Back to Journals » OncoTargets and Therapy » Volume 12
Ultra-Low-Dose Bevacizumab For Cerebral Radiation Necrosis: A Prospective Phase II Clinical Study
Authors Zhuang H, Zhuang H, Shi S, Wang Y
Received 15 July 2019
Accepted for publication 9 September 2019
Published 11 October 2019 Volume 2019:12 Pages 8447—8453
DOI https://doi.org/10.2147/OTT.S223258
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Hongqing Zhuang,1 Hongxia Zhuang,2 Siyu Shi,3 Yuxia Wang1
1Department of Radiation Oncology, Peking University Third Hospital, Beijing, People’s Republic of China; 2Department of Hematology, Weifang People’s Hospital, Weifang, Shandong Province, People’s Republic of China; 3Stanford University School of Medicine, Stanford, CA 94305, USA
Correspondence: Hongqing Zhuang
Peking University Third Hospital, 49 North Garden Road, Haidian District, Beijing 100191, People’s Republic of China
Tel +86 10 8226 6699
Email [email protected]
Objective: To investigate the treatment efficacy of ultra-low-dose bevacizumab for cerebral radiation necrosis.
Methods: Patients with cerebral radiation necrosis after stereotactic radiotherapy (SRT) confirmed by imaging were included. Bevacizumab (1 mg/kg, once every three weeks, for at least three continuous treatments) was administered. The primary endpoints included change in cerebral necrosis symptoms, volume of intracranial edema, and changes in MRI signals. The secondary endpoints were adverse reactions of bevacizumab treatment.
Results: In total, 21 patients were included in this study, all of whom received SRT between December 2016 and February 2019, developed cerebral radiation necrosis, and were treated with bevacizumab. Twenty patients were symptomatic from radiation necrosis, and the symptoms were alleviated in 18 patients (90%). Twenty patients had intracranial edema, and the grade of edema index (EI) was improved in 19 patients (95%). The intensity of the intracranial-enhanced MRI signals was significantly reduced in 20 patients (95.24%). The adverse reactions of bevacizumab treatment were mild, and no adverse reactions more severe than grade 2 were found.
Conclusion: The preliminary results showed that ultra-low-dose bevacizumab had high efficacy for treating cerebral radiation necrosis, and could be a valid alternative to the standard-dose bevacizumab.
Clinical registry: Chinese clinical trial registry (ChiCTR-IOD-16009803).
Keywords: bevacizumab, cerebral radiation necrosis, stereotactic radiotherapy, drug dose, edema index
Introduction
Cerebral radiation necrosis is a common complication from stereotactic radiotherapy (SRT) for intracranial tumors.1–3 However, no effective treatments for this disorder are available to date. One of the major causes of cerebral radiation necrosis is vascular changes.4,5 As an important anti-angiogenic drug, bevacizumab could potentially treat cerebral radiation necrosis. Bevacizumab (Roche, Switzerland) is a humanized monoclonal immunoglobulin G antibody that is 93% human and 7% murine in protein sequence. Bevacizumab maintains the high specificity and affinity of the parental antibody for VEGF-A with reduced immunogenicity and a longer half-life. Bevacizumab inhibits endothelial-cell mitogenic activity, vascular permeability-enhancing activity, and other angiogenesis-promoting biologic functions of VEGF. Neutralization of VEGF has 2 cytostatic effects on tumor biology: prevention of neovasculature formation by limiting the blood supply and “normalization” or pruning of immature and abnormal blood vessels.6,7 However, the doses of bevacizumab were mainly decided as anti-tumor therapy in previous studies, while no study has investigated whether these doses were suitable for the treatment of cerebral radiation necrosis. This preliminary clinical study explored the efficacy of ultra-low-dose bevacizumab for the treatment of cerebral radiation necrosis following SRT, which could provide evidence for optimizing doses of bevacizumab for treating cerebral radiation necrosis.
Patients And Methods
Patients
This study was prospectively approved and supervised by the Peking University Third Hospital Ethics Committee. All participants signed informed consent. The inclusion criteria of the patients were: (1) patient had primary or secondary intracranial lesions; (2) the intracranial lesions were treated with SRT; (3) the cerebral radiation necrosis after SRT was diagnosed by imaging; (4) patient did not have a history of anti-vascular medication. Patients with contradictions for bevacizumab treatment, such as history or risk for bleeding, were excluded from the study. In total, 21 patients with cerebral radiation necrosis after SRT for primary or secondary intracranial tumors were included between December 2016 and February 2019. Patient characteristics are described in Table 1.
