the missing limb. It may be constant but has various intensities and can be described in different terms (shooting, burning, cramping, and aching). Although these 3 categories are described separately, amputees usually experience at least one3 and, in most cases, have difficulty distinguishing one category from another.4
The treatment of PAP is quite difficult as it has multifactorial mechanisms, and the pathophysiological causes of PAP often remain unclear. The interactions between peripheral, spinal, and supraspinal mechanisms are thought to contribute to PAP phenomena.1 These mechanisms include somatosensory cortical reorganization, central sensitivity, and catastrophizing factors. Spinal reorganization in dorsal horns, expansion of receptive fields, loss of inhibitory interneurons, and activation of glial cells often occur at the spinal level after a peripheral nerve injury. Among all the peripheral mechanisms, the generation of neuromas can lead to changes of ion channel expression, alteration of receptor proteins, and ectopic discharges from severed nerve endings.1 Notably, once the nerves are transected by trauma, neuromas can develop at the ends after 6–10 weeks. Neuromas may be regarded as a normal part of the healing process, but the development of amputation stump neuromas is a common and frequent cause of PAP.5 Injection therapy is widely used in many pain management centers. The target of the injection is sometimes myofascial tissue (at a trigger point) and sometimes the neuroma stumps. Injected therapeutic agents can be local anesthetics,6 steroids,7 chemo-denervation substances (botulinum toxin),8 phenol,9,10 alcohol,11 etc. However, in most cases, local injection therapy seems to be more efficacious in the treatment of stump pain than in phantom pain. A separate, small case series found that for patients who experienced relief from a diagnostic local anesthetic injection, pulsed radiofrequency ablation (RFA) was effective in relieving PAP.12
The use of high-resolution ultrasound guidance to assist with the injection procedure is becoming increasingly popular because of real-time visualization of the stump neuroma in soft tissue.13,14 Using this technique, alcohol injection11 and RFA12,15 can be performed more easily and accurately.
However, knowledge of effective management of PAP with alcohol neurolysis and RFA is limited, and their procedural techniques have not been standardized. The differences in outcome between alcohol injection and RFA have not been reported. Here, we present a case series of 12 PAP patients in whom we used alcohol neurolysis first and, when necessary, secondarily performed RFA. We tried to obtain preliminary data on safety, efficacy, side effects, and complications of injection therapy guided by ultrasound on the different types of symptoms reported by PAP patients. We are also attempting to develop a standard protocol for alcohol neurolysis and RFA of neuroma.
Methods
Subjects
The Institutional Review Board of Shanghai Jiaotong University Affiliated Shanghai Sixth People’s Hospital approved the study protocol. Informed consent for participation in the study was obtained from each patient, and a consent form was reviewed and signed, which included the risks, possible adverse consequences of alcohol neurolysis and RFA. Written informed consent was obtained for publication of this paper and accompanying images. Twelve adult patients (7 men, 5 women; median age, 57.5 years; range, 32–82 years) who had undergone limb amputation (upper extremity, n = 4; lower extremity, n = 8) and presented to the Shanghai Sixth People’s Hospital Pain Management Center with PAP between March 2014 and April 2015 were recruited as study subjects (Table 1). The clinical assessments for this prospective study were performed by an experienced pain physician, who selected study subjects with stable general condition at the stump (no local inflammation or other acute tissue alterations).
  | Table 1 Patient characteristics Abbreviations: NRS, numerical rating scale; F, female; M, female; L, left; R, right. |
Data recording
Data regarding each patient’s pain symptoms were recorded carefully. We divided stump pain into 2 categories. One category, “paroxysmal” pain, was defined as intermittent sharp pain of high intensity that had a sudden onset. The frequency of paroxysmal pain was recorded. The other category, “abiding” pain, was defined as continuous burning pain of low intensity that lasted for at least 1 hour. Each kind of stump pain was recorded with a numerical rating scale (NRS, 10 maximum and 0 minimum). Because patients often had difficulty in distinguishing phantom pain from stump pain, we only recorded whether the phantom pain was present (Table 1). The patients’ NRS scores and the frequency of paroxysmal sharp pain were recorded at 3 time points (before treatment and 2 weeks and 6 months after the final treatment). As the frequency of paroxysmal pain in all the patients was different, we only recorded the change (less, equal, or more frequent than before) for the follow-up evaluations (Table 3). During the treatment period, before each operation, pain relief was recorded carefully. Because almost all patients said they could not provide an accurate and comparable NRS score during the ongoing treatment, we replaced the NRS score with the following 4-step scale for the evaluation of pain relief during treatments (Table 2):
  | Table 2 The assessment of pain relief during treatment period Abbreviations: RFA, radiofrequency ablation; L, left; R, right. |
Excellent – when the pain is completely resolved or decreased by ≥75%
Good – when the pain decreased by 50%–74%
Fair – when the pain decreased by 25%–49%
Poor – when the pain decreased by <25% or increased
Examinations
Before operation, a skillful physician performed a careful physical and ultrasound examination on each patient. In all cases, pressing the stump evoked a positive Tinel’s sign, and the neuroma could be detected in the stump area by a high-resolution ultrasound machine (S-Nerve; SonoSite, Bothell, WA, USA). Ultrasonography revealed discrete hypoechoic masses in the distal stump, directly contiguous to the injured nerve. When this amputation neuroma was pressed, extreme pain was evoked. Usually, in lower extremity amputees, the ultrasound examination will reveal 2 obvious bulbous-shaped neuromas in the distal end of injured femoral nerve and sciatic nerve. In upper extremity amputees, 3 separate neuromas in the distal end of injured ulnar nerve, radial nerve, and median nerve usually can be identified.
