Back to Journals » Cancer Management and Research » Volume 11
Type 2 diabetes mellitus increases the risk of hepatocellular carcinoma in subjects with chronic hepatitis B virus infection: a meta-analysis and systematic review
Authors Tan Y, Wei S, Zhang W, Yang J, Yang J, Yan L
Received 20 September 2018
Accepted for publication 21 December 2018
Published 14 January 2019 Volume 2019:11 Pages 705—713
DOI https://doi.org/10.2147/CMAR.S188238
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Rituraj Purohit
Yifei Tan,1 Shiyou Wei,2 Wei Zhang,1 Jian Yang,1 Jiayin Yang,1 Lunan Yan1
1Liver Transplantation Center, Department of Liver Surgery, West China Hospital of Sichuan University, Chengdu, Sichuan, China; 2Department of Thoracic Surgery, West China Hospital of Sichuan University, Chengdu, Sichuan, China
Background: Type 2 diabetes mellitus has been proved to be a risk factor of hepatocellular carcinoma, but how diabetes affects incidence of hepatocellular carcinoma among patients with chronic hepatitis B virus infection remains controversial.
Methods: A comprehensive search of Medline and Embase was performed. Incidence of hepatocellular carcinoma in chronic hepatitis B patients was the primary outcome. Pooled HRs and 95% CIs were calculated to assess the correlation between diabetes and incidence of hepatocellular carcinoma.
Results: Five cohort studies and two case–control studies were identified, with a total of 21,842 chronic hepatitis B patients. The diabetes mellitus cohort was found to have increased incidence of hepatocellular carcinoma (pooled HR 1.77, 95% CI 1.28–2.47; fixed effect) and worse overall mortality (pooled RR 1.93, 95% CI 1.64–2.27; fixed effect) in comparison with those without diabetes. In case–control studies, hepatocellular carcinoma cases were found to have an insignificantly elevated diabetes mellitus rate in comparison with the control group.
Conclusion: Type 2 diabetes mellitus is significantly associated with increased risk of hepatocellular carcinoma among patients with chronic hepatitis B virus infection, and aggressive management of diabetes mellitus is strongly suggested.
Keywords: type 2 diabetes mellitus, hepatocellular carcinoma risk, HBV-infected
Introduction
Hepatocellular carcinoma (HCC) is the fifth-most common cancer worldwide, leads to nearly 1 million deaths every year,1 and is the third-most frequent cause of cancer-related death. The incidence of HCC is particularly high in Asia (over 20 in 100,000 men and over ten in 100,000 women) and in Africa, intermediate in southern Europe, and much lower in most developed countries.2 Hepatitis virus infection, mainly hepatitis B virus (HBV) and hepatitis C virus (HCV), has been widely accepted as the major recognized risk factor of HCC globally, accounting for over three-quarters of primary HCC cases.2,3 However, when HBV or HCV is not involved, the etiologic factor of HCC varies, of which diabetes mellitus (DM),4 heavy alcohol drinking,5 smoking,6 obesity,7 and aflatoxin8 are relatively important.
DM, which has been proved to be a risk factor of various kinds of malignancies, is strongly associated with nonalcoholic fatty-liver disease and many other metabolic processes.9 Insulin resistance10 was believed to play an important role in hepatocarcinogenesis in HBV patients with type 2 DM or even prediabetes.11 The association between DM and HCC risk was indicated to be independent of cirrhosis, though most HCC cases presented with cirrhosis.12 A recent systematic review demonstrated that concurrent DM is strongly associated with increased HCC risk among chronic HCV patients,13 but scanty evidence is available about the correlation between DM and HCC in chronic HBV (CHB) patients. The clinical landscape of HCV is currently facing a great change, such that its cure would be universal for patients for whomever has access to effective therapy, which will definitely result in a decrease in HCC developments. Therefore, HBV infection, alcohol consumption, and metabolic disorders, such as DM and obesity, are supposed to be the leading etiologic factors of HCC in the coming future. There are mixed results14–17 of the few studies on the association between DM and the risk of HCC in patients with CHB. As such, we performed this meta-analysis and systematic review of the literature to achieve further understanding of the impact of DM on the risk of developing HCC in patients with CHB.