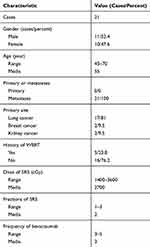 |
Table 1 Baseline Of Patient And Treatment Characteristics |
Diagnostic Criteria For Cerebral Radiation Necrosis
Pathological examination is the gold standard for diagnosis of cerebral radiation necrosis. However, this method has several issues in clinical practice: (1) many intracranial tumors are close to the skull base or other important functional regions, and thus surgical resection or stereotactic biopsy and subsequent pathological examination cannot be conducted; (2) very few patients consent to biopsy following SRT; and (3) the stereotactic biopsy does not necessarily reflect the overall pathological changes of the entire tissue. In addition, it is almost impossible to conduct the craniotomy if cerebral necrosis is suspected in patients receiving palliative treatments for multiple intracranial metastases. Furthermore, craniotomy in such patients also violates the goals of SRT treatment, which is to increase survival and improve life quality. Therefore, pathological examination, the gold standard for diagnosis of cerebral radiation necrosis, is difficult to conduct in clinical practice. Instead, comprehensive imaging examination is practical and also the most commonly used method for diagnosing cerebral radiation necrosis in clinical practice.7–9 In this study, cerebral radiation necrosis was diagnosed based on a comprehensive analysis of medical history, symptoms, signs, MRI findings, spectrum analysis results, and PET-CT findings.10–12 MRI scanning or spectrum analysis was initially conducted for all patients, and PET-CT scanning was performed for those cases that could not be diagnosed by MRI alone.10,13–18 Each case cerebral radiation necrosis was diagnosed by three physicians independently according to the imaging findings.
Bevacizumab Treatment
Bevacizumab, at a dose of 1 mg/kg body weight,6,7 was used to treat cerebral radiation necrosis. The regimen included three cycles of treatment, with one infusion every three weeks. Antiallergic treatment using diphenhydramine and low-dose glucocorticoids was used for the patients before the first bevacizumab treatment (In the first application of bevacizumab, in order to avoid allergic reactions to monoclonal antibodies, diphenhydramine and low-dose hormones were used in the initial application.). The time of bevacizumab administration was >90 mins in the first treatment, and shortened to >60 mins in the following treatments. Electrocardiogram monitoring was conducted for the patients, and the adverse reactions were recorded during the treatment.
Efficacy Assessment
Intracranial MRI scanning was routinely conducted at one month after the three cycles of bevacizumab treatment were completed. Afterwards, re-examinations were conducted every 2–3 months within one year, and then as per the conditions of the patients, at intervals ≤6 months. For patients with intracranial symptoms, immediate re-examination was conducted. The changes in the symptoms after bevacizumab treatment were assessed according to the CTCAE4.0 criteria.19 Edema area was measured on FRFSE-T2WI image, and the volume was calculated by edema area marked on MRI images × layer thickness. EI, which was calculated by the equation of EI = volume of (edema + necrosis)/volume of necrosis, was used for the assessment. Changes in the signals of cerebral necrosis area were measured in the enhanced T1 phase. The signal values in the enhanced area of cerebral necrosis were measured, and the mean value was calculated, which was compared with the signals of the white matter on the same MRI image to minimize the influences of different enhancements. This value was used for assessing the changes in the signals of cerebral necrosis area before and after treatment.
Statistical Analysis
SPSS 17.0 software was used for statistical analysis. Student’s t-test was used for the analysis of the changes in symptoms, EI, and signals in cerebral necrosis area after bevacizumab treatment. P <0.05 was considered statistically significant.
Results
Adverse Reactions Of Bevacizumab Treatment
The median follow-up was 22.7 months (range 6.1–38.4 months). The median time to onset of cerebral radiation necrosis from radiotherapy was 17.6 months (range 8.2–32.4 months). Among the 21 patients, mild allergy was found in one patient. Grade 1 blood pressure increase was found in one patient, which resolved spontaneously. The overall incidence of adverse reactions was 9.52% (2/21). No adverse reaction of grade 2 or more was found among the patients. No other adverse reactions of bevacizumab treatment, such as skin rash, weakness, proteinuria, thromboembolism, bleeding, gastrointestinal perforation, delayed wound healing, reversible posterior leukoencephalopathy syndrome, and congestive heart failure, were found.