Protocol
All the subjects received alcohol neurolysis treatment once every 2 weeks until pain relief reached the “excellent” level. After 3 injections of alcohol solution, if the patients’ pain relief did not reach the excellent level, RFA would be performed once every 2 weeks, with 2 procedures in all. During the treatment period, before each operation, the patients’ pain relief level was recorded (Table 2). Two post-treatment surveys were also done to assess the patients’ stump pain NRS scores for the frequency of paroxysmal sharp pain and the phantom pain relief level (Table 3). The first survey was obtained 2 weeks after final treatment, and the other was done 6 months after the final treatment.
Alcohol injection
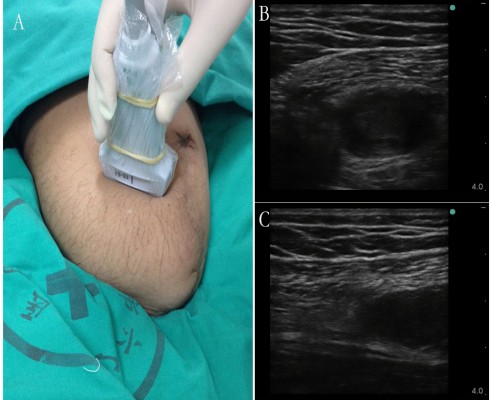
As described by Gruber et al,9 alcohol injection was performed according to the following algorithm in each subject: patients were placed in a convenient position for the intervention, which varied with the location of the neuroma. After skin preparation with antiseptic solution, a linear high-frequency ultrasound probe (6 MHz; S-Nerve; SonoSite) was covered by a sterile plastic bag and placed on the subjects’ stump transversely (Figure 1A) to obtain a transverse axial view (Figure 1B). The hypoechoic neuroma could be easily detected. Then the probe was rotated vertically to reveal the longitudinal image of the neuroma. In this image, we could see the intact nerve tract leading to the neuroma (Figure 1C). According to the method described by Gruber et al,9 the analgesic drug should be injected into the nerve proximal to the neuroma with the in-plane technique in longitudinal image (Figure 1C). In our clinical experience, the nerve tract proximal to the neuroma cannot be distinguished precisely in some cases. It is known that if the injection was not performed successfully, alcohol can damage the surrounding soft tissue. Our alcohol injections have been usually performed in the transverse image, and the injection needle was advanced toward the neuroma body directly. When the needle penetrated the body of the neuroma, the operator would adjust the tip position to evoke the extreme pain that the patient could suffer (Figure 2). After aspiration, a small amount of normal saline (0.9% NaCl) solution would be injected to evoke the pain and reconfirm that the needle tip was in the proper position. When the target was confirmed, 10 mL of local anesthetic was administered around the nerve proximal to the neuroma with another sterile syringe under ultrasound guidance. When the local anesthetic worked, 2–5 mL of dehydrated alcohol solution would be injected into the neuroma body.
RFA
Our RFA procedures were performed as described by Kim et al16 with slight modification. After obtaining written informed consent from each subject, the subject was placed in a convenient position for the intervention, as mentioned earlier. After skin preparation with antiseptic solution, an ultrasound probe was placed on the subjects’ stump transversely to obtain a transverse axial view (Figure 3). When the stump neuroma was detected, slide the probe proximal to the intact nerve pathway for ~5 mm. In this transverse image, the response nerve diameter usually was ~4–8 mm. After attachment to a radiofrequency (RF) generator (Baylis Corporation, Montreal, Canada), a 10 cm RF needle with a 5 mm active tip would be advanced toward and positioned just outside the nerve. Then, the needle was used to stimulate the site at 0.4 mA in the sensory mode (50 Hz) to evoke the patient’s pain. When the target was confirmed, 2 mL of the local anesthetic solution with 10 mg of triamcinolone would be injected through the needle. When the local anesthetic worked, the needle would be advanced into the responding nerve to perform RFA at 80°C for 90 seconds twice, separated by a 60 second interval (Figure 3C).
