Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 8
Type 2 diabetes in Brazil: epidemiology and management
Authors Almeida-Pititto B , Dias ML, Franco de Moraes AC, Ferreira SR, Franco DR, Eliaschewitz F
Received 11 August 2014
Accepted for publication 14 October 2014
Published 5 January 2015 Volume 2015:8 Pages 17—28
DOI https://doi.org/10.2147/DMSO.S72542
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Video abstract presented by Denise Reis Franco
Views: 366
Bianca de Almeida-Pititto,1 Monike Lourenço Dias,2 Ana Carolina Franco de Moraes,3 Sandra RG Ferreira,3 Denise Reis Franco,4 Freddy Goldberg Eliaschewitz4,5
1Department of Preventive Medicine, Federal University of São Paulo, São Paulo, Brazil; 2Department of Endocrinology, Federal University of Goiás, Goiânia, Goiás, Brazil; 3Department of Epidemiology, School of Public Health, University of São Paulo, São Paulo, Brazil; 4CPClin Clinical Research Center, 5Albert Einstein Hospital, São Paulo, Brazil
Abstract: Type 2 diabetes mellitus (T2DM) is one of the most important epidemic diseases in the world this century, and accounts for 90% of cases of diabetes globally. Brazil is one of the most important examples of the alarming picture of T2DM in emergent societies, being the country with the fourth largest number of people with diabetes. The aim of this paper is to review the literature on diabetes in Brazil, specifically looking at the epidemiology and management of T2DM. A literature search was conducted using PubMed and LILACS to identify articles containing information on diabetes in Brazil. Official documents from the Brazilian government, World Health Organization, and International Diabetes Federation were also reviewed.
Keywords: type 2 diabetes, Brazil, epidemiology, management
Introduction
Type 2 diabetes mellitus (T2DM) is one of the most important epidemic diseases of this century. In 1985, estimates were that 30 million adults had diabetes, but this number grew to 135 million in 1995, and reached 173 million in 2002.1 This increasing prevalence is predicted to continue, with the World Health Organization projecting that there will be 366 million adults with diabetes in 2030.2 T2DM accounts for 90% of cases of diabetes around the world,3 and 80% of people with diabetes live in low-income or middle-income countries.4
T2DM occurs when the pancreas does not produce enough insulin to maintain a normal blood glucose level or when the body is unable to use the insulin that is produced (insulin resistance). This type of diabetes is caused by a combination of factors, including genetic susceptibility, obesity, and physical inactivity.5 In parallel with population ageing, several other factors associated with modern lifestyle have contributed to the increased trend in prevalence of T2DM over recent decades. A sedentary lifestyle, unhealthy eating habits, and obesity are established risk factors for development of the disease.
According to the International Diabetes Federation, Brazil ranks fourth among the countries with the largest number of people with diabetes, being 11.9 million in 2013. The International Diabetes Federation also estimated the prevalence of diabetes in Brazil to be 10.3% in 2012.6
We reviewed the literature to provide an overview of diabetes in Brazil, including epidemiological data, national guidelines, and current glycemic control status of the Brazilian diabetes population, as well as to describe health policies for the management of T2DM and its complications.
Methodology
A literature search was conducted to identify articles containing information on T2DM in Brazil until June 15, 2014. Official documents from the Brazilian government, World Health Organization, and International Diabetes Federation were also examined. We searched PubMed with the terms “diabetes AND type 2 AND (Brasil* OR Brazil*)”. A search of the LILACS (Literatura Latinoamericana y del Caribe en Ciencias de la Salud) database was also performed, using the terms “diabetes” and “Brazil”. The most recent and relevant articles were selected.
T2DM in Brazil
Prevalence
The definition of T2DM and prediabetes provided by the Brazilian Society of Diabetes (BDS) follows the same criteria as those of the American Diabetes Association (ADA).7 To diagnose diabetes, two fasting plasma glucose values ≥126 mg/dL or a casual glycemia ≥200 mg/dL are necessary. After undergoing a 2-hour 75 g oral glucose tolerance test, the diagnostic criterion for diabetes is a plasma glucose ≥200 mg/dL. For impaired fasting glycemia, the diagnostic criteria are ≥100 mg/dL and <126 mg/dL, and for impaired glucose tolerance, the criterion is a 2-hour plasma glucose ≥140 mg/dL and <200 mg/dL.7
Nutritional transition and the population aging occurring in Brazil have contributed to greater incidence and prevalence rates of noncommunicable chronic diseases (NCCD), including T2DM.8,9 Similarly to other emergent societies, T2DM, combined with cardiovascular risk factors such as hypertension and dyslipidemia, appears as one of the most important public health problems among Brazilians.10
In the 1980s, a multicenter prevalence study including 21,847 individuals aged 30–69 years found that 7.6% of these individuals were diabetic based on an oral glucose tolerance test.11 Other smaller studies were conducted in urban regions in Brazil to estimate the prevalence of diabetes using methods similar to those used in the nationwide study. Among individuals aged 30–69 or 30–79 years, 12.1%–13.5% had diabetes and 5.0%–7.7% had impaired glucose tolerance (Figure 1).12 A relevant finding of this multicenter study was that almost half of the individuals with a diagnosis of diabetes did not know they had the condition.11
  | Figure 1 Distribution of prevalence (according to regions) and trends (in the region of São Paulo) of diabetes diagnosed by oral glucose tolerance testing in adults living in urban areas in Brazil. |