Methods
Literature-search strategy
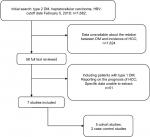
A comprehensive search of Medline and Embase was performed to retrieve studies published in English (cutoff date February 5, 2018) using the keywords “diabetes” or “diabetes mellitus, type 2” or “DM”, “hepatitis B” or “HBV”, and “hepatocellular carcinoma” or “HCC” or “liver cancer”. We also examined the reference lists of eligible studies to identify additional articles, in order to guarantee a systemic search. Figure 1 depicts the search strategy in detail.
Inclusion and exclusion criteria
Inclusion criteria from the literature search were: studies that focused on the relationship between DM and the risk of HCC: HCC incidence and/or related mortality as outcomes; results of HR/RR/OR and their corresponding 95% CIs for DM and incidence of HCC; and if two or more studies were reported on the same cohort and objectives, either the higher-quality publication or more recent publication was included in the analysis. Studies were excluded if they had not presented data on the relationship between DM and incidence of HCC in patients with HBV infection or specific results were unable to extract. Studies reporting on the effect of DM on the prognosis of HCC or where HCC was not the only outcome (eg, including cholangiocarcinoma) or including patients with type 1 DM were not considered. Reviews, case reports, letters, animal or in vitro studies, conference abstracts, and non-peer-reviewed articles were also excluded from the meta-analysis.
Assessment of quality of articles according to the Newcastle–Ottawa Scale, and data extraction were independently performed by YT and SW, as well as the literature search. Quality assessment of both cohort studies and case–control studies included three main characteristics: selection of groups, comparability of cohorts/cases and controls, and outcome/exposure. Studies with seven or more scores were defined as highly qualified.
Data extraction
Incidence of HCC was the main outcome assessed in this meta-analysis, while overall mortality or HCC-related mortality was the secondary main outcome. HR, RR, and OR values with their corresponding 95% CIs were extracted from each study. Other information included (but was not limited to) study design, population characteristics, source of cases and controls, ascertainment of type 2 DM, treatment for HBV infection, anti-HCV status, variables adjusted for in the multivariate regression models, and the measure of association between DM and HCC incidence.
Statistical analysis and exploration of heterogeneity
We used pooled HRs for primary outcomes to assess the relationship between type 2 DM and incidence of HCC in HBV patients. RRs and weighted mean differences, both with corresponding 95% CIs, were computed for binary data and continuous data, respectively. Funnel plots were visually inspected to identify publication bias. Sensitivity analysis was conducted by removing a single study each time and recalculating pooled results of the remaining studies. The χ2 test was used to explore potential heterogeneity with I2 and P-values. I2>50% or P<0.10 was defined as increased heterogeneity and a random-effect model18 used, while a fixed model was used when P>0.10. Results are presented as P-values and 95% CIs, where appropriate, and two-sided P<0.05 was considered to indicate statistical significance. Meta-analyses were performed using RevMan software (version 5.3.5; Cochrane Collaboration, Copenhagen). Quality of evidence was evaluated using the software GradePro, comprising four levels: high quality, moderate quality, low quality, and very low quality.