Results
Subject characteristics are presented in Table 1. Twelve subjects (male, n = 7; female, n = 5) were enrolled. Subject 8 had a bilateral lower extremity amputation; so 13 amputation sites are discussed. All the subjects reported 2 kinds of stump pain (intermittent sharp pain and continuous burning pain) and 10 of 13 (77%) had phantom pain combined with stump pain. But almost all the patients had difficulty distinguishing the 2 syndromes accurately.
Treatment period
We recorded a 4-step scale for the evaluation during treatments. As shown in Table 2, all the patients received 1–5 operative interventions. Seven (54%) of 13 subjects gained pain relief ranked as excellent after 1–3 alcohol neurolysis attempts (1 treatment session, n = 1; 2 sessions, n = 4; 3 sessions, n = 2). The remaining 6 (48%) subjects did not achieve excellent pain relief level and then received RFA. After administration of RFA, all the remaining subjects reported excellent pain relief.
Final assessment
Two post-treatment surveys of the NRS scores were obtained from the subjects to quantify the frequency of paroxysmal sharp pain and the level of phantom pain relief at 2 weeks and 6 months after the final treatments. Stump pain was divided into intermittent sharp pain and continuous burning pain, and each kind of pain has been recorded as NRS score. Two weeks after the final treatment, the subjects had an overall decrease in median NRS score for intermittent sharp pain assessment from 10.0 ± 0.5 to 2.0 ± 0.9 and for continuous burning pain assessment from 7.0 ± 1.0 to 2.0 ± 1.0. The frequency of paroxysmal pain exhibited a large difference among the subjects. Therefore, we only recorded the change (less, equal, or more frequency than before treatment) for the follow-up evaluation. Two weeks after treatments, only 1 subject (8%) reported that the frequency did not change, and 12 (92%) subjects’ paroxysmal pain frequency was less than before treatment (Table 3).
In the survey performed at 6 months after treatment, the NRS scores were recorded again. The scores did not change significantly during the follow-up period (from 2.0 ± 0.9 to 2.0 ± 0.9 for intermittent sharp pain assessment and from 2.0 ± 1.0 to 2.0 ± 1.2 for continuous burning pain assessment). Paroxysmal pain frequency at 6 months was recorded and compared to that at 2 weeks after treatment. Compared with 2 weeks after treatments, 11 (85%) of 13 subjects’ sharp pain frequency decreased, while 2 subjects’ (15%) frequency remained the same (Table 3).
The characteristics of phantom pain also cannot be quantified accurately. We used a 3-scale method (stronger pain, ++; weaker pain, +; pain not present, −) to assess the changes of phantom pain, but this pain recording was complicated. Before treatment, 10 of 13 (77%) subjects had phantom pain symptoms, whereas 2 weeks after the final treatment, 5 (50%) of the 10 subjects were free of phantom pain, 4 (40%) reported less pain, and only 1 (10%) reported unchanged phantom pain. Notably, at the 6-month follow-up, 3 of 5 (60%) phantom pain-free subjects remained phantom pain free, whereas 2 (40%) of the 5 had recrudescent phantom pain, although their pain levels were lower than before treatment. In the 4 phantom pain relief subjects, mild pain remained at an intensity equal to the level reported 2 weeks after the final treatment. There was only 1 subject (10%) whose phantom pain did not change during the observation period. The 3 subjects who had no phantom pain at the outset of the study remained free of this pain throughout the observation period (Table 3).
Discussion
PAP is of neuropathic origin, and its treatment can be very challenging. The underlying mechanisms for this type of pain are multifactorial, including supraspinal-, spinal-, and peripheral-level components.1 As indicated by multiple studies, both stump pain and phantom pain can be controlled by peripheral nerve block to some extent.6,17 Treatments focused on peripheral nerve might be an effective method, such as local injection therapy, RFA, peripheral nerve stimulation, and surgery. In this study, we used 2 methods – alcohol neurolysis and RFA – to block the peripheral ectopic inputs from amputation neuromas. Both procedures can relieve PAP.
