Back to Journals » Clinical Ophthalmology » Volume 11
Ultra-wide-field fluorescein angiography in diabetic retinopathy: a narrative review
Authors Rabiolo A, Parravano M, Querques L, Cicinelli MV , Carnevali A, Sacconi R, Centoducati T, Vujosevic S, Bandello F , Querques G
Received 1 February 2017
Accepted for publication 23 March 2017
Published 27 April 2017 Volume 2017:11 Pages 803—807
DOI https://doi.org/10.2147/OPTH.S133637
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Alessandro Rabiolo,1 Mariacristina Parravano,2 Lea Querques,1,2 Maria Vittoria Cicinelli,1 Adriano Carnevali,1,3 Riccardo Sacconi,1,4 Teresa Centoducati,1 Stela Vujosevic,5 Francesco Bandello,1 Giuseppe Querques1
1Department of Ophthalmology, University Vita-Salute, Scientific Institute San Raffaele, Milan, 2G. B. Bietti Foundation – IRCCS, Rome, 3Department of Ophthalmology, University of “Magna Graecia,” Catanzaro, 4Department of Neurological and Movement Sciences, University of Verona, Verona, 5Department of Neuroscience, Ophthalmology Clinic, University of Padova, Padova, Italy
Abstract: Fluorescein angiography (FA) is a useful examination in patients suffering from diabetic retinopathy (DR). Traditional angiograms explore 30°–50° of the retina at once; however, visualization of peripheral retina is fundamental in order to assess nonperfused areas, vascular leakage, microvascular abnormalities, and neovascularizations. In order to expand the field of view, wide-field and ultra-wide-field imaging has been developed allowing to image up to 200° of retinal surface in one single shot. The aim of this narrative review was to provide an overview of the role of the most recent technique of ultra-wide-field fluorescein angiography in DR.
Keywords: ischemic index, targeted retinal photocoagulation, diabetic macular edema, diabetic macular ischemia, peripheral vessel leakage, capillary nonperfusion
Introduction
Almost 55 years after its introduction, fluorescein angiography (FA) is still a helpful test in most retinal diseases, including diabetic retinopathy (DR).1 Traditional angiograms explore 30°–50° of the retina at once; however, visualization of peripheral retina is fundamental in order to assess nonperfused areas, vascular leakage, microvascular abnormalities, and neovascularizations (NVs).2 Hence, the evaluation of the peripheral retina is crucial for screening, diagnosis, monitoring, treatment, and prognosis of DR.3
In order to extend the field of view and, thus, to obtain wide-field (>30° and <200°) and ultra-wide-field (≥200°) fundus photography and ultra-wide-field fluorescein angiography (UWFA), 3 different strategies have been applied in the past years, namely 1) photomontage of traditional angiograms (eg, 7 standard field [7SF] and 2 field protocols exploring 75° and 45°, respectively);4 2) additional lens applied to a standard fundus camera or confocal selective laser ophthalmoscope (cSLO) extending the field of view (eg, Staurenghi lens [150°]5 and Spectralis ultra-wide-field lens [105°]6); 3) dedicate instrumentation (eg, Pomerantzeff camera [148°],7 Panoret [100°],8 and Retcam [130°]9). Unfortunately, all these instruments did not break into clinical practice due to several drawbacks, including need of mydriasis, technical skill by photographer, patient cooperation, limited resolution, need of clear media, and contact lens.
Commercialized for the first time in the year 2000, Optos ultra-wide-field camera (Optos, PLC, Scotland) is a cSLO device with a panoramic ellipsoid mirror, which allows the imaging of 82% of the retina (200°) in a single image with no need of mydriasis or contact lens. Compared to conventional digital acquisition systems, Optos showed a 2-fold increase of field of view.10 Furthermore, UWFA through Optos system visualizes a significantly wider total retinal surface compared to Heidelberg Spectralis ultra-wide-field lens.6
Almost 15 years from its introduction, Optos UWFA proved to be a useful tool in several retinal diseases, including DR. The aim of this narrative review was to provide an overview of the role of the most recent technique of UWFA in DR.
Methods
A PubMed engine search was carried out using the term “diabetic retinopathy” paired with “ultra wide field fluorescein angiography,” “ultra-wide field fluorescein angiography,” “ultra wide-field fluorescein angiography,” “ultra-wide-field fluorescein angiography,” “ultrawide field fluorescein angiography,” “ultra widefield fluorescein angiography,” and “ultrawide angiography.” All studies published in English up to July 2016 irrespective of their publication status were reviewed, and relevant publications were included in this review.