Changes In Radiation Necrosis Symptoms
Among the 21 patients, 20 had headache, dizziness, nausea, vomiting, mental disorders (such as memory loss), and visual impairment (such as visual field defects) before bevacizumab treatment. However, severity of the symptoms decreased after bevacizumab treatment in 18 patients (90%) (t = 5.657, p < 0.001). The detailed data are shown in Table 2.
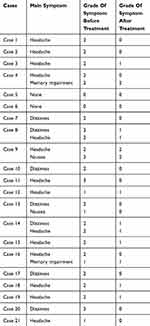 |
Table 2 The Symptom Changes Of The Patients Before And After Treatment (CTC4.0) |
Changes In Edema Severity
EI was calculated according to the edema severity of the patients, and the changes were compared before and after the treatment. The results showed that among the 20 patients with edema, EI in 19 patients (95%) significantly improved after bevacizumab treatment (t = 3.8, p= 0.002) (Figure 1).
 |
Figure 1 The EI of the patient before and after Bev treatment. |
Changes In MRI Signals Of Cerebral Necrosis Area
The relative intensity on the enhanced intracranial T1 phase MRI images was measured to assess the changes of blood flow in the cerebral necrosis lesions after bevacizumab treatment. The results showed that the intensity of the enhanced intracranial MRI signals in 20 patients (95.24%) decreased significantly after bevacizumab treatment (t = 5.9, p = 0.001) (Figure 2).
 |
Figure 2 The MRI signals of the patient before and after Bev treatment. |
Discussion
The findings of this preliminary study showed that ultra-low-dose (1 mg/kg body weight) bevacizumab had high efficacy in treating cerebral radiation necrosis.
The mechanisms involved in the treatment of cerebral radiation necrosis by bevacizumab could be discussed from two aspects, namely the pathogenesis of cerebral radiation necrosis and anti-angiogenic effects of bevacizumab. Blood vessel damage plays an important role in the pathogenesis of cerebral radiation necrosis.20 The irradiation on blood vessels induces fibrinoid degeneration of vascular endothelial cells, which in turn induces hypoxia and necrosis. Cytokines (such as VEGF) that affect blood vessels are over-expressed during the process, which gradually induce blood-brain barrier dysfunction and encephaledema, and consequently affect the functions of neurons.21 In addition, irradiation also damages astrocytes to further induce release of VEGF, and thereby worsens blood-brain barrier dysfunction and encephaledema. Bevacizumab binds VEGF and inhibits its action on blood vessels, reducing the permeability of the capillaries, decreasing extracellular release of inflammatory factors in plasma through endothelial cells of capillaries, and alleviating blood-brain barrier damages and encephaledema. Therefore, bevacizumab can be an effective method for the treatment of cerebral radiation necrosis. These mechanisms have already been established based on the findings of increased VEGF expression in animal models of cerebral radiation necrosis. In addition, the treatment efficacy of bevacizumab is mainly associated with the duration of the anti-angiogenic effects, but not the dose of bevacizumab. Further, in the previous case report, there was a report of using 3mg/kg,22 which showed preliminary evidence that dose lower than the initial dose (5 or 7.5mg/kg) can still be effective. Therefore, the underlying mechanisms of cerebral radiation necrosis and the dose-independent anti-angiogenic effects of bevacizumab23,24 provide the theoretical basis of this study on ultra-low dose of bevacizumab as an effective treatment for cerebral radiation necrosis.
In this study, brain radiation necrosis diagnosis was based mainly on imaging. The gold standard for the diagnosis of radiation cerebral necrosis is pathological diagnosis; however, there have been many issues in clinical practice.13,14 First, the locations of many intracranial tumors in stereotactic radiotherapy were close to the cranial base or in important function areas; therefore, surgical resection or stereotactic puncture could not be performed to obtain pathological diagnosis. Next, patients had very low intention for puncture after stereotactic radiotherapy. In addition, even if the stereotactic puncture was performed, the results might not completely represent the overall tissue pathology. For patients who received palliative treatment after multiple intracranial metastases, it was almost impossible to persuade patients to undergo craniotomy to confirm cerebral necrosis; furthermore, craniotomy in patients receiving palliative treatment for brain metastasis contradicted the clinical treatment purpose of prolonging survival and increasing quality of life. Therefore, although pathological diagnosis after surgery is the gold standard for radiation cerebral necrosis, it could not be achieved in clinical works. Thus, comprehensive imaging measures are the most practical and have been the most commonly applied diagnosis methods in our clinical works. Therefore comprehensive imaging is the most realistic and most frequently used method in the diagnosis of brain radiation necrosis.