Alcohol11 and phenol10 are the most commonly used neurolytic agents for chemical ablation and have been proved efficacious in the management of chronic neuropathic pain, including stump neuromas. Gruber et al10 described the technique of chemical neurolysis for neuroma in detail in 2003. They used an ultrasound probe to scan the stump limb to gain a longitudinal image of the tumor, from which they could see hypoechoic terminal stump neuromas continued from the nerves of origin. Then they injected phenol into the nerve stalk just proximal to the neuroma under an in-plane approach. The phenol volume was ~0.3–1 mL. Lim et al11 reported using dehydrated alcohol (volume of 1.2 mL) for injections into nerve tracts proximal to neuromas. In our study, alcohol was injected directly into the neuroma body other than the stalk, and we found it easier to inject the agent into the body than into the nerve itself. In the longitudinal view, the neuroma stalk that is in continuity with original nerve cannot be easily identified in every patient. Normally, the stalk is small and obscure. Therefore, injection into the stalk is not assured. If the chemical agent is not accurately injected into the stalk, ectopic impulses from the neuroma may not be blocked completely and surrounding soft tissues may be damaged. As the volume of the neuroma body is far higher than that of the neuroma stalk, we need more of the chemical agent to effectuate complete neurolysis. However, the texture of neuroma bodies can be very compact, so that even with the application of a copious amount of chemical agent, it cannot be guaranteed that the agent will diffuse well throughout the tumor. Hence, during the procedure we adjust the position of the needle tip little by little until the needle evokes the exact pain that replicates the subject’s spontaneous events. Injecting agent into that specific area of the neuroma can block the abnormal discharge of neuroma completely and improve the success rate of neurolysis.
Neuroma development is a part of a normal reparative process following peripheral nerve injury. Usually, the distal terminal area of the injured nerve will generate a neuroma. However, in some cases, several neuromas can grow at the end of a nerve terminal. These neuromas can form a grape-like cluster or gathering at the end of a single nerve fiber (Figure 4A). In this setting, the issue of how to identify the specific neuroma responsible for the generation of pain is a significant problem that needs to be addressed. Use of a nerve stimulator might be useful for this, using sensory mode stimulation (50 Hz) at 0.4 mA to reproduce the patient’s symptoms to help identify the target neuroma.
Ultrasound-guided RFA is also used for the treatment of PAP. West and Wu12 reported a case series showing that for patients who experienced relief from a diagnostic lidocaine injection, pulsed radiofrequency (PRF) ablation was effective in relieving PAP. Kim et al16 also described this method, placing the PRF needle on the neuroma stalk as close as possible to the distal part of the associated nerve. However, observations in this study suggest that some nerve tissue, those close to the neuroma stalk, can show significant pathological changes. As shown in Figure 4B and C, the nerve tissue close to neuroma stalk shows significant swelling, with a diameter as large as 1 cm or more (Figure 4B and C). However, the PRF needle normally has a 5 mm active tip. It is very difficult to use such a tiny tip to disrupt a large-diameter nerve completely. Therefore, we revised the procedure to try to completely block the connection between the impaired neuroma tissue and the healthy nerve tissue. We targeted the nerve fiber at 5 mm proximal to the neuroma stalk, which in most cases is healthy and has a diameter of 5–8 mm. At this point, we can block the responsible nerve input completely to relieve PAP. In most studies, the PRF was performed at 42°C.15,16 However, in our procedures, we raised the temperature to 80°C, so that we could block abnormal inputs completely and gain great pain relief. In this case series, no complication has been observed after PRF ablation.
PAP includes phantom sensation, stump pain, and phantom pain. In our general understanding, stump pain is mainly contributed by the peripheral mechanisms, and phantom pain is mainly contributed by the central mechanisms. Alcohol neurolysis and RFA are procedures that block the ectopic peripheral nerve inputs. Theoretically, both procedures should benefit stump pain more effectively than phantom pain. But in reality, these 2 kinds of pain often have similar simultaneous outbreak patterns, and patients usually cannot distinguish them clearly. In this case series, the 10 subjects had both stump pain and phantom pain. They obtained great relief of the stump pain by blocking peripheral nerve discharges through alcohol neurolysis or RFA. Further, 9 of 10 subjects’ phantom pain also eased, though phantom pain is thought to originate from the central nervous system. Notably, amputation of the peripheral nerves resulted in hyperexcitability and spontaneous action potential discharge in the damaged nerve tracts, which may be a potential source of the stump pain, including phantom pain.18 This mechanism may help to explain why the phantom pain was also relieved in our study. There is also evidence from the study of Borghi et al17 to show that peripheral nerve blocks can control phantom pain.