UWFA in DR
In 2008, Friberg et al10 were the first to report the feasibility of UWFA in 30 eyes of 30 patients affected by DR. Compared to standard systems, they observed that UWFA allowed to image a greater area of both retinal surface (8.7±1.6 vs 3.4±0.76 disc diameter [DD], P<0.001) and retinal ischemia (16.9±15 vs 3.4±4.26 sectors, P<0.05), albeit with a reduction in image quality.
In a retrospective case series including 218 eyes of 118 DR patients, Wessel et al2 compared UWFA to a simulated 7SF. UWFA disclosed 3.2 times more total retinal surface, 3.9 times more nonperfusion, 1.9 times more NV, and 3.8 times more laser panretinal photocoagulation (PRP)-treated area. Notably, UWFA revealed retinal pathology in 10% of the eye judged normal with 7SF. The study by Wessel et al2 implies that UWFA may change the degree of DR by revealing more retinal pathology and it may even lead to the diagnosis of DR in patients judged normal using standard angiograms.
Since capillary nonperfusion upregulates pro-angiogenic and pro-inflammatory factors (eg, vascular endothelial growth factor [VEGF], insulin growth factor, angiopoietin 1 and 2, fibroblast growth factor 2, tumor necrosis factor α, interleukin 1 [IL-1], IL-8, monocyte chemoattractant protein 1) thought a hypoxia-induced mechanism, it has been postulated that peripheral ischemia can lead to NV and diabetic macular edema (DME).11,12 In a retrospective case series by Oliver and Schwartz13 involving 218 eyes of 118 patients, peripheral nonperfusion was significantly linked to both anterior and posterior NV, conversely no significant association with DME was found. In the same study, the authors described a novel angiographic features of DR, termed peripheral vessel leakage (PVL), that is the late leakage from retinal vessels seen as hyperfluorescence extending beyond vessel wall occurring in the setting of active retinopathy. PVL has been linked to peripheral nonperfusion and NV, but not to DME. However, the association between DME and peripheral ischemia was supported by other studies where capillary nonperfusion was assessed through a quantitative approach, generating a percentage of ischemia over the total retina area called ischemic index (ISI).
To estimate ISI, pixels contained in nonperfused areas are calculated and divided by the number of pixels of the whole retina surface. Since Optos relies on a parabolic mirror and converts a 3 dimensional image to a 2 dimensional image, it induces peripheral distortion, and this has an impact on ISI calculation; in order to minimize this phenomenon and calculate the precise area of nonperfusion, Tan et al14 proposed a corrected ISI expressed in mm2 based on stereographic projection software, which strongly correlated with the uncorrected index.
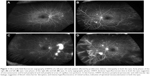
Wessel et al15 retrospectively reviewed 122 eyes of 70 naïve patients and found a positive correlation between ISI and DME. Interestingly, peripheral ischemia turned out to be an independent risk factor for DME development. In a retrospective case series involving 148 eyes of 76 patients, Patel et al16 found recalcitrant DME to be worse in patients with higher DR severity and ISI. Correlation among capillary nonperfusion, PVL, macular leakage, and epiretinal NVs is shown in Figure 1.
Sim et al17 retrospectively investigated the relationship between peripheral and diabetic macular ischemia (DMI) quantified by means of ISI and foveal avascular zone area, respectively. They found a positive correlation between these 2 variables, indicating how both the conditions share a common pathogenesis, that is, capillary nonperfusion.
In a retrospective case–control study, Kim et al18 observed that peripheral nonperfusion, together with NV and PVL, had higher incidence in eyes with recurrent post-vitrectomy diabetic vitreous hemorrhage (PVDVH) compared to non-PVDVH ones. Once again, such difference was not appreciated with 7SF. Moreover, peripheral ischemia turned out to be associated with DR severity and predominantly peripheral lesions, defined as >50% of a specific DR lesions outside the 7SF.
Although UWFA has been extensively studied as a diagnostic tool, it may also have a role in DR treatment. Since PRP has been associated with several side effects (ie, visual acuity reduction, visual field constriction, DME onset/worsening, choroidal detachment, angle-closure glaucoma, and decrease in color vision), targeted retinal photocoagulation (TRP) of ischemic areas has been proposed. Reddy et al19 reported 2 cases of TRP with NV regression and no PRP-related side effects, and this observation was further corroborated by a prospective study by Muqit et al.20 In a pilot randomized study comparing TRP, minimally traumatic (MT) PRP and standard intensity (SI) PRP, Muqit et al21 demonstrated that TRP was as effective as SI-PRP in inducing NV regression, but with higher reduction in CMT. Table 1 summarizes most relevant studies in the field.
