Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Ultrasound Evaluation of the Quadriceps Muscle Contractile Index in Patients with Stable Chronic Obstructive Pulmonary Disease: Relationships with Clinical Symptoms, Disease Severity and Diaphragm Contractility
Authors Maynard-Paquette AC, Poirier C, Chartrand-Lefebvre C , Dubé BP
Received 20 July 2019
Accepted for publication 11 November 2019
Published 9 January 2020 Volume 2020:15 Pages 79—88
DOI https://doi.org/10.2147/COPD.S222945
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Anne-Catherine Maynard-Paquette,1 Claude Poirier,2 Carl Chartrand-Lefebvre,3,4 Bruno-Pierre Dubé2,5
1Centre Hospitalier de l’Université de Montréal (CHUM), Montreal, Quebec, Canada; 2Centre Hospitalier de l’Université de Montréal (CHUM), Department of Medicine, Respiratory Medicine Division, Montreal, Quebec, Canada; 3Centre Hospitalier de l’Université de Montréal (CHUM), Department of Radiology, Montréal, Québec, Canada; 4Research Center of the CHUM (CRCHUM) – Imaging and Engineering Axis, Montreal, Quebec, Canada; 5Research Center of the CHUM (CRCHUM) - Health Innovation and Evaluation Hub, Montreal, Quebec, Canada
Correspondence: Bruno-Pierre Dubé
Centre de Recherche du Centre Hospitalier de l’Université de Montréal (CRCHUM), 850 Rue St-Denis, Montréal, Qc H2X0A9, Canada
Tel +1 514 890-8000
Email [email protected]
Rationale: Chronic obstructive pulmonary disease (COPD) is associated with changes in the composition and function of peripheral and respiratory muscles, which can negatively impact quality of life. Ultrasonography can provide a non-invasive evaluation of the integrity of both peripheral muscles and diaphragm, but its use in patients with COPD is still being investigated. We aimed at evaluating the relationship between quadriceps size, using ultrasonography and symptoms, lung function and diaphragm contractility in a cohort of patients with COPD.
Methods: COPD patients were prospectively recruited and ultrasonography of the dominant quadriceps and of the diaphragm was performed. Quadriceps size was evaluated using three measurements: 1) cross-sectional area of the rectus femoris (Qcsa), 2) thickness (Qthick) and 3) contractile index (Qci), defined as the ratio of quadriceps thickness/total anterior thigh thickness. Diaphragm contractility was evaluated using thickening fraction (TFdi). Clinical characteristics and number of moderate-to-severe exacerbations in the previous year were retrieved from medical files. Dyspnea (mMRC scale) and disease impact on health status (COPD Assessment Test (CAT)) were measured at inclusion. Fat-free mass index (FFMI) was assessed using bioelectrical impedance.
Results: Forty patients were recruited (20 males, mean age and FEV1 66±6 years and 49±17%predicted, respectively). Mean Qcsa, Qthick and Qci were 336±145 mm2, 1.55±0.53 cm and 64±16%, respectively, and mean TFdi was 91±36%. Qci was significantly correlated with FFMI (rho=0.59, p=0.001), TFdi (rho=0.41, p=0.008), FEV1 (rho=0.43, p=0.001) but not with age (rho=0.18, p=0.28). Qci was significantly correlated to CAT score (rho=−0.47, p=0.002), even when controlled for FEV1, and was lower in patients with an mMRC score ≥2 (55±15 vs 70±14%, p=0.002). Qcsa and Qci were significantly lower in patients with frequent exacerbations. In a multiple linear regression analysis that included age, gender, FFMI, FEV1 and TFdi, only FFMI and TFdi were found to be significantly related to lower Qci values.
Conclusion: In patients with COPD, ultrasound evaluation of the quadriceps contractile index is feasible and related to disease severity, clinical symptoms, exacerbation history and diaphragm contractility. As such, it may provide a novel tool for the evaluation of the severity and burden of the disease in this population. Further studies are required to better delineate its potential role as a prognostic marker in this population.