Results
The online search initially found 1,682 studies, and 1,624 were excluded after screening of titles and abstracts. A total of 58 full-text articles were reviewed. Finally, two case–control studies17,19 and five cohort studies14–16,20,21 were identified, of which six were conducted in Asia14–17,19,20 and one in New Zealand.21 In all cohort studies, patients diagnosed with HCC before the inception point were excluded. The number of eligible CHB patients ranged from 223 to 6,545, with a total of 21,842. Diagnosis of type 2 DM was obtained from patient self-report, abnormal fasting/random glucose, positive oral glucose tolerance test, and DM management (oral hyperglycemic agent or insulin injection). HCC cases were confirmed with the combination of increased AFP and imaging findings (ultrasound, enhanced computed tomography, or angiography) in five studies,14,15,17,19,21 and positive histology or cytology was also used to define HCC in the two case–control studies.17,19 Two cohort studies16,20 in Taiwan defined HCC cases according to the national cancer registry alone (Table 1). Only Hsiang et al21 reported details of dropouts and withdrawals. In their study, seven patients were lost to follow-up or moved overseas, resulting in a loss to or unavailability for follow-up rate of 3%. Other studies, however, did not provided data about details of follow up.
No statistical differences were found between subjects with and without type 2 DM for average age (mean difference 2.81, 95% CI –2.91 to 8.52), male sex (RR 0.99, 95% CI 0.91–1.08), years of follow-up (mean difference –0.35, 95% CI –1.02 to 0.32), or HBV-treatment rate (RR 1.07, 95% CI 0.73–1.55).
Type 2 DM and risk of HCC in CHB subjects
Of the seven studies included in this meta-analysis, three cohort studies15,20,21 and one case–control study17 demonstrated a positive association between type 2 DM and risk of HCC in CHB patients, while other two cohort studies14,16 and one case–control study19 failed to find a statistical difference between the two groups. Four in seven studies reported correlations between DM and HCC with HRs,14,16,20,21 two with ORs,17,19 and another with RRs,15 all with corresponding 95% CIs (Table 2). All studies had different variables adjusted in multivariate regression analysis (Table 3).
  | Table 2 DM and incidence of HCC risk Abbreviations: DM, diabetes mellitus; HCC, hepatocellular carcinoma; NDM, non-DM; NA, not applicable. |
  | Table 3 Factors adjusted in the multivariate regression analysis Abbreviations: HCV, hepatitis C Virus; BMI, body-mass index, MELD, model for end-stage liver disease. |
The incidence of HCC in DM cohorts varied from 3.29% to 26.0% in cohort studies, while the rate was 2.02%–13.3% in the non-DM group. The four cohort studies reporting on HR14,16,20,21 found increased incidence of HCC among patients with DM over those without DM among CHB patients (Figure 2), resulting in a pooled HR of 1.77 (95%CI 1.28–2.47, heterogeneity I2=0; fixed effect). In case–control studies,17,19 an elevated DM rate was indicated in HCC cases in comparison with control groups (12.35% vs 6.53%), but not statistically, with an RR of 2.10 (95% CI 0.84–5.25; random-effect). Among the three studies reporting RRs or ORs, Chen et al15 and Ko et al17 also found significant associations between type 2 DM and risk of HCC in HBV subjects, with an RR of 2.41 (95% CI 1.17–4.95) and an OR of 4.32 (95% CI 1.92–9.70), respectively, but Li et al19 found no significant relationships between DM and risk of HCC when comparing all HCC cases with cross-sectional controls, with an OR of 0.9 (95%CI 0.7–1.2). The publication bias of studies assessing the relation between DM and risk of HCC is shown in Figure 3.
  | Figure 2 Forest plot of meta-analysis results comparing the incidence of HCC between patients with DM and those without DM. Abbreviations: HCC, hepatocellular carcinoma; DM, diabetes mellitus. |
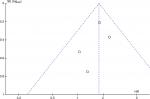  | Figure 3 Funnel plot of studies assessing the relationship between DM and risk of HCC. Abbreviations: DM, diabetes mellitus; HCC, hepatocellular carcinoma. |
Analysis of sensibility was carried out by excluding one article at a time to guarantee stability of the meta-analysis. As a result, pooled results remained statistically positive when any of the cohort studies was excluded from the meta-analysis. For example, when Wang et al was excluded, the pooled HR of remaining studies remained at 1.80 (95%CI 1.29–2.53). When it came to heterogeneity, I2 remained at 0 when any study was excluded.