In 2006, Brazil launched VIGITEL (Surveillance of Risk and Protective Factors for Chronic Diseases Telephone Survey), a nationwide telephone surveillance system aiming to provide epidemiological data about the risk and protective factors for NCCD in individuals aged ≥18 years. VIGITEL is conducted yearly, and shows a trend toward increasing prevalence rates since its first edition (Figure 2). In 2013, the survey found a rate of 6.9% for self-reported diabetes mellitus.13 In the same year, VIGITEL also showed increasing rates with aging (8.5% in the group aged 45–54 years, 17.1% in the group aged 55–64 years, and 22.1% in the group aged ≥65 years; Figure 3).14
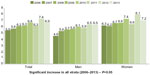  | Figure 2 Trend in prevalence of self-reported diabetes mellitus in Brazil. |
  | Figure 3 Distribution of prevalence of self-reported diabetes mellitus according to age. |
Regional variations in self-reported diabetes mellitus in Brazil were detected. Rates ranged from 3.6% to 5.5% in individuals from the northern region, while in the southeast region, characterized by a higher income, the rates varied from 6.7% to 8.2%.13 This variation according to regions was also seen in the nationwide study performed in the 1980s (Figure 1).10
Different methodologies were used in the multicenter study to determine the prevalence of diabetes (diagnosis based on an oral glucose tolerance test) and in VIGITEL (self-reported diabetes), which limits any comparison and evaluation of trends in the rates. However, the multicenter study provided self-reported data showing a prevalence of 3.2% and 11.6% in the age groups 30–69 years and >70 years, respectively, with these rates being lower than those found in VIGITEL in recent years.
The prevalence of disturbances in glucose metabolism among specific populations in Brazil was also investigated. A population-based study in Japanese-Brazilians living in a city in the state of São Paulo reported that this population has one of the highest prevalence rates of T2DM detected worldwide, involving 35% of individuals aged 45–79 years.15 Similar data were confirmed by other investigators in a more recent study showing the rate of T2DM to be 37.4% in Japanese-Brazilians aged ≥60 years.16
Few cases of disturbed glucose metabolism were confirmed in the Native American population between 1970 and 1990.17,18 However, recent studies indicate changes in this scenario, since at least 5% of Indians were shown to have disturbances of glucose metabolism, which may be a consequence of increased rates of obesity (14.2% in men and 30.8% in women). Among the Xavantes Indians, this frequency is even higher, with 28.2% having T2DM, 32.3% having impaired glucose tolerance, and 50.8% being obese.19
Risk factors
Considering the high prevalence of diabetes and its relevant impact on population health, there is an effort to identify risk factors and associated comorbidities that contribute to the occurrence of diabetes and its complications (mainly cardiovascular events). These include hypertension, dyslipidemia, and modifiable risk factors that collaborate for the development of T2DM, such as smoking, alcohol abuse, physical inactivity, an unhealthy diet, and obesity. The four main groups of NCCD (cardiovascular disease, cancer, chronic respiratory disease, and diabetes) share these modifiable risk factors.10
Obesity, especially central adiposity, plays a key role in the physiopathology of insulin resistance and diabetes. Accumulation of body fat during adulthood is directly associated with the incidence of cardiovascular disease and T2DM.20,21
Population-based studies conducted in Brazil have shown escalating rates of obesity in all age groups, including adolescents, with a decrease in the prevalence of under nutrition,8 creating a high burden of disease and associated costs.22 Serial data from the Household Budget Survey showed that excessive weight (body mass index >25 kg/m2) increased from 16% to 50% in men and from 28% to 48% in women in the past 36 years.23 Considering the last 6 years, the prevalence of obesity (body mass index >30 kg/m2) increased from 8.9% to 12.5% in men and from 13.1% to 16.9% in women.
Some data suggest that carbohydrates with a lower glycemic index may decrease the risk of T2DM, as well as improve glucose and lipid concentrations in patients with impaired glucose tolerance or T2DM.24,25 Studies provide evidence that saturated fat significantly worsens insulin resistance, while monounsaturated and polyunsaturated fatty acids improve it through modifications in the composition of cell membranes, reflecting at least in part dietary fat composition.26,27 Additionally, dietary fiber has been inversely associated with inflammatory markers and insulin resistance. Therefore, international dietary recommendations reinforce the benefits of high consumption of high-fiber food and the importance of reducing the intake of sugar and saturated fat.26,28–30
Physical inactivity and sedentary lifestyle are considered important modifiable risk factors for T2DM and cardiovascular disease.31,32 Epidemiological studies suggest that physical activity may slow the onset and progression of insulin resistance, and improve glycemic control, blood pressure, and the lipid profile.31,32 In Brazil, it is estimated that 41% of the adult population is not sufficiently active to achieve health benefits.33
The rates of chronic diseases have been attributed in part to modifiable environmental factors, such as dietary habits, physical inactivity, smoking, and a stressful lifestyle, that contribute to accumulation of body fat, which is involved in the genesis of insulin resistance. VIGITEL found high frequencies of soft drink (23.3%) and fatty meat (31%) consumption, alcohol abuse (16.4%), cigarette smoking (11.3%), and low fruit and vegetable intake according to World Health Organization recommendations (23.6%), and also little physical activity (33.8%). The frequency of these risky behaviors was associated with gender, age, and level of education, indicating that these variables should be taken into consideration by health policies13 (Table 1).
  | Table 1 Prevalence of selected risk factors for chronic diseases as ascertained by VIGITEL, 2006–2013 |
Improvement in knowledge and health professionals’ ability to target a reduction of cardiometabolic risk and also raise population awareness concerning healthy living habits might help to tackle diabetes and its associated disorders and complications.