Keywords: COPD, ultrasound, quadriceps, diaphragm, contractile index
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death worldwide,1 and both its incidence and its burden are bound to increase in the coming years. In the last decades, the spectrum of comorbidities and non-pulmonary complications arising from COPD has generated growing interest. Numerous studies have addressed this aspect of the disease, which is now recognized as a multifaceted entity.2–4 Peripheral muscle dysfunction/weakness and sarcopenia are well-recognized complications of COPD,5–9 and are associated with disease severity, exercise (in)tolerance and vital prognosis.6,10–13 As such, the evaluation of peripheral muscle integrity is an attractive marker of disease severity in patients with COPD. However, routine direct evaluation of this marker is difficult because of the need to rely on imaging techniques such as computed tomography12 or dual-energy X-ray absorptiometry which are expensive, ionizing, time-consuming and not readily available at the bedside.
In recent years, ultrasonography has emerged as a point-of-care tool that allows access to a rapid, simple, widely available and non-ionizing evaluation of many physiological variables, including peripheral muscle and diaphragm function and integrity.14–17 When considering peripheral muscle strength, ultrasound-derived measurement of rectus femoris cross-sectional area has been shown to be a reliable estimator of muscle strength in patients with COPD,18 and the ultrasound measurement of a contractile index of the quadriceps muscle19 was recently shown to be a reliable, reproducible and non-volitional predictor of functional decline, hospitalization and death in elderly subjects.20 As such, these markers may prove a simple tool to assess disease severity and prognosis in the COPD population. However, a formal comparison of these techniques to other variables usually associated with disease severity in COPD is lacking.
In this pilot study, we aimed at evaluating the feasibility and potential clinical usefulness of ultrasound-derived measurements of peripheral muscle integrity in patients with COPD by comparing it to usual markers of disease severity and respiratory muscle function.
Methods
Study Design and Participants
COPD patients were prospectively recruited from the Centre Hospitalier de l’Université de Montréal (CHUM, Montréal, Canada) on the day of the appointment for pulmonary function testing. Included individuals had to have a clinician-confirmed diagnosis of COPD (according to GOLD criteria1) and be ≥18 years old. Individuals suffering from asthma, known neurological, rheumatological or musculoskeletal degenerative disease limiting physical capacity were excluded from the study, as were subjects who reported an episode of acute exacerbation (AE) in the last 6 weeks. The study received approval from the ethics board of the CHUM and was conducted in compliance with the Declaration of Helsinki. Informed written consent was obtained from participants by a member of the study team.
Data Collection
Data collection including age, gender, height, weight, smoking history, medical comorbidities, inhaled medication, perception of dyspnea (as evaluated by the mMRC score21) and disease impact on health status (using the COPD Assessment Test (CAT)) were obtained. The number of moderate to severe AE [defined according to Global Initiative for COPD (GOLD) guidelines22] in the year before inclusion in the study was retrieved from medical files, as were pulmonary function tests, including values of forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), residual volume (RV) and diffusion capacity of the lung for carbon monoxide (DLCO). All pulmonary function tests were performed according to American Thoracic Society/European Respiratory Society guidelines.23–25
Bioelectrical Impedance
Body composition was assessed using bioelectrical impedance (Tanita TBF-300A, Arlington Heights, IL, USA). Patients were asked to stand upright on the bioelectrical scale for approximately 5 seconds to ensure valid measurements by the device. Raw data including body fat (%), total body weight (TBW), muscle mass (kg), basal metabolic rate (BMR), bone mass (kg) and visceral fat (%) were measured. Fat-free mass was calculated TBW*(1–percentage body fat). The fat-free mass index (FFMI) was calculated as fat-free mass/height squared.
Ultrasound Measurements
Ultrasonography of the dominant quadriceps (as defined by the leg individuals usually use to go up a flight of stairs or kick a ball20 and of the right hemidiaphragm) was performed. All measurements were performed using a linear 4–12 MHz probe (Sparq, Phillips Healthcare, USA).
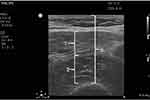
Measurements of the rectus femoris were made by placing the transducer perpendicular to the long axis of the thigh (precisely at 3/5 of the distance from the anterior superior iliac spine to the superior patellar border), therefore isolating the muscle (Figure 1). Individuals were placed in a supine position with the rested leg in extension. Prior to image acquisition, contraction-relaxation maneuvers were performed in order to delineate the muscle septa separating the vastus intermedius and rectus femoris muscles. Three comparative measurements were performed, all in the relaxation state: 1) the cross-sectional area (Qcsa) of the rectus femoris muscle (as acquired on frozen image using electronic calipers, see Figure 4), 2) the total thickness of the quadriceps muscle (Qthick, defined as the addition of the measurement of the rectus femoris muscle and that of the vastus intermedius muscle), and 3) its contractile index (Qci).19,20 Qci was obtained by dividing Qthick by the total anterior thigh thickness (distance from the skin to the anterior border of the femur). Therefore, Qci=[100*(Qthick/total anterior thigh thickness)]. All measurements were performed at the level of the most anterior, central part of the femur bone, in perpendicular axis to the skin. To assess reliability, repeated measurements on the frozen images were performed after an interval of 3 weeks by the same operator.