Future directions
UWFA is a fascinating tool extremely useful in the diagnosis, staging, management, and therapy of DR. The ability of UWFA to show more retinal pathology even in eyes judged normal with 7SF is stimulating. Since most of the present knowledge comes from clinical trials based on 7SF protocol, those results need to be reconsidered in view of UWFA revolution, as simple transposition of prior information could be misleading. At the state of the art, most of the studies involving UWFA in DR lacks high quality features, including being prospective, randomized, with large sample, and long-term follow-up. The Diabetic Retinopathy Clinical Research Network (DRCRnet) protocol AA is currently investigating whether evaluation of retinal far periphery on UWFA improves the ability to assess DR and predicts rates of DR worsening over time compared to 7SF.
The possibility to precisely quantify the peripheral ischemia (ie, ISI) is extremely appealing especially for research purposes. ISI has already been correlated with PVL, DME, DMI, and DR severity; however, little is known about the variation of ISI after intravitreal (IV) injections. Mir et al22 reported a reduction of retinal nonperfusion in patients with retinal vein occlusion undergoing ranibizumab IV injections. However, these observations are based on qualitative assessment; moreover, so far, such evaluation has not been performed in eyes with DR. The impact of anti-VEGF agents and steroids on peripheral ischemia in DR has to be still determined. In a pilot study23 this group of authors showed how dexamethasone sustained release implant reduces capillary nonperfusion in DR. Despite the small sample size, this study opens a new field in retinal imaging and evaluation of the effect of IV drugs on 1) severity and progression of DR and 2) peripheral retinal vessels and their correlation to macular status. Quantitative assessment of retinal ischemia could also be useful in the clinical setting, for example, to further classify patients with severe nonproliferative DR based on the risk of developing NV given a certain ISI score. Technological improvements will further refine UWFA. Through the photomontage of UWFA images acquired in different steered positions, it is now possible to evaluate almost the entire retinal vascular up to the ora serrata, as proposed by Singer et al.24 It seems that single image can underestimate the real peripheral ischemia, especially in case of DR.25 Algorithms to correct the image distortion created by UWFA are very useful in order to rectify ISI values.26 Although really fascinating, the role of TRP needs to be clarified in large, prospective, randomized trial with long follow-up against PRP.
Conclusion
UWFA is going to revolutionize DR on multiple aspects. Several interesting observations have been pointed out by recent studies; however, further high-quality trials are warranted in order to confirm the prior investigations and to translate them into the everyday clinical practice.
Disclosure
Giuseppe Querques has the following disclosures: Allergan (financial support), Alimera (financial support), Bayer (financial support), Alcon (consultant), Allergan (consultant), Alimera (consultant), Bausch and Lomb (consultant), Novartis (consultant), Bayer (consultant), Ophthotech (consultant). Francesco Bandello has the following disclosures: Allergan (financial support), Alimera (financial support), Bayer (financial support), Farmila-Thea (financial support), Schering Pharma (financial support), Sanofi-Aventis (financial support), Novagali (financial support), Pharma (financial support), Hoffmann-LA Roche (financial support), Genetech (financial support), Novartis (financial support). The other authors report no conflicts of interest in this work.
References
Novotny HR, Alvis DL. A method of photographing fluorescence in circulating blood in the human retina. Circulation. 1961;24:82–86. | ||
Wessel MM, Aaker GD, Parlitsis G, Cho M, D’Amico DJ, Kiss S. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32(4):785–791. | ||
Soliman AZ, Silva PS, Aiello LP, Sun JK. Ultra-wide field retinal imaging in detection, classification, and management of diabetic retinopathy. Semin Ophthalmol. 2012;27(5–6):221–227. | ||
Ghasemi Falavarjani K, Wang K, Khadamy J, Sadda SR. Ultra-wide-field imaging in diabetic retinopathy; an overview. J Curr Ophthalmol. 2016;28(2):57–60. | ||