Previous studies used 5–7.5 mg/kg of bevacizumab for the treatment of cerebral radiation necrosis.25–27 These doses were decided according to the doses used for the treatment of tumors, which have limited reference value for the treatment of cerebral radiation necrosis. In addition, such high doses also increase the financial burden on patients. There are very limited studies and case reports on the efficacy of low-dose bevacizumab on cerebral radiation necrosis, but further research is warranted.28,29 The treatment efficacy of ultra-low-dose bevacizumab in this study provides a potential alternative for the effective treatment of radiation necrosis in clinical practice, as well as new evidence for differing doses of bevacizumab based on clinical application.
Conclusion
In summary, the preliminary results showed that ultra-low-dose bevacizumab was the efficacy for treating cerebral radiation necrosis with acceptable side effects, and could be a valid alternative to the standard-dose bevacizumab. Other study with higher number of patients and longer follow-up are necessary to confirm this finding.
Abbreviations
CRN, cerebral radiation necrosis; BED, biological effective dose; NSCLC, non-small-cell lung cancer; MRI, magnetic resonance imaging; CTC, common toxicity criteria.
Copyright/Ethics
The study was approved and supervised by Tianjin Medical University Cancer Institute and Hospital and Peking University Third Hospital ethics committee, and this trial was conducted in accordance with the Declaration of Helsinki.
Data Sharing Statement
Because the clinical trial is not finished yet, the authors did not intend to share individual deidentified participant data now. After the clinical trial was completed and with the final data, it will be made available. Please contact the corresponding author for data requests.
Acknowledgment
The authors thank the groups of the Peking University Third Hospital Cyberknife Center.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This Paper was supported by Clinical Key Project of Peking University Third Hospital (BYSY2017030).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Du Four S, Hong A, Chan M, et al. Symptomatic histologically proven necrosis of brain following stereotactic radiation and ipilimumab in six lesions in four melanoma patients. Case Rep Oncol Med. 2014;2014:417913.
2. Rogers LR. Neurologic complications of radiation. Continuum (Minneap Minn). 2012;18(2):343–354. doi:10.1212/01.CON.0000413662.35174.a8
3. Lacy J, Saadati H, Yu JB. Complications of brain tumors and their treatment. Hematol Oncol Clin North Am. 2012;26(4):779–796. doi:10.1016/j.hoc.2012.04.007
4. Rinne ML, Lee EQ, Wen PY. Central nervous system complications of cancer therapy. J Support Oncol. 2012;10(4):133–141. doi:10.1016/j.suponc.2011.11.002
5. Gallet P, Phulpin B, Merlin JL, et al. Longterm alterations of cytokines and growth factors expression in irradiated tissues and relation with histological severity scoring. PLoS One. 2011;6(12):e29399. doi:10.1371/journal.pone.0029399
6. Schüttrumpf LH, Niyazi M, Nachbichler SB, et al. Prognostic factors for survival and radiation necrosis after stereotactic radiosurgery alone or in combination with whole brain radiation therapy for 1–3 cerebral metastases. Radiat Oncol. 2014;9:105. doi:10.1186/1748-717X-9-105
7. Lubelski D, Abdullah KG, Weil RJ, Marko NF. Bevacizumab for radiation necrosis following treatment of high grade glioma: a systematic review of the literature. J Neurooncol. 2013;115(3):317–322. doi:10.1007/s11060-013-1233-0
8. Matuschek C, Bölke E, Nawatny J, et al. Bevacizumab as a treatment option for radiation-induced cerebral necrosis. Strahlenther Onkol. 2011;187(2):135–139. doi:10.1007/s00066-010-2184-4
9. Wang Y, Wang E, Pan L, et al. A new strategy of CyberKnife treatment system based radiosurgery followed by early use of adjuvant bevacizumab treatment for brain metastasis with extensive cerebral edema. J Neurooncol. 2014;119(2):369–376. doi:10.1007/s11060-014-1488-0
10. Kickingereder P, Dorn F, Blau T, et al. Differentiation of local tumor recurrence from radiation-induced changes after stereotactic radiosurgery for treatment of brain metastasis: case report and review of the literature. Radiat Oncol. 2013;8:52. doi:10.1186/1748-717X-8-52
11. Parvez K, Parvez A, Zadeh G. The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int J Mol Sci. 2014;15(7):11832–11846. doi:10.3390/ijms150711832
12. Pallavi T, Prateek P, Lisa R, et al. Texture descriptors to distinguish radiation necrosis from recurrent brain tumors on multi-parametric. MRI Proc SPIE. 2014;9035:90352B.