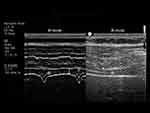
Ultrasound assessment of diaphragm thickness and thickening was performed by placing the probe perpendicular to the right chest wall at the midaxillary line between the 9th and 10th intercostal spaces, at the level at which diaphragm fibers run parallel to the abdominal wall (zone of apposition). The right diaphragm could be identified as the three-layered structure comprising two hyperechoic (i.e. more dense) lines representing the pleural and peritoneal membranes and a middle hypoechoic (i.e. less dense) layer representing the diaphragmatic muscle fibers (Figure 4). Then, using M-mode at a sweep speed of 10mm/s, three spontaneous quiet breathing cycles were recorded and saved to a frozen image. Diaphragm thickness was measured at end-expiration (TDE) and end-inspiration (TDI) using electronic calipers. The thickening fraction (TFdi) was then calculated offline as [100*(TDI−TDE)/TDI].14
All ultrasound measurements were performed jointly with the same two operators present (BPD and ACMP, one with a thorough diaphragmatic ultrasound training and the other also with an ultrasound training but with limited practice). All evaluations made by the junior trainee were directly supervised by the other operator. Although the operators had limited experience with musculoskeletal ultrasound, the technique described by Guerreiro et al20 was closely followed. Operators were blinded to diaphragmatic thickening fraction measurement (as calculations were performed later on frozen images) and to pulmonary function test results.
Statistical Analyses
Data are reported as mean (standard deviation) or n (percent). Relationships between ultrasound variables and the other clinical markers of disease severity (FFMI, FEV1, CAT score, TFdi) were assessed using Spearman correlation, in order to facilitate the identification of a potentially non-linear relationship between variables. To control for the potential effect of lung hyperinflation on the measurement of TFdi in subjects with advanced disease, partial correlation for RV was used when comparing TFdi to quadriceps ultrasound values, and linear regression was used to adjust the comparison of CAT score and Qci for FEV1 value. Lung function test results and quadriceps and diaphragm ultrasound measurements were compared between groups with frequent (≥2/year) or less frequent AE using independent t-tests (or Mann–Whitney U-test for non-parametric data). Ultrasound variables were compared between patients with high and low dyspnea score (≥2 units of mMRC scale) using independent t-tests. Univariate analyses of the relationship between Qci and its potential predictors (age, gender, FFMI, FEV1, RV and TFdi) were performed using linear regression. A multiple linear regression analysis (“enter” model) that included all aforementioned variables with a p<0.05 in univariate analysis, in addition to age and gender was then performed to identify independent predictors of Qci. Due to the high correlation between RV and FEV1 (r=0.68, p<0.001) and FFMI (r=0.78, p<0.001), RV was excluded from the final multiple variable model.
Intra-observer agreement for Qcsa, Qthick and Qci was assessed with the intraclass correlation coefficient for absolute concordance of unique measurements, using a two-way mixed model. An ICC <0.40 implies poor agreement; 0.40–0.59, fair agreement; 0.60–0.74, good agreement and 0.75–1.00, excellent agreement.26
All analyses were performed using SPSS (version 21, IBM, Armonk, NY, USA). P-values <0.05 were considered statistically significant.
Results
Forty subjects (20 males) were included in the study. Subject characteristics are shown in Table 1. The majority of patients had moderate to severe airflow obstruction and mean dyspnea and CAT scores showed a moderate to severe symptom burden. Valid ultrasound images could be obtained in all subjects, for all measurements, and image acquisition lasted less than 5 mins in all cases. Measures of FFMI for the first 11 individuals undergoing assessment could not be obtained because of unavailability of the bioimpedance scale at the time of patient visit.