Impact
The age of individuals with diabetes along with late diagnosis contributes to development of the chronic complications associated with T2DM, which correlates with the duration of exposure to hyperglycemia. This scenario has a direct impact on the occurrence of long-term complications, including macroangiopathy, retinopathy, nephropathy, and neuropathy, which implies a deterioration of quality of life and high economic costs. Diabetes accounts for 5.1% and cardiovascular disease accounts for 13.3% of disability-adjusted life years, according to the Global Burden of Disease Project in Brazil.34 In comparison with other countries, Brazil showed a higher proportion of life years with disability among total disability-adjusted life years for diabetes.10
In Brazil, the public health system (Sistema Único de Saúde; SUS) has been progressively increasing the assistance available for individuals with diabetes. Hospitalization data from the Ministry of Health indicate that T2DM and hypertension in adults are involved directly or indirectly in the great majority of cases, being responsible for 7.4% of all admissions not related to pregnancy and for 9.3% of all hospital assistance costs10 (Figure 4).
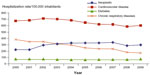  | Figure 4 Hospitalization rate according to selected chronic diseases, Brazil 2000–2009. |
Some decrease in mortality due to cardiovascular diseases has been observed in Brazil. Diabetes as the underlying cause of death rose by 11% from 1996 to 2000, but decreased by 8% from 2000 to 2007 (Figure 4).10 This pattern has been attributed to the control of certain risk factors, including hypertension, tobacco use, and dyslipidemia.
A national registry for diabetes and hypertension (SisHiperDia) found that 4.3% of diabetic patients had foot disorders, 2.2% had a previous amputation, 7.8% had renal disease, 7.8% had a previous myocardial infarction, and 8% had a previous stroke.10 Moreover, registry data suggest that mortality standardized for age and gender in people with diabetes was 57% higher than that of the general population.10
The Brazilian Study on Diabetes Costs (ESCUDI) estimated the costs of diabetes care in the public health care system based on data collected retrospectively from diabetic individuals assisted at different levels of care in eight Brazilian cities during 2007.35 A total of 1,000 patients were interviewed and had their medical records data analyzed. The self-reported prevalence of coronary artery disease was 70.4%, 79.6% for arterial hypertension, 7.6% for cerebrovascular disease, and 70.4% for hypercholesterolemia. Information on chronic diabetes complications from medical records showed that 28.9% had at least one microvascular complication, 17% had at least one macrovascular complication, and 16% had at least one microvascular and one macrovascular complication. ESCUDI estimated that the total (direct and indirect) cost of diabetes care was US$ 1,144 per patient at the primary care level, reaching US$ 2,810 at the tertiary level.35
International studies have shown that approximately half of the total costs of diabetes management are accounted for by hospital admissions.36,37 In Brazil, the costs of public hospital admissions between 1999 and 2001 due to diabetes were significant, ie, 2.2% of the Brazilian Ministry of Health budget.38
Although NCCD are a great health problem in Brazil, some decrease in mortality has been observed. Age-standardized cardiovascular mortality has declined by 20%; however, when associated with diabetes, the mortality due to cardiovascular disease increased by 2% (Figure 5). Nevertheless, strategies to control risk factors such as excessive body weight and hyperglycemia have failed to reduce cardiovascular mortality in diabetic individuals, who have a more severe atherosclerotic process and a worse prognosis with regard to cardiovascular events.10,39
  | Figure 5 Trends in mortality from NCCD for 1996–2000 and 2007. |
Glycemic control in Brazilian patients with T2DM
Given that blood glucose levels are recognized as indicators of quality in diabetes care,40,41 it is relevant to know how Brazilian patients fare in this sense. A nationwide cross-sectional study, evaluating 5,750 T2DM patients attending the public health care system, was conducted between 2006 and 2011.42 This study found a mean glycosylated hemoglobin (HbA1c) of 8.6%±2.2%, with a median of 8.1%. Regarding the targets advised by the ADA and BDS, only a minority of patients reached the recommended HbA1c target of <7%, and even when a less stringent target was considered, only 48.5% of patients had an HbA1c level <8%, indicating a poorer glycemic control rate than that observed in Europe and the USA.42
Although there are limited data on glycemic control in patients using the private health care system in Brazil, their picture seems better than that observed in the public health care system. A cross-sectional multicenter study conducted in Latin American countries showed that approximately 40% of 878 Brazilian private practice patients had HbA1c levels <7%.43 Therefore, the Brazilian public health care system has not been achieving adequate glycemic control in patients with T2DM.