DM, overall mortality, and HCC-related mortality
Two cohort studies20,21 reported overall mortality between two groups, revealing that subjects with type 2 DM suffered significantly higher overall mortality in comparison with those without DM (Figure 4), with a pooled RR of 1.93 (95% CI 1.64–2.27, I2=18%; fixed effect). Only Hsiang et al21 reported on HCC-related mortality in patients with CHB, and patients assigned to the DM group had significantly higher HCC-related mortality than those in the non-DM group (27.9 vs 8.8 per 1,000 patient-years, P=0.02). They also demonstrated increased liver-related mortality or orthotopic liver-transplantation rate in the DM group, at 23.4%, compared to the non-DM group, at 9.4% (P=0.009).
  | Figure 4 Forest plot of results comparing overall mortality between DM and non-DM subjects. Abbreviations: DM, diabetes mellitus; NDM, non-DM; M–H, Mantel–Haenszel. |
Risk of bias and quality evaluation of evidence
We conducted a quality evaluation on all five cohort studies and two case–control studies included, based on the coding manual for cohort-studies and for case–control studies. All studies scored at least 6, and five studies scored 7 or more. The main outcomes and some other results are summarized in Table 4. The evidence was considered to be of low quality.
  | Table 4 Quality evaluation of evidence |
Discussion
Chronic hepatitis virus infection, including HBV and HCV,22 has always been widely acknowledged as a major risk factor of primary HCC. DM, as well as other metabolic abnormalities, has been proved to be associated with quite a few kinds of malignancies,23,24 and this association was suggested not to be mediated by body-mass index.25 A recent meta-analysis26 and a systemic review13 demonstrated a strong associations between concurrent DM and risk of HCC among chronic HCV patients. While well studied in anti-HCV-positive subjects, the potential relationship of type 2 DM and risk of HCC in the HBV-infected population remains unclear. In addition, with effective treatment for HCV infection, HCV-related HCC cases are expected to decrease worldwide.27 Therefore, HBV infection and metabolic factors, including obesity, DM, and hyperlipidemia, are predicted to account for most of the increase in HCC.
Five cohort studies and two case–control studies were included in the current meta-analysis, with >20,000 individuals enrolled. A recent meta-analysis by Chen et al26 summarized three studies (two reporting with HRs and the other with RRs), revealing a similar risk of HCC in diabetics without DM among CHB patients. However, in the current meta-analysis, we found a significant association between type 2 DM and increasing incidence of HCC among CHB patients, with a pooled HR of 1.77 (95% CI 1.28–2.47) and no heterogeneity detected (I2=0). Previous meta-analyses have failed to find statistical differences between the two groups, possibly due to the small number of studies included and statistical issues, as not all results were presented with HRs. The biological mechanism for how type 2 DM influences HCC development is not well understood and remains controversial. Most researchers have suggested that type 2 DM contributes to HCC development independently or synergetically with other risk factors, such as HBV28 or HCV29,30 infection and alcohol consumption.31
Though relatively uncommon in the setting of HCC, DM is of growing importance, because of its rapidly increasing incidence among adults, as well as nonalcoholic fatty-liver disease, especially in developed countries.9 It has been reported that type 2 DM and/or obesity had the greatest population-attributable fractions (36.6%) and proportion of cases that could be attributed to specific risk factors: to HCC, higher than alcohol-related disorders (23.5%) or HCV (22.4%).32 Insulin resistance9,33 and hyperinsulinemia34,35 are believed to be key factors of HCC oncogenesis in diabetics, mostly through the process of inflammation and cellular injury.36–38 While playing an important role in glucose and lipid metabolism, insulin also has pleiotropic effects on regulation of inflammation and cell proliferation. IGF1, the most powerful activator of cellular proliferation, and IRS1 are crucial downstream targets of insulin.36 HCC cells have been found to overexpress39 IGF1 and IRS1, revealing that they are of great importance in the process of HCC development. On the other hand, the inflammatory milieu caused by insulin resistance and nonalcoholic steatohepatitis leads to multiple pathways of inflammatory processes, which in return activated oncogenic signaling pathways, such as PI3K–PTEN–Akt and JAK–STAT.9 Insulin resistance also causes hepatic inflammation and fibrosis by the accumulation of fat within hepatocytes, which produces oxidative stress and results in hepatosteatosis.40 All these activations of pathways are associated with cellular proliferation promotion, increased angiogenesis, and decreased apoptosis, which finally lead to HCC development.