Studies elucidating the differences between the effects of alcohol neurolysis and RFA on PAP are lacking. In our study, after alcohol neurolysis, 6 of 13 subjects reported that their pain relief did not reach the excellent level. When we later performed RFA on these 6 subjects, they all reported excellent pain relief. It seems that RFA can be taken as an alternative method to treat PAP patients. Further studies are needed to further clarify the differences between the 2 methods in more depth.
Conclusion
Our case series reports data on the feasibility, safety, and efficacy of ultrasound-guided alcohol injection and RFA with PAP patients. We conclude that the use of ultrasound guidance for alcohol neurolysis and RFA is a promising tool for the treatment of PAP. Although limited by a small sample size, our observations suggest 2 important conclusions: 1) alcohol injection and RFA are safe and effective procedures to treat PAP, including stump pain and phantom pain; and 2) RFA might be an effective alternative method to alcohol injection.
Acknowledgment
This work was supported in part by the General Program of National Natural Science Foundation of China (81370933, 81400803, and 81672237).
Disclosure
The authors report no conflicts of interest in this work.
References
Hsu E, Cohen SP. Postamputation pain: epidemiology, mechanisms, and treatment. J Pain Res. 2013;6:121–136. | ||
Pirowska A, Wloch T, Nowobilski R, Plaszewski M, Hocini A, Menager D. Phantom phenomena and body scheme after limb amputation: a literature review. Neurol Neurochir Pol. 2014;48(1):52–59. | ||
Ephraim PL, Wegener ST, MacKenzie EJ, Dillingham TR, Pezzin LE. Phantom pain, residual limb pain, and back pain in amputees: results of a national survey. Arch Phys Med Rehabil. 2005;86(10):1910–1919. | ||
Sherman RA, Sherman CJ. Prevalence and characteristics of chronic phantom limb pain among American veterans. Results of a trial survey. Am J Phys Med. 1983;62(5):227–238. | ||
Henrot P, Stines J, Walter F, Martinet N, Paysant J, Blum A. Imaging of the painful lower limb stump. Radiographics. 2000;20(Spec No):S219–S235. | ||
Fischler AH, Gross JB. Ultrasound-guided sciatic neuroma block for treatment of intractable stump pain. J Clin Anesth. 2007;19(8):626–628. | ||
Kesikburun S, Yasar E, Dede I, Goktepe S, Tan AK. Ultrasound-guided steroid injection in the treatment of stump neuroma: pilot study. J Back Musculoskeletal Rehabil. 2014;27(3):275–279. | ||
Wu H, Sultana R, Taylor KB, Szabo A. A prospective randomized double-blinded pilot study to examine the effect of botulinum toxin type A injection versus Lidocaine/Depomedrol injection on residual and phantom limb pain: initial report. Clin J Pain. 2012;28(2):108–112. | ||
Gruber H, Kovacs P, Peer S, Frischhut B, Bodner G. Sonographically guided phenol injection in painful stump neuroma. AJR Am J Roentgenol. 2004;182(4):952–954. | ||
Gruber H, Glodny B, Kopf H, et al. Practical experience with sonographically guided phenol instillation of stump neuroma: predictors of effects, success, and outcome. AJR Am J Roentgenol. 2008;190(5):1263–1269. | ||
Lim KB, Kim YS, Kim JA. Sonographically guided alcohol injection in painful stump neuroma. Ann Rehabil Med. 2012;36(3):404–408. | ||
West M, Wu H. Pulsed radiofrequency ablation for residual and phantom limb pain: a case series. Pain Pract. 2010;10(5):485–491. | ||
Brown MR, Farquhar-Smith P, Williams JE, Ter Haar G, deSouza NM. The use of high-intensity focused ultrasound as a novel treatment for painful conditions-a description and narrative review of the literature. Br J Anaesth. 2015;115(4):520–530. | ||
Sivan M, Stoppard E. Sonographically guided phenol instillation of stump neuroma. AJR Am J Roentgenol. 2008;191(5):W208; author reply W209. | ||
Restrepo-Garces CE, Marinov A, McHardy P, Faclier G, Avila A. Pulsed radiofrequency under ultrasound guidance for persistent stump-neuroma pain. Pain Pract. 2011;11(1):98–102. | ||
Kim YK, Jung I, Lee CH, Kim SH, Kim JS, Yoo BW. Pulsed radiofrequency ablation under ultrasound guidance for huge neuroma. Korean J Pain. 2014;27(3):290. | ||
Borghi B, D’Addabbo M, Borghi R. Can neural blocks prevent phantom limb pain? Pain Manag. 2014;4(4):261–266. | ||
Flor H, Nikolajsen L, Staehelin Jensen T. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci. 2006;7(11):873–881. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.