Staurenghi G, Viola F, Mainster MA, Graham RD, Harrington PG. Scanning laser ophthalmoscopy and angiography with a wide-field contact lens system. Arch Ophthalmol. 2005;123(2):244–252. | ||
Witmer MT, Parlitsis G, Patel S, Kiss S. Comparison of ultra-widefield fluorescein angiography with the Heidelberg Spectralis((R)) noncontact ultra-widefield module versus the Optos((R)) Optomap((R)). Clin Ophthalmol. 2013;7:389–394. | ||
Pomerantzeff O. Equator-plus camera. Invest Ophthalmol. 1975;14(5):401–406. | ||
Shields CL, Materin M, Shields JA. Panoramic imaging of the ocular fundus. Arch Ophthalmol. 2003;121(11):1603–1607. | ||
Azad R, Chandra P, Khan MA, Darswal A. Role of intravenous fluorescein angiography in early detection and regression of retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2008;45(1):36–39. | ||
Friberg TR, Gupta A, Yu J, et al. Ultrawide angle fluorescein angiographic imaging: a comparison to conventional digital acquisition systems. Ophthalmic Surg Lasers Imaging. 2008;39(4):304–311. | ||
Capitao M, Soares R. Angiogenesis and inflammation crosstalk in diabetic retinopathy. J Cell Biochem. 2016;117(11):2443–2453. | ||
Owen LA, Hartnett ME. Soluble mediators of diabetic macular edema: the diagnostic role of aqueous VEGF and cytokine levels in diabetic macular edema. Curr Diab Rep. 2013;13(4):476–480. | ||
Oliver SC, Schwartz SD. Peripheral vessel leakage (PVL): a new angiographic finding in diabetic retinopathy identified with ultra wide-field fluorescein angiography. Semin Ophthalmol. 2010;25(1–2):27–33. | ||
Tan CS, Chew MC, van Hemert J, Singer MA, Bell D, Sadda SR. Measuring the precise area of peripheral retinal non-perfusion using ultra-widefield imaging and its correlation with the ischaemic index. Br J Ophthalmol. 2016;100(2):235–239. | ||
Wessel MM, Nair N, Aaker GD, Ehrlich JR, D’Amico DJ, Kiss S. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96(5):694–698. | ||
Patel RD, Messner LV, Teitelbaum B, Michel KA, Hariprasad SM. Characterization of ischemic index using ultra-widefield fluorescein angiography in patients with focal and diffuse recalcitrant diabetic macular edema. Am J Ophthalmol. 2013;155(6):1038.e2–1044.e2. | ||
Sim DA, Keane PA, Rajendram R, et al. Patterns of peripheral retinal and central macula ischemia in diabetic retinopathy as evaluated by ultra-widefield fluorescein angiography. Am J Ophthalmol. 2014;158(1):144.e1–153.e1. | ||
Kim DY, Kim JG, Kim YJ, Joe SG, Lee JY. Ultra-widefield fluorescein angiographic findings in patients with recurrent vitreous hemorrhage after diabetic vitrectomy. Invest Ophthalmol Vis Sci. 2014;55(11):7040–7046. | ||
Reddy S, Hu A, Schwartz SD. Ultra wide field fluorescein angiography guided targeted retinal photocoagulation (TRP). Semin Ophthalmol. 2009;24(1):9–14. | ||
Muqit MM, Marcellino GR, Henson DB, et al. Optos-guided pattern scan laser (Pascal)-targeted retinal photocoagulation in proliferative diabetic retinopathy. Acta Ophthalmol. 2013;91(3):251–258. | ||
Muqit MM, Young LB, McKenzie R, et al. Pilot randomised clinical trial of Pascal TargETEd Retinal versus variable fluence PANretinal 20 ms laser in diabetic retinopathy: PETER PAN study. Br J Ophthalmol. 2013;97(2):220–227. | ||
Mir TA, Kherani S, Hafiz G, et al. Changes in retinal nonperfusion associated with suppression of vascular endothelial growth factor in retinal vein occlusion. Ophthalmology. 2016;123(3):625.e1–634.e1. | ||
Querques L, Parravano MC, Sacconi R, et al. Ischemic index changes in diabetic retinopathy after dexamethasone implant using ultra wide-field fluorescein angiography: a pilot study. Poster presented at: Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO); May 07; 2017; Baltimore, MD. | ||
Singer M, Sagong M, van Hemert J, Kuehlewein L, Bell D, Sadda SR. Ultra-widefield Imaging of the Peripheral Retinal Vasculature in Normal Subjects. Ophthalmology. 2016;123(5):1053–1059. | ||
Franco-Cardenas V, Shah SU, Apap D, et al. Assessment of ischemic index in retinal vascular diseases using ultra-wide-field fluorescein angiography: single versus summarized image. Semin Ophthalmol. 2016. | ||
Kim JH, Jung HG, Chung HJ, Lee K, Sohn J. Simplified correction of ischemic index in diabetic retinopathy evaluated by ultra-widefield fluorescein angiography. Korean J Ophthalmol. 2015;29(3):168–172. | ||
Silva PS, Dela Cruz AJ, Ledesma MG, et al. Diabetic Retinopathy Severity and Peripheral Lesions Are Associated with Nonperfusion on Ultrawide Field Angiography. Ophthalmology. 2015;122:2465–2472. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.