DM was also found to be a poor prognostic factor for overall mortality in the cohort of patients with CHB infection (RR 2.33, 95% CI 1.64–3.31) in the current meta-analysis, which echoed previous studies of worse overall mortality and HCC recurrence after potential curative therapy in patients with DM.41,42 DM has been attributed to increases in both HCC related mortality and mortality from other causes, such as cardiovascular factors.21 Since insulin resistance and hyperinsulinemia are key factors in the process of hepatocarcinogenesis, any other underlying disease or medication that affects the insulin level is a potential risk factor for the progression of HCC. Several studies21,43,44 have demonstrated higher incidence of HCC among HBV subjects who were treated with insulin or antidiabetic drugs that increase circulating insulin, while metformin, which is supposed to improve insulin sensibility, has been reported to be related to declined HCC risk and mortality.45,46 Therefore, we recommend better management of DM and cautious selection of therapy for DM in CHB patients.
In our meta-analysis, five studies were conducted in Taiwan. Although they were conducted using different databases in different periods (Table 1), it is still difficult to confirm whether the cohort pool used in each study was completely different. Besides, methods used to identify HCC and DM were different in the studies included. Therefore, we think this may be a limitation of our study, and our results should be interpreted with caution.
The major limitation involves the variability of the adjustments within the studies. As is well known, HCC is related to quite a few independent risk factors, of which HBV DNA levels, antiviral therapy, and liver cirrhosis are of great importance. However, specific data about these effecting factors were not available in most of the studies included in this meta-analysis. Three studies19–21 clearly adjusted for cirrhosis in the multivariate analysis, two20,21 adjusted for antiviral therapy, and one19 just excluded patients who accepted antiviral therapy, while only one21 adjusted sustained viral suppression (HBV viral load <3log IU/mL on at least two occasions 6 months apart). Also, the evaluation of evidence turned out to be of low quality. All seven studies included were observational: five cohort studies and two case–control studies. Many of the researches did not report on the details of follow-up, including precise follow-up years of both groups and loss rate. More well-designed research and randomized controlled trials are needed to confirm further the association between type 2 DM and the risk of HCC. Though the evidence was evaluated as low quality, most studies were designed to minimize potential bias by adjusting for age, sex, and other covariates that were possibly influential (Table 3).
Consequently, the findings of type 2 DM significantly related to HCC risk may shed some light on the prevention of HCC. Better management of DM and correlated metabolic factors is strongly recommended, and HBV patients with DM should be tested more frequently, in order to improve early detection of HCC or other malignancies.