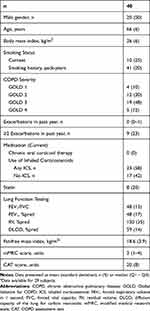 |
Table 1 Patient Characteristics |
Relationship Between Peripheral Muscle Measurements and Markers of Disease Severity
Mean values of Qcsa, Qthick and Qci are reported in Table 2. Patients with frequent AE had significantly lower values of FEV1, RV, diffusion capacity, FFMI, mMRC score, CAT score, Qcsa, Qci and TFdi (Table 2).
 |
Table 2 Patients Characteristics and Ultrasound Quadriceps and Diaphragm Measurements According to Exacerbation Frequency |
Statistically significant correlations were found between Qci and FFMI (rho=0.59, p=0.001), FEV1 (rho=0.43, p=0.001), RV (rho=−0.54, p=0.005) and CAT score (rho=−0.47, p=0.002), but not age (rho=−0.18, p=0.28) (Figure 2). The relationship between Qci and CAT score remained significant even when adjusting for FEV1 [B=−0.75 (95% CI −1.43 to −0.06), p=0.03]. Qci was not significantly correlated to BMI (rho=0.25, p=0.12).
Qthick was significantly related to FFMI (rho=0.57, p=0.001), FEV1 (rho=0.41, p=0.01), RV (rho=−0.39, p=0.048), DLCO (rho=0.58, p=0.002) and CAT score (rho=−0.38, p=0.02).
In contrast, Qcsa was only significantly correlated to FFMI (rho=0.46, p=0.01) and to RV (rho=−0.47, p=0.02), but not to FEV1 or CAT score (both p>0.05).
Relationship Between Peripheral Muscle Measurements and Respiratory Muscle Activity
Qcsa and Qci were statistically correlated with TFdi (rho=0.35, p=0.03 and rho=0.41, p=0.008, respectively, see Figure 2), but Qthick was not (rho=0.14, p=0.39). When controlling for RV, only Qci remained significantly correlated to TFdi (r=0.46, p=0.02). There were no significant correlations between TDE and either Qcsa, Qthick or Qci (all p>0.05).
Relationship Between Peripheral Muscle Measurements and Dyspnea
Subjects with higher dyspnea scores had significantly lower values of Qcsa, Qthick and Qci (2.76±1.32 vs 3.85±1.39 cm2, p=0.02, 1.29±0.50 vs 1.77±0.46 cm, p=0.003 and 55±15 vs 71±14%, p=0.002, respectively) (Figure 3).
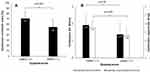 |
Figure 3 Comparison between dyspnea levels (n=22 for mMRC ≤2 and n=18 for mMRC >2) and Qci (A) and Qthick and Qcsa (B). |
Predictors of Lower Qci Values
Based on the results of univariate analysis (Table 4), a multiple linear regression analysis was performed, which included age, gender, FFMI, FEV1 and TFdi. Only FFMI and TFdi were found to be significantly related to worse Qci values (Table 5).
 |
Table 3 Intra-Observer Reliability of Quadriceps Ultrasound Measurements |
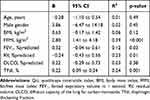 |
Table 4 Univariate Analyses of the Relationship Between Qci and Markers of Disease Severity |
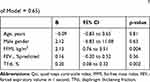 |
Table 5 Multiple Regression Analysis of the Predictors of Qci (R2 of Model = 0.65) |
Reliability
Intra-observer reliability was high for all ultrasound measurements, but Qcsa had slightly lower ICC values than Qthick and Qci (Table 3).
Discussion
While the evaluation of the quadriceps muscle using Qcsa or Qthick in patients with COPD has already been reported,18,27–35 this is the first study to evaluate the use of ultrasonography to assess the quadriceps contractile index (Qci) in this population. Our main results could be summarized as follows: 1) the measurement of Qthick, Qcsa and Qci is feasible, simple, rapid and reliable in this population, 2) although all three indices showed some degree of relationship to other clinically significant markers of disease severity, Qci performed best with regards to its correlation to airflow obstruction, hyperinflation, dyspnea and CAT score/quality of life and 3) Qci was independently related not only to FFMI but also to diaphragm contractile activity.
Taken together, these results further strengthen the notion that peripheral muscle integrity is an important marker of disease severity in patients with COPD, and support a role for ultrasonography as a clinical tool for its evaluation.