Current guidelines for T2DM in Brazil
The BDS is comprised of specialists (endocrinologists, cardiologists, and general practitioners), residents, and medical students. Periodically, the BDS releases guidelines for the prevention and treatment of diabetes. Those recommendations were updated in 2014, aiming to adapt ADA, European Association for the Study of Diabetes (EASD), and American Association of Clinical Endocrinologists guidelines for use in private and public health care practice in Brazil. Aside from the BDS guidelines, the public health system has its own recommendations based on the therapies available in the public system. Guidance on patient education and individualization of treatment regarding comorbidities, adherence, adverse drug events, and costs is also provided in this guideline.
Diagnosis and prevention of prediabetes and diabetes
The diagnostic criteria for diabetes and prediabetes used in Brazil are those recommended by the ADA and EASD guidelines. BDS guidelines are based on evidence of the positive effects of modifying lifestyle to prevent onset of diabetes. The guidelines also include the proven benefits of acarbose, metformin, and pioglitazone, each of which has been shown to decrease the incidence of diabetes to various degrees. Therefore, the recommendations of the BDS for prevention of T2DM are:
- T2DM can be prevented, or at least delayed, through intervention in individuals with prediabetes; these patients should change their lifestyle, including eating habits (balanced diet with high fiber content), weight loss (at least 5%–10% of total weight) if overweight or obese, and moderate physical activity such as walking for at least 150 minutes per week.
- In addition to lifestyle modification, in individuals with prediabetes, metformin (first option, essentially in patients aged <65 years or obese), or alternatively, acarbose or pioglitazone, can be considered for young patients with a moderate/high risk of developing T2DM, if no contraindications are present.
Glycemic goals
In 2014, the BDS reviewed its recommendations for glycemic targets in the treatment of T2DM in order to make them more applicable and realistic. Glycemic goals were defined as “recommended” (fasting and preprandial glycemia <100 mg/dL, and postprandial glycemia <160 mg/dL) or “acceptable” (fasting and preprandial glycemia <130 mg/dL and postprandial glycemia <180 mg/dL) for therapeutic adjustment. The HbA1c goal for nonpregnant adults is <7.0%, which may vary with age, clinical comorbidities, and risk of hypoglycemia. Higher tolerance for glycemic and HbA1c goals for children and the elderly, and more strict goals for diabetes during pregnancy are established, as in the ADA/EASD guidelines1 (Table 2).
Treatment algorithm for T2DM
The 2014 BDS guidelines1 are based on the ADA/EASD,7 the 2013 American Association of Clinical Endocrinologists,44 and the previous version of the BDS guidelines,45 which improve previous algorithms in prioritizing treatment individualization and patient involvement in therapeutic decision-making (patient-centered approach). However, US and European entities recommend a 3-month interval between each of the three initial steps of treatment adjustment. This interval can be too long, as it may take 9–12 months before the physician can perceive failure of a therapeutic strategy. Nevertheless, glycemic status before treatment is not considered in the ADA/EASD algorithm. The BDS guidelines suggest shortening the 3-month interval to 1 month for re-evaluation of treatment, and also suggest considering the patient’s glycemic status and clinical condition in the initial therapeutic decision (Figure 6).
  | Figure 6 Algorithm for treatment of type 2 diabetes mellitus. |
BDS guidelines recommend the use of a computer-based evaluation of glycemia data to generate more precise parameters such as glycemic profiles, average glycemia, and glycemic variability. Software for analysis of glycemic data is available free of charge from manufacturers of glucometers, facilitating the use of these glycemic parameters in clinical decision-making and more frequent and safer therapeutic interventions. With regard to addition of a third antihyperglycemic drug, there is no proven difference in glycemic control between the available drug options when patients are being already treated with metformin and sulfonylureas. The most appropriate option depends on the individual patient’s characteristics.