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
Lombardi A, Grimaldi A, Zappavigna S, Misso G, Caraglia M. Hepatocarcinoma: genetic and epigenetic features. Minerva Gastroenterol Dietol. 2018;64(1):14–27. | ||
Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol. 2014;28(5):753–770. | ||
Güthle M, Dollinger MM. Epidemiología y factores de riesgo de carcinoma hepatocelular. [Epidemiology and risk factors of hepatocellular carcinoma]. Radiologe. 2014;54(7):654–659. German | ||
Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28(2):109–122. | ||
Chuang SC, Lee YC, Wu GJ, Straif K, Hashibe M. Alcohol consumption and liver cancer risk: a meta-analysis. Cancer Causes Control. 2015;26(9):1205–1231. | ||
Lee YC, Cohet C, Yang YC, Stayner L, Hashibe M, Straif K. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol. 2009;38(6):1497–1511. | ||
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. | ||
Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr. 2004;80(5):1106–1122. | ||
Noureddin M, Rinella ME. Nonalcoholic fatty liver disease, diabetes, obesity, and hepatocellular carcinoma. Clin Liver Dis. 2015;19(2):361–379. | ||
Yu J, Shen J, Sun TT, Zhang X, Wong N. Obesity, insulin resistance, NASH and hepatocellular carcinoma. Semin Cancer Biol. 2013;23(6 Pt B):483–491. | ||
Huang Y, Cai X, Qiu M, et al. Prediabetes and the risk of cancer: a meta-analysis. Diabetologia. 2014;57(11):2261–2269. | ||
Kasmari AJ, Welch A, Liu G, Leslie D, McGarrity T, Riley T. Independent of cirrhosis, hepatocellular carcinoma risk is increased with diabetes and metabolic syndrome. Am J Med. 2017;130(6):746.e1–74746. | ||
Dyal HK, Aguilar M, Bartos G, et al. Diabetes mellitus increases risk of hepatocellular carcinoma in chronic hepatitis C virus patients: a systematic review. Dig Dis Sci. 2016;61(2):636–645. | ||
Lai MS, Hsieh MS, Chiu YH, Chen TH. Type 2 diabetes and hepatocellular carcinoma: a cohort study in high prevalence area of hepatitis virus infection. Hepatology. 2006;43(6):1295–1302. | ||
Chen CL, Yang HI, Yang WS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135(1):111–121. | ||
Wang CS, Yao WJ, Chang TT, Wang ST, Chou P. The impact of type 2 diabetes on the development of hepatocellular carcinoma in different viral hepatitis statuses. Cancer Epidemiol Biomarkers Prev. 2009;18(7):2054–2060. | ||
Ko WH, Chiu SY, Yang KC, Chen HH. Diabetes, hepatitis virus infection and hepatocellular carcinoma: a case-control study in hepatitis endemic area. Hepatol Res. 2012;42(8):774–781. | ||
Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. | ||
Li Q, Li WW, Yang X, et al. Type 2 diabetes and hepatocellular carcinoma: a case-control study in patients with chronic hepatitis B. Int J Cancer. 2012;131(5):1197–1202. | ||
Fu SC, Huang YW, Wang TC, Hu JT, Chen DS, Yang SS. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with new onset diabetes: a nationwide cohort study. Aliment Pharmacol Ther. 2015;41(11):1200–1209. | ||
Hsiang JC, Gane EJ, Bai WW, Gerred SJ. Type 2 diabetes: a risk factor for liver mortality and complications in hepatitis B cirrhosis patients. J Gastroenterol Hepatol. 2015;30(3):591–599. | ||
El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160(21):3227–3230. | ||
Sasazuki S, Charvat H, Hara A, et al. Diabetes mellitus and cancer risk: pooled analysis of eight cohort studies in Japan. Cancer Sci. 2013;104(11):1499–1507. | ||
Starup-Linde J, Karlstad O, Eriksen SA, et al. CARING (CAncer Risk and INsulin analoGues): the association of diabetes mellitus and cancer risk with focus on possible determinants - a systematic review and a meta-analysis. Curr Drug Saf. 2013;8(5):296–332. | ||
King LY, Khalili H, Huang ES. Diabetes mellitus is associated with an increased risk of HCC in a large prospective cohort with long term follow-up. Hepatology. 2014;60:281A. | ||