When comparing the various ultrasound measurements, Qci seemed to present stronger and more prominent correlations to the physiological and clinical variables studied. In the study by Guerreiro et al,20 Qci also outperformed Qthick as a marker of functional decline in elderly subjects (defined as a decrease in basic activities of daily living at 3-months follow-up). This advantage may relate to the fact that Qci intrinsically reports Qthick as a fraction of total thigh thickness, instead of relying only on the measurement of the muscle’s thickness. As such, Qci may provide a better estimation of the muscle’s contractile capacity than Qthick, which in itself is possibly more dependent on other factors such as body weight and height.
Our findings that Qci is significantly related to FFMI was expected, and the absence of relationship between Qci and BMI echoes the findings of Marquis et al,12 which also reported on the poor predictive value of BMI for midthigh muscle size. Similarly, we observed significant relationships between Qci and the severity of airflow obstruction and static hyperinflation, which confirms previous findings on the higher prevalence of peripheral muscle weakness and wasting in patients with more severe airflow obstruction.36–39
Although exploratory in nature, our finding that Qci was significantly related to diaphragm activity is thought-provoking. This relationship remained significant even after adjusting for the severity of airflow obstruction and gas trapping, suggesting that factors other than the mechanical effect of prominent hyperinflation on diaphragm contractility may be implicated. It must be noted, however, that TFdi values in our subjects remained largely in the normal range, with no patients displaying overt diaphragm dysfunction.16 Whether the pathophysiological mechanisms responsible for peripheral muscle wasting in COPD also affect the respiratory muscles in a similar manner remains unclear,40 and should be the target of future research.
Finally, we also observed that patients with lower Qci values had higher dyspnea levels, higher CAT scores and that patients who had been frequent exacerbators in the previous year had statistically lower values of both Qcsa and Qci. Whether quadriceps wasting is the cause or the consequence of repeated AEs cannot be ascertained from our data, but this finding echoes that of others who have reported on a higher prevalence of AEs in COPD patients with sarcopenia and muscle weakness.5,38,41,42 Taken together, these findings highlight the potential clinical usefulness of Qci as a global marker of disease severity, and as a potential prognostic tool.
Our study has several limitations that need to be acknowledged. First, we did not formally compare our ultrasound measurements to gold standard assessments of muscle size (assessed using CT scan or magnetic resonance imaging) or strength (assessed using one-repetition maximum strength). This limits the physiological exploration of the relationship between the different ultrasound variables that we measured and direct measurement of muscle function and composition. Such a comparison would be desirable to further delimit the applicability and physiological determinants of quadriceps ultrasound, and others have already reported on the significant relationship between rectus femoris area and muscle strength in patients with COPD, albeit on a small number of subjects.28 Our findings still signal a true clinically relevant value to ultrasound markers in the context of COPD, as evidenced by the relationship we observed between these markers and other relevant markers of disease severity.
Second, although our finding of a high intra-observer reliability is reassuring, a thorough analysis of the inter-observer in the COPD population would be desirable before clinical implementation can be considered. The pilot nature of our study limited this aspect of our evaluation, but high intra- and inter-observer reliability for the measurement of Qci has already been described in the elderly population,20 while Qcsa has been shown to be reproducible in patients with COPD.31,43
Third, the cross-sectional design of our pilot study did not allow for a validation of the potential prognostic value of quadriceps ultrasound in our population. Our results, coupled to the strong prognostic value of quadriceps cross-sectional area assessed using CT scan in COPD patients12 and the validated ability of quadriceps ultrasound to act as a marker of functional decline and hospitalisations in elderly patients20 highlight the need for a formal longitudinal evaluation of the potential of quadriceps ultrasound to predict clinically relevant outcomes in COPD patients. Future studies aiming at predicting or quantifying the response to interventions such as pulmonary rehabilitation-based ultrasound measurements of peripheral muscles should be encouraged. Lastly, the measurement of FFMI was not possible in a number of subjects for technical reasons and, while other studies have applied disease-specific equations on bioelectrical impedance studies in order to obtain more reliable results, our bioelectrical impedance scale did not include this function.
Conclusion
In patients with COPD, ultrasound evaluation of the quadriceps contractile index (Qci) is feasible, rapid, simple and reliable. It is related to disease severity, clinical symptoms and respiratory muscle activity. It could prove a novel and promising tool for the evaluation of the severity and burden of the disease in this population. Further studies should aim at better delineating inter-rater reliability of the technique, further validate its use against objective measurements of muscle strength and, eventually, at quantifying the role of quadriceps ultrasound as a marker of prognosis and response to interventions in this population.