13. Shah R, Vattoth S, Jacob R, et al. Radiation necrosis in the brain: imaging features and differentiation from tumor recurrence. Radiographics. 2012;32:1343–1359. doi:10.1148/rg.325125002
14. Stockham AL, Ahluwalia M, Reddy CA, et al. Results of a questionnaire regarding practice patterns for the diagnosis and treatment of intracranial radiation necrosis after SRS. J Neurooncol. 2013;115:469–475. doi:10.1007/s11060-013-1248-6
15. Chernov MF, Hayashi M, Izawa M, et al. Multivoxel proton MRS for differentiation of radiation-induced necrosis and tumor recurrence after gamma knife radiosurgery for brain metastases. Brain Tumor Pathol. 2006;23:19–27. doi:10.1007/s10014-006-0194-9
16. Kang TW, Kim ST, Byun HS, et al. Morphological and functional MRI, MRS, perfusion and diffusion changes after radiosurgery of brain metastasis. Eur J Radiol. 2009;72:370–380. doi:10.1016/j.ejrad.2008.08.009
17. Reddy K, Westerly D, Chen C. MRI patterns of T1 enhancing radiation necrosis versus tumour recurrence in high-grade gliomas. J Med Imaging Radiat Oncol. 2013;57:349–355. doi:10.1111/j.1754-9485.2012.02472.x
18. Ozsunar Y, Mullins ME, Kwong K, et al. Glioma recurrence versus radiation necrosis? A pilot comparison of arterial spin-labeled, dynamic susceptibility contrast enhanced MRI, and FDG-PET imaging. Acad Radiol. 2010;17:282–290. doi:10.1016/j.acra.2009.10.024
19. Chen AP, Setser A, Anadkat MJ, et al. Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol. 2012;67(5):1025–1039. doi:10.1016/j.jaad.2012.02.010
20. Jiang X, Ding M, Qiao Y, Liu Y, Liu L. Recombinant human endostatin combined with radiotherapy in the treatment of brain metastases of non-smallcell lung cancer. Clin Transl Oncol. 2014;16(7):630–636. doi:10.1007/s12094-013-1129-7
21. Coderre JA, Morris GM, Micca PL, et al. Late effects of radiation on the central nervous system: role of vascular endothelial damage and glial stem cellsurvival. Radiat Res. 2006;166(3):495–503. doi:10.1667/RR3597.1
22. Alessandretti M, Buzaid AC, Brandão R, Brandão EP. Low-dose bevacizumab is effective in radiation-induced necrosis. Case Rep Oncol. 2013;6(3):598–601. doi:10.1159/000357401
23. Williams KJ1, Telfer BA, Shannon AM, Babur M, Stratford IJ, Wedge SR. Inhibition of vascular endothelial growth factor signalling using cediranib (RECENTIN; AZD2171) enhancesradiation response and causes substantial physiological changes in lung tumour xenografts. Br J Radiol. 2008;81:S21–S27. doi:10.1259/bjr/59853976
24. Gonzalez J1, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67(2):323–326. doi:10.1016/j.ijrobp.2006.10.010
25. Kuffler DP. Hyperbaric oxygen therapy: can it prevent irradiation-induced necrosis? Exp Neurol. 2012;235(2):517–527. doi:10.1016/j.expneurol.2012.03.011
26. Rahmathulla G, Marko NF, Weil RJ. Cerebral radiation necrosis: a review of the pathobiology, diagnosis and management considerations. J Clin Neurosci. 2013;20(4):485–502. doi:10.1016/j.jocn.2012.09.011
27. Chao ST, Ahluwalia MS, Barnett GH, et al. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys. 2013;87(3):449–457. doi:10.1016/j.ijrobp.2013.05.015
28. Delishaj D, Ursino S, Pasqualetti F, et al. The effectiveness of bevacizumab in radionecrosis after radiosurgery of a single brain metastasis. Clin Med Res. 2017;9(4):273–280. doi:10.14740/jocmr2936e
29. Delishaj D, Ursino S, Pasqualetti F, et al. The effectiveness of bevacizumab in radionecrosis after radiosurgery of a single brain metastasis. Rare Tumors. 2015;7(4):6018. doi:10.4081/rt.2015.6018
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