Chen J, Han Y, Xu C, Xiao T, Wang B. Effect of type 2 diabetes mellitus on the risk for hepatocellular carcinoma in chronic liver diseases: a meta-analysis of cohort studies. Eur J Cancer Prev. 2015;24(2):89–99. | ||
Cholankeril G, Patel R, Khurana S, Satapathy SK. Hepatocellular carcinoma in non-alcoholic steatohepatitis: current knowledge and implications for management. World J Hepatol. 2017;9(11):533–543. | ||
Zheng Z, Zhang C, Yan J, et al. Diabetes mellitus is associated with hepatocellular carcinoma: a retrospective case-control study in hepatitis endemic area. PLoS One. 2013;8(12):e84776. | ||
Hung C-H, Lee C-M, Wang J-H, et al. Impact of diabetes mellitus on incidence of hepatocellular carcinoma in chronic hepatitis C patients treated with interferon-based antiviral therapy. Int J Cancer. 2011;128(10):2344–2352. | ||
Arase Y, Kobayashi M, Suzuki F, et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology. 2013;57(3):964–973. | ||
Hassan MM, Hwang LY, Hatten CJ, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36(5):1206–1213. | ||
Welzel TM, Graubard BI, Quraishi S, et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol. 2013;108(8):1314–1321. | ||
Farrell G. Insulin resistance, obesity, and liver cancer. Clin Gastroenterol Hepatol. 2014;12(1):117–119. | ||
Balkau B, Kahn HS, Courbon D, Eschwège E, Ducimetière P; Paris Prospective Study. Hyperinsulinemia predicts fatal liver cancer but is inversely associated with fatal cancer at some other sites: the Paris prospective study. Diabetes Care. 2001;24(5):843–849. | ||
Baba H, Tsuneyama K. Hyperinsulinemia rather than hyperglycemia can accelerate the progression of hepatocellular carcinoma. Hepatology. 2015;9(1 Suppl 1):S318–S19. | ||
Moore MA, Park CB, Tsuda H. Implications of the hyperinsulinaemia-diabetes-cancer link for preventive efforts. Eur J Cancer Prev. 1998;7(2):89–107. | ||
Tanaka S, Mohr L, Schmidt EV, Sugimachi K, Wands JR. Biological effects of human insulin receptor substrate-1 overexpression in hepatocytes. Hepatology. 1997;26(3):598–604. | ||
Longato L, de La Monte S, Kuzushita N, et al. Overexpression of insulin receptor substrate-1 and hepatitis Bx genes causes premalignant alterations in the liver. Hepatology. 2009;49(6):1935–1943. | ||
Ampuero J, Romero-Gomez M. Prevention of hepatocellular carcinoma by correction of metabolic abnormalities: role of statins and metformin. World J Hepatol. 2015;7(8):1105–1111. | ||
Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2 Suppl 1):S99–S112. | ||
Kawamura Y, Ikeda K, Arase Y, et al. Diabetes mellitus worsens the recurrence rate after potentially curative therapy in patients with hepatocellular carcinoma associated with nonviral hepatitis. J Gastroenterol Hepatol. 2008;23(11):1739–1746. | ||
Ting CT, Chen RC, Chen CC, Liu MH, Chu D, Kuo NW. Diabetes worsens the surgical outcomes in cirrhotic patients with hepatocellular carcinoma. Tohoku J Exp Med. 2012;227(1):73–81. | ||
Tateishi R, Okanoue T, Okita K, et al. P572 insulin use is related to the development of hepatocellular carcinoma at younger age with non-viral etiology. J Hepatol. 2014;60(1):S260. | ||
Bosetti C, Franchi M, Nicotra F, et al. Insulin and other antidiabetic drugs and hepatocellular carcinoma risk: a nested case-control study based on Italian healthcare utilization databases. Pharmacoepidemiol Drug Saf. 2015;24(7):771–778. | ||
Zhang X, Harmsen WS, Mettler TA, et al. Continuation of metformin use after a diagnosis of cirrhosis significantly improves survival of patients with diabetes. Hepatology. 2014;60(6):2008–2016. | ||
Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30(5):750–758. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.