Disclosure
Dr Carl Chartrand-Lefebvre reports research collaborations with Terarecon and Seimens outside of the submitted work. The authors report no other conflicts of interest in this work.
References
1. Singh D, Agusti A, Anzueto A, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi:10.1183/13993003.00164-2019
2. Barr RG, Celli BR, Mannino DM, et al. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Am J Med. 2009;122(4):348–355. doi:10.1016/j.amjmed.2008.09.042
3. Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. doi:10.1164/rccm.201201-0034OC
4. Patel AR, Hurst JR. Extrapulmonary comorbidities in chronic obstructive pulmonary disease: state of the art. Expert Rev Respir Med. 2011;5(5):647–662. doi:10.1586/ers.11.62
5. Decramer M, Gosselink R, Troosters T, Verschueren M, Evers G. Muscle weakness is related to utilization of health care resources in COPD patients. Eur Respir J. 1997;10(2):417–423. doi:10.1183/09031936.97.10020417
6. Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med. 1996;153(3):976–980. doi:10.1164/ajrccm.153.3.8630582
7. Gosselink R, Troosters T, Decramer M. Distribution of muscle weakness in patients with stable chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2000;20(6):353–360. doi:10.1097/00008483-200011000-00004
8. Remels AH, Gosker HR, Langen RC, Schols AM. The mechanisms of cachexia underlying muscle dysfunction in COPD. J Appl Physiol (1985). 2013;114(9):1253–1262. doi:10.1152/japplphysiol.00790.2012
9. Wust RC, Degens H. Factors contributing to muscle wasting and dysfunction in COPD patients. Int J Chron Obstruct Pulmon Dis. 2007;2(3):289–300.
10. Yin HL, Yin SQ, Lin QY, Xu Y, Xu HW, Liu T. Prevalence of comorbidities in chronic obstructive pulmonary disease patients: a meta-analysis. Medicine (Baltimore). 2017;96(19):e6836. doi:10.1097/MD.0000000000006836
11. Maltais F, Decramer M, Casaburi R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15–62. doi:10.1164/rccm.201402-0373ST
12. Marquis K, Debigare R, Lacasse Y, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(6):809–813. doi:10.1164/rccm.2107031
13. Seymour JM, Spruit MA, Hopkinson NS, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J. 2010;36(1):81–88. doi:10.1183/09031936.00104909
14. Matamis D, Soilemezi E, Tsagourias M, et al. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med. 2013;39(5):801–810. doi:10.1007/s00134-013-2823-1
15. Dube BP, Dres M, Mayaux J, Demiri S, Similowski T, Demoule A. Ultrasound evaluation of diaphragm function in mechanically ventilated patients: comparison to phrenic stimulation and prognostic implications. Thorax. 2017;72:811–818. doi:10.1136/thoraxjnl-2016-209459
16. Baria MR, Shahgholi L, Sorenson EJ, et al. B-mode ultrasound assessment of diaphragm structure and function in patients with COPD. Chest. 2014;146(3):680–685. doi:10.1378/chest.13-2306
17. Mourtzakis M, Parry S, Connolly B, Puthucheary Z. Skeletal muscle ultrasound in critical care: a tool in need of translation. Ann Am Thorac Soc. 2017;14(10):1495–1503. doi:10.1513/AnnalsATS.201612-967PS
18. Seymour JM, Ward K, Sidhu PS, et al. Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax. 2009;64(5):418–423. doi:10.1136/thx.2008.103986
19. Agyapong-Badu S, Warner M, Samuel D, Narici M, Cooper C, Stokes M. Anterior thigh composition measured using ultrasound imaging to quantify relative thickness of muscle and non-contractile tissue: a potential biomarker for musculoskeletal health. Physiol Meas. 2014;35(10):2165–2176. doi:10.1088/0967-3334/35/10/2165
20. Guerreiro AC, Tonelli AC, Orzechowski R, Dalla Corte RR, Moriguchi EH, de Mello RB. Bedside ultrasound of quadriceps to predict rehospitalization and functional decline in hospitalized elders. Front Med (Lausanne). 2017;4:122. doi:10.3389/fmed.2017.00122
21. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. doi:10.1378/chest.93.3.580
22. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