Treatment of diabetes in Brazil
Achieving glycemic goals and preventing the development and progression of chronic complications in patients with T2DM requires access to skilled health care providers, antihyperglycemic treatments, and supplies for self-monitoring of blood glucose, as well as appropriate education in order to actively participate in the control and treatment of the disease.46
In SUS, the initial evaluation of a diabetic patient is usually undertaken in a primary health center by a primary care physician,47 who is often not adequately trained to manage this condition.46 In a recent study conducted in Brazil, primary care physicians reported that they did not feel prepared to implement educational practices for people with diabetes.46,48 Furthermore, up to 80% of diabetic patients are continuously treated in primary care centers,47 so general practitioners are increasingly responsible for providing adequate metabolic control and for timely initiation and titration of insulin therapy. Moreover, public health care systems in Brazil and Latin America do not use a comprehensive multidisciplinary team approach for the treatment of people with diabetes. Implementation of easily accessible educational programs for primary care physicians, focusing on patient care and insulin prescription and management, could improve this scenario.49
Referral to an endocrinologist is recommended for patients with T1DM, those with T2DM and diabetes-related complications, and especially those on multiple doses of insulin,47 but the scarcity of specialists in public health care hinder the proper assistance of referred cases. In 2005, 16.1% of cities with >25,000 inhabitants did not provide clinical care or appointments by endocrinology specialists.50
SUS provides antihyperglycemic agents such as regular and neutral protamine Hagedorn insulin, metformin, glibenclamide, and gliclazide. The Ministry of Health suggests a diabetes treatment guideline comprising only these antihyperglycemic agents.47
The first-line antihyperglycemic agent recommended is metformin, after a 3-month period of changes in lifestyle or sooner in nonresponsive, overweight, or obese patients (except when creatinine clearance is <30 mL/min/1.73 m2). The recommended second-line agent is sulfonylurea, which can be added after 3–6 months of treatment with metformin, or sooner (4–8 weeks) in the event of a poor response to metformin. Sulfonylureas can also be the first treatment option when there is unexplained weight loss and higher glycemic values. Insulin therapy is recommended after failure of 3–6 months of treatment with metformin and sulfonylureas, and also when glycemia is >300 mg/dL at first evaluation, at diagnosis, or when combined with weight loss, ketonuria, and/or ketonemia. If the HbA1c goal is not achieved after 2–3 months, treatment with regular and/or neutral protamine Hagedorn insulin is recommended.47
Oral antihyperglycemic agents can be obtained through the Brazilian Popular Pharmacy Program, a federal program that partially subsidizes some brands of medications, including metformin and glibenclamide. This program is very important in Brazil due to the limited availability of drugs provided by the public sector and because of the high prices in private drugstores, so is a useful alternative to access low-cost medications in the country.51
Appropriate epidemiological and quality of care data on diabetes could contribute to making policymakers aware of the impact and burden of the disease. An Internet-based system of registration and follow-up of hypertensive and diabetic patients (SisHiperDia) was instituted by the Ministry of Health in 2002, allowing any person to access monthly updated reports on anthropometry, blood pressure, and glycemic data, and the profile of the followed patient population.52 Satisfactory accuracy was found in 68.7% of the variables, and although most of the variables revealed good usage conditions, greater involvement of managers and health professionals in the information production process is required in order to generate more accurate and reliable data.53
Prevention strategies in public health
Large interventional studies have shown that T2DM can be prevented or delayed in insulin-resistant individuals by adopting a healthier lifestyle.54,55 The relevance of lifestyle changes has been proven by their beneficial impact on cardiovascular risk factors in interventions carried out in Brazil, such as community-based health promotion programs.56–58
Because of the high number of diabetic individuals in Brazil, a major concern in diabetes care is secondary prevention. The data have consistently shown the benefits of intensive glycemic control in preventing microvascular complications in individuals with T1DM or T2DM.59,60 However, for prevention of macrovascular complications, intensive control of cardiovascular risk factors (hypertension, dyslipidemia, and smoking) is necessary.60,61 Intensive glucose control in older individuals with a long-term disease and more complications is associated with an increased risk of hypoglycemia and cardiovascular event.62–64 These findings show that strict control of glycemia may harm high-risk T2DM patients and emphasize the importance of intensive treatment in the early stages of diabetes.60
The health of the Brazilian population is guaranteed by the Federal Constitution to provide universal and free coverage through the SUS. Federal, state, and municipal governments share responsibility for accountability, funding, and carrying out SUS procedures. Private services are regulated by the Brazilian government through a National Agency for Private Health Insurance. The Family Health Strategy, based on a family health care team, is the main sponsor of primary care, being present in approximately 90% of municipalities. Each family health care team, comprising one physician, one nurse, one or two nursing assistants, and five or six community health agents, serves up to 3,500–4,000 people. Availability of health care units and number of families was used to map the needs of each municipality. Family Health Strategy teams have helped the monitoring of major diseases and risk factors in the families they assist and have helped communities to make healthier choices regarding lifestyle, targeting to avoid a significant set of diseases.
In 2011–2022, the Brazilian Ministry of Health launched a strategic action plan to tackle NCCD,14 as a result of a collaborative proposal designed by educational and research institutions, several ministries, members of nongovernmental organizations from the health sector, and patient associations, among others. The plan aims, during the next 10 years, to prepare Brazil to cope with and limit progression of the four main groups of NCCD (cardiovascular disease, cancer, chronic respiratory disease, and diabetes) as well as their shared risk factors (smoking, alcohol abuse, physical inactivity, an unhealthy diet, and obesity). It also proposes to outline national guidelines and measures to be introduced concerning surveillance, information, evaluation, and monitoring of health promotion, as well as integral care. Furthermore, Brazil has been preparing to enter the United Nations’ global effort and mobilization toward fighting NCCD.
The National Health at a Distance Program, considered to be an instrument for support of primary health care, was recently implemented.65,66 However, this interactive education model based on motivation has been underutilized. Other similar programs have been developed in selected strategic areas of the country to train lay individuals to work with people with chronic diseases including diabetes care, but the impact of these initiatives has not been evaluated as yet.
On the other hand, isolated primary prevention programs have failed to cover the continental dimension of Brazil, and their efficacy has been challenged due to lack of adequate resources in the health services and poor adherence of participants.56,57 The unsuccessful prevention initiatives can be partly explained by misdirected population targeting and inadequate screening procedures and evaluation tools.
There is an urgent need to optimize preventive strategies that are both effective and sustainable in the Brazilian public health care system. In the long term, reductions in direct and indirect costs due to T2DM and cardiovascular disease in Brazil should be expected.
