Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 6
Tympanostomy tubes: patient selection and special considerations
Authors Whittemore Jr K
Received 22 January 2015
Accepted for publication 7 March 2015
Published 6 May 2015 Volume 2015:6 Pages 41—43
DOI https://doi.org/10.2147/PHMT.S61938
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Laurens Holmes, Jr
Kenneth R Whittemore Jr
Department of Otolaryngology and Communication Enhancement, Boston Children's Hospital, Boston, MA, USA
Abstract: Indications for tympanostomy tube (TT) placement in children with chronic serous effusions and hearing loss are fairly well established. The use of TTs for children with recurrent acute otitis media (rAOM) has not been studied as extensively as for those with hearing loss from middle-ear effusions. There is a subset of children who fall outside established guidelines for either of the preceding categories who may benefit from TT placement. The indications for TT placement in children with middle-ear disease are discussed.
Keywords: tympanostomy tubes, pediatrics, otitis media with effusion, acute otitis media, hearing loss
Middle-ear disease and tympanostomy tubes
Middle-ear disease in children is one of the most common ailments that a child is seen for on sick visit in a pediatric practice. The diagnosis of middle-ear disease falls into two major categories that have some overlap, as a child may have either recurrent acute otitis media (rAOM) or chronic serous otitis media (CSOM) alone or in combination. The medical treatment for episodes of acute otitis media in the United States is generally the administration of antibiotics and analgesics. The medical treatment for children with CSOM is observation and assessment of language development, hearing, and speech. Surgical treatment for rAOM and CSOM generally includes the placement of tympanostomy tubes (TTs). The purpose of this article is to discuss the clinical situations in which the placement of TTs may be considered for treating CSOM, rAOM, and special clinical circumstances where TTs can be considered outside the purview of those classic diagnoses.
The American Academy of Otolaryngology – Head and Neck Surgery (AAO-HNS) has published clinical practice guidelines related to the placement of TTs.1 For treatment of CSOM, they recommend against surgical treatment for children with a single episode of otitis media with effusion (OME) which has lasted less than 3 months from date of onset (or diagnosis, if onset is not known), as spontaneous resolution is likely