23. Miller MR, Brusasco V, Burgos F, et al. Standardisation of spirometry. Eur Respir J. 2005;296:319–338. doi:10.1183/09031936.05.00034805
24. Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522. doi:10.1183/09031936.05.00035005
25. Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720–735. doi:10.1183/09031936.05.00034905
26. Cicchetti DV.Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 1994;6:284–290. doi:10.1037/1040-3590.6.4.284
27. Menon MK, Houchen L, Harrison S, Singh SJ, Morgan MD, Steiner MC. Ultrasound assessment of lower limb muscle mass in response to resistance training in COPD. Respir Res. 2012;13:119. doi:10.1186/1465-9921-13-119
28. Hammond K, Mampilly J, Laghi FA, et al. Validity and reliability of rectus femoris ultrasound measurements: comparison of curved-array and linear-array transducers. J Rehabil Res Dev. 2014;51(7):1155–1164. doi:10.1682/JRRD.2013.08.0187
29. Cruz-Montecinos C, Guajardo-Rojas C, Montt E, et al. Sonographic measurement of the quadriceps muscle in patients with chronic obstructive pulmonary disease: functional and clinical implications. J Ultrasound Med. 2016;35(11):2405–2412. doi:10.7863/ultra.15.11032
30. Greening NJ, Harvey-Dunstan TC, Chaplin EJ, et al. Bedside assessment of quadriceps muscle by ultrasound after admission for acute exacerbations of chronic respiratory disease. Am J Respir Crit Care Med. 2015;192(7):810–816. doi:10.1164/rccm.201503-0535OC
31. Mandal S, Suh E, Thompson A, et al. Comparative study of linear and curvilinear ultrasound probes to assess quadriceps rectus femoris muscle mass in healthy subjects and in patients with chronic respiratory disease. BMJ Open Respir Res. 2016;3(1):e000103. doi:10.1136/bmjresp-2015-000103
32. Buttery SC, Mohan D, Fisk M, et al. Longitudinal follow-up of quadriceps strength and function in a COPD cohort after 3 years. Eur Respir J. 2017;50(2):1700707. doi:10.1183/13993003.00707-2017
33. Seymour JM, Ward K, Raffique A, et al. Quadriceps and ankle dorsiflexor strength in chronic obstructive pulmonary disease. Muscle Nerve. 2012;46(4):548–554. doi:10.1002/mus.v46.4
34. Shrikrishna D, Patel M, Tanner RJ, et al. Quadriceps wasting and physical inactivity in patients with COPD. Eur Respir J. 2012;40(5):1115–1122. doi:10.1183/09031936.00170111
35. Parry S, Burtin C, Denehy L, Puthucheary Z, Bear D. Ultrasound evaluation of quadriceps muscle dysfunction in respiratory disease. Cardiopulm Phys Ther J. 2019;30(1):15–23. doi:10.1097/CPT.0000000000000102
36. Smith BM, Hoffman EA, Basner RC, Kawut SM, Kalhan R, Barr RG. Not all measures of hyperinflation are created equal: lung structure and clinical correlates of gas trapping and hyperexpansion in COPD: the Multi-Ethnic Study of Atherosclerosis (MESA) COPD study. Chest. 2014;145(6):1305–1315. doi:10.1378/chest.13-1884
37. Dubé B-P, Guerder A, Morelot-Panzini C, Laveneziana P. The clinical relevance of the emphysema-hyperinflated phenotype in COPD. COPD Res Pract. 2016;2(1):1–11.
38. Bernard S, Blanc PLE, Whittom F, et al. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:629–634. doi:10.1164/ajrccm.158.2.9711023
39. Jones SE, Maddocks M, Kon SS, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax. 2015;70(3):213–218. doi:10.1136/thoraxjnl-2014-206440
40. Barreiro E, Gea J. Respiratory and limb muscle dysfunction in COPD. COPD. 2015;12(4):413–426. doi:10.3109/15412555.2014.974737
41. Poberezhets V, Mostovoy Y, Demchuk H. Exacerbation of chronic obstructive pulmonary diseases as a risk factor of the skeletal muscle dysfunction. Lung India. 2019;36(3):188–192. doi:10.4103/lungindia.lungindia_185_18
42. Spruit MA, Gosselink R, Troosters T, et al. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax. 2003;58(9):752–756. doi:10.1136/thorax.58.9.752
43. Zaidman CM, Wu JS, Wilder S, Darras BT, Rutkove SB. Minimal training is required to reliably perform quantitative ultrasound of muscle. Muscle Nerve. 2014;50(1):124–128. doi:10.1002/mus.v50.1
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.