Conclusion
Brazil has been experiencing a gradual increase in the prevalence of T2DM over the last 3 decades, as well as an increase in the prevalence of important risk factors, such as obesity. The discrepant findings with regard to diabetes prevalence in recent studies, with lower self-referral numbers compared with the prevalence of diabetes observed in studies that tested blood glucose, seems to reinforce the notion that diabetes among Brazilians is still an underdiagnosed condition, a situation that can foster the occurrence of chronic complications.59,60,67
Glycemic control among Brazilian patients with T2DM is poor, especially when compared with data from high-income countries, particularly in patients treated in the public health care system. Policymakers and health professionals are aware of the current scenario of T2DM in Brazil, and have been making efforts to maximize existing programs and create new prevention strategies to modify this course.
Research institutions and the government are trying to reverse the growth of T2DM, diabetic complications, and other associated cardiovascular risk factors (such as obesity, hypertension, and dyslipidemia) in the population. However, some challenges will have to be faced, such as the unequal distribution of infrastructure throughout the country. In addition, the impact of previous actions seeking to prevent diabetes has been restricted to a small number of high-risk individuals, highlighting the urgent need to expand preventive actions nationwide.
Acknowledgment
The authors are grateful for the support of Christiane Bueno and Dr Luciano Paladini in the preparation of this manuscript, which was supported by a grant from Sanofi.
Disclosure
FGE is a member of the scientific advisory board member of and delivers conferences for Merck Sharpe and Dome, Sanofi, Novo-Nordisk, and Eli Lilly. DRF is both a member of the scientific advisory board and delivers conferences for Sanofi, Novo-Nordisk, E Lilly. The remaining authors report no conflicts of interest in this work.
References
Sociedade Brasileira de Diabetes. Diretrizes da Sociedade Brasieira de Diabetes: 2013–2014. Barueri, Brazil: AC Farmacêutica Ltda.; 2014. Available from: http://www.nutritotal.com.br/diretrizes/files/342--diretrizessbd.pdf. Accessed December 12, 2014. Portuguese. | |
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. | |
World Health Organization. Diabetes Fact Sheet. 2013. Available from: http://www.who.int/mediacentre/factsheets/fs312/en/. Accessed October 19, 2014. | |
International Diabetes Federation. IDF Diabetes Atlas. 6th ed. Brussels, Belgium: International Diabetes Federation; 2013. | |
National Diabetes Information Clearinghouse. Causes of diabetes. 2014. Available from: http://diabetes.niddk.nih.gov/dm/pubs/causes/. Accessed October 19, 2014. | |
Bertoldi AD, Kanavos P, Franca GV, et al. Epidemiology, management, complications and costs associated with type 2 diabetes in Brazil: a comprehensive literature review. Global Health. 2013;9:62. | |
American Diabetes Association. Standards in medical care in diabetes – 2014. Diabetes Care. 2014;37 Suppl 1:S14–S80. | |
Batista Filho M, Rissin A. [Nutritional transition in Brazil: geographic and temporal trends]. Cad Saude Publica. 2003;19 Suppl 1:S181–S191. Portuguese. | |
Sartorelli DS, Franco LJ. [Trends in diabetes mellitus in Brazil: the role of the nutritional transition]. Cad Saude Publica. 2003;19 Suppl 1:S29–S36. Portuguese. | |
Schmidt MI, Duncan BB, Azevedo e Silva G, et al. Chronic non-communicable diseases in Brazil: burden and current challenges. Lancet. 2011;377(9781):1949–1961. | |
Malerbi DA, Franco LJ. Multicenter study of the prevalence of diabetes mellitus and impaired glucose tolerance in the urban Brazilian population aged 30–69 yr. The Brazilian Cooperative Group on the Study of Diabetes Prevalence. Diabetes Care. 1992;15(11):1509–1516. | |
Bosi PL, Carvalho AM, Contrera D, et al. Prevalence of diabetes and impaired glucose tolerance in the urban population of 30 to 79 years of the city of Sao Carlos, Sao Paulo. Arq Bras Endocrinol Metabol. 2009;53(6):726–732. | |
Brasil, Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância de Doenças e Agravos não transmissíveis e Promoção da Saúde. VIGITEL Brasil 2013: Vigilância de fatores de risco e proteção para doenças crônicas por inquérito telefônico. Brasília (DF):Ministério da Saúde; 2014. Available from: http://www.prefeitura.sp.gov.br/cidade/secretarias/upload/saude/arquivos/morbidade/Vigitel-2013.pdf. Accessed december 12, 2014. Portuguese. | |
Brasil, Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Análise de Situação de Saúde. [Strategic Action Plan to Tackle Noncommunicable Diseases (NCD) in Brazil, 2011–2022]. Brasília (DF):Ministério da Saúde; 2011. Available from: http://bvsms.saude.gov.br/bvs/publicacoes/plano_acoes_enfrent_dcnt_2011.pdf. Accessed December 12, 2014. Portuguese. | |
Gimeno SGA, Ferreira SRG, Franco LJ, Harima H, Moisés RCS; Japanese-Brazilian Diabetes Study Group. Cardiovascular mortality in Japanese-Brazilians according to category of glucose tolerance. Diabetes. 2002;51 Suppl 2:A225. | |
Andrade RCG, Figueiredo RC, Foss-Freitas MC, et al. Prevalence of diabetes mellitus in the Japanese-Brazilian community of Mombuca, Guatapará, SP. Arq Bras Endocrinol Metabol. 2011;55(2):127–133. | |
Vieira Filho JPB. [O diabetes mellitus e as glicemias de jejum dos índios Caripuna e Palikur]. Revista da Associação Médica Brasileira. 1977;23:175–178. Portuguese. | |
Bloch KV, Coutinho ESF, Lobo MEC, Oliveira JEP, Milech A. [Pressão arterial, glicemia capitar e medidas antropométricas em uma população Yanomámi]. Cad Saúde Pública. 1993;9:428–438. Portuguese. | |
Dal Fabbro AL, Franco LJ, Silva AS, et al. High prevalence of type 2 diabetes mellitus in Xavante indians from Mato Grosso, Brazil. Ethn Dis. 2014;24:35–40. | |
Cabrera MAS, Jacob Filho W. [Obesidade em Idosos: Prevalência, Distribuição e Associação Com Hábitos e Co-Morbidades]. Arq Bras Endocrinol Metabol. 2001;45(5):494–501. Portuguese. | |
Palaniappan L, Carnethon MR, Wang Y, et al. Predictors of the incident metabolic syndrome in adults: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2004;27(3):788–793. | |
Rtveladze K, Marsh T, Webber L, et al. Health and economic burden of obesity in Brazil. PLoS One. 2013;8(7):e68785. | |
Instituto Brasileiro de Geografia e Estatística IBGE. Pesquisa de Orçamentos Familiares 2008–2009: análise da disponibilidade domiciliar de alimentos e do estado nutricional no Brasil. Rio de Janeiro: IBGE; 2010. Available from: http://www.ibge.gov.br/home/estatistica/populacao/condicaodevida/pof/2008_2009_aval_nutricional/pof20082009_avaliacao.pdf. Accessed October 20, 2014. | |
McKeown NM, Meigs JB, Liu S, Saltzman E, Wilson PW, Jacques PF. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care. 2004;27(2):538–546. | |
van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Dietary patterns and risk for type 2 diabetes mellitus in US men. Ann Intern Med. 2002;136(3):201–209. | |
Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr. 2004;23(4):447–456. | |
Salas-Salvado J, Martinez-Gonzalez MA, Bullo M, Ros E. The role of diet in the prevention of type 2 diabetes. Nutr Metab Cardiovasc Dis. 2011;21 Suppl 2:B32–B48. | |
Wannamethee SG, Whincup PH, Thomas MC, Sattar N. Associations between dietary fiber and inflammation, hepatic function, and risk of type 2 diabetes in older men: potential mechanisms for the benefits of fiber on diabetes risk. Diabetes Care. 2009;32(10):1823–1825. | |
Pimentel GD, Portero-McLellan KC, Oliveira EP, et al. Long-term nutrition education reduces several risk factors for type 2 diabetes mellitus in Brazilians with impaired glucose tolerance. Nutr Res. 2010;30(3):186–190. | |
Esposito K, Ceriello A, Giugliano D. Diet and the metabolic syndrome. Metab Syndr Relat Disord. 2007;5(4):291–296. | |
Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999;282(15):1433–1439. | |
Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30(3):744–752. | |
Santos ALT, Weiss T, Duarte CK, Azevedo MJ, Zelmanovitz T. [Análise crítica das recomendações da Associação Americana de Diabetes para doença cardiovascular no diabetes melito]. Arq Bras Endocrinol Metabol. 2009;53(5):657–666. Portuguese. | |
Gadelha AMJ, Leite IC, Valente JG, Schramm JMA, Portela MC, Campos MR. Relatório Final do Projeto Estimativa da Carga de Doença do Brasil – 1998. Rio de Janeiro: ENSP/Fiocruz-FENSPTEC; 2002. | |
Bahia LR, Araujo DV, Schaan BD, et al. The costs of type 2 diabetes mellitus outpatient care in the Brazilian public health system. Value Health. 2011;14(5 Suppl 1):S137–S140. | |
[No authors listed] Economic costs of diabetes in the US in 2012. Diabetes Care. 2013;36(4):1033–1046. | |
Jonsson B. Revealing the cost of type II diabetes in Europe. Diabetologia. 2002;45(7):S5–S12. | |
Rosa RS, et al. [Internaçóes por Diabetes Mellitus como diagnóstico principal na Rede Pública do Brasil]. Rev Bras Epidemiol. 9007;10(4):465–478. Portuguese. | |
Gerstein HC, Pogue J, Mann JF, et al. The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysis. Diabetologia. 2005;48(9):1749–1755. | |
Calsbeek H, Ketelaar NA, Faber MJ, Wensing M, Braspenning J. Performance measurements in diabetes care: the complex task of selecting quality indicators. Int J Qual Health Care. 2013;25(6):704–709. | |
Ahmann AJ. Guidelines and performance measures for diabetes. Am J Manag Care. 2007;13 Suppl 2:S41–S46. | |
Viana LV, Leitao CB, Kramer CK, et al. Poor glycaemic control in Brazilian patients with type 2 diabetes attending the public healthcare system: a cross-sectional study. BMJ Open. 2013;3(9):e003336. | |
Lopez Stewart G, Tambascia M, Rosas Guzman J, Etchegoyen F, Ortega Carrion J, Artemenko S. [Control of type 2 diabetes mellitus among general practitioners in private practice in nine countries of Latin America]. Rev Panam Salud Publica. 2007;22(1):12–20. Spanish. | |
American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists’ Comprehensive Diabetes Management Algorithm 2013. Endocr Pract. 2013;19 Suppl 1:1–48. | |
Sociedade Brasileira de Diabetes. Diretrizes da Sociedade Brasileira de Diabetes 2012–2013. Available from: http://www.diabetes.org.br/para-profissionais/diretrizes-da-sbd. Accessed October 20, 2014. | |
Escalante M, Gagliardino JJ, Guzman JR, Tschiedel B. Call-to-action: timely and appropriate treatment for people with type 2 diabetes in Latin America. Diabetes Res Clin Pract. 2014;104(3):343–352. | |
Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de Atenção Básica. Estratégias para o cuidado da pessoa com doença crônica: diabetes mellitus. Cadernos de Atenção Básica, n 36. Brasília; 2013. | |
Torres HC, Rozemberg B, Amaral MA, Bodstein RC. Perceptions of primary healthcare professionals towards their role in type 2 diabetes mellitus patient education in Brazil. BMC Public Health. 2010;10:583. | |
Scain SF, Friedman R, Gross JL. A structured educational program improves metabolic control in patients with type 2 diabetes: a randomized controlled trial. Diabetes Educ. 2009;35(4):603–611. | |
Robles C, Mirosevic V. Social protection systems in Latin America and the Caribbean: Brazil. Economic Commission for Latin America, September 2013. Available from: http://www.cepal.org/publicaciones/xml/7/51397/SPS_Brasil_esp.pdf. Accessed July 8, 2014. | |
Pinto Cdu B, Miranda ES, Emmerick IC, Costa Ndo R, Castro CG. Medicine prices and availability in the Brazilian Popular Pharmacy Program. Rev Saude Publica. 2010;44(4):611–619. | |
Brasil. Ministério da Saúde. SISHIPERDIA [Internet]. DATASUS Departamento de Informática do SUS. 2011. Available from: http://hiperdia.datasus.gov.br/. Acessed July 8, 2014. | |
Correia LO, Padilha BM, Vasconcelos SM. [Accuracy of registration data for patients with arterial hypertension and diabetes mellitus recorded in the Hiperdia system in a state in the northeast of Brazil]. Ciencia and Saude Coletiva. 2014;19(6):1685–1697. Portuguese. | |
Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. | |
Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. | |
Almeida-Pititto B, Griffin SJ, Sharp SJ, Hirai AT, Gimeno SG, Ferreira SR. A behavioral intervention in a cohort of Japanese-Brazilians at high cardiometabolic risk. Rev Saude Publica. 2012;46(4):602–609. | |
Barros CR, Cezaretto A, Salvador EP, Santos TC, Siqueira-Catania A, Ferreira SRG. [Um programa estruturado, factível e promissor de hábitos de vida saudáveis para redução de risco cardiometabólico]. Arq Brás Endocrinol Metabol. 2013;57(1):7–18. Portuguese. | |
Sartorelli DS, Sciarra EC, Franco LJ, Cardoso MA. Primary prevention of type 2 diabetes through nutritional counseling. Diabetes Care. 2004;27(12):3019. | |
[No authors listed]. Diabetes Control and Complications Trial: results of feasibility study. The DCCT Research Group. Diabetes Care. 1987;10(1):1–19. | |
King P, Peacock I, Donnelly R. The UK prospective diabetes study (UKPDS):clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 1999;48(5):643–648. | |
Pedersen O, Gaede P. Intensified multifactorial intervention and cardiovascular outcome in type 2 diabetes: the Steno-2 study. Metabolism. 2003;52(8 Suppl 1):19–23. | |
Margolis KL, O’Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care. 2014;37(6):1721–1728. | |
Hirakawa Y, Arima H, Zoungas S, et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care. 2014;37(8):2359–2365. | |
Azad N, Agrawal L, Emanuele NV, Klein R, Bahn GD, Reaven P. Association of blood glucose control and pancreatic reserve with diabetic retinopathy in the Veterans Affairs Diabetes Trial (VADT). Diabetologia. 2014;57(6):1124–1131. | |
Campos FE, Haddad AE, Chao LW, Alkmim MBM, Cury PM. The National Telehealth Program in Brazil: an instrument of support for primary health care. Latin American Journal of Telehealth. 2009;1(1):39–59. | |
Chao LW. [Telemedicina e Telessaúde – Um panorama no Brasil. IP (Informática Pública)]. Prefeitura de Belo Horizonte. 2008;10(2):7–15. | |
Engelgau MM, Narayan KM, Herman WH. Screening for type 2 diabetes. Diabetes Care. 2000;23(10):1563–1580. | |
SIHD [homepage on the Internet]. Brazil: SUS Hospital Information System, Ministry of Health; 2014. Available from: http://www2.datasus.gov.br/SIHD. Accessed December 12, 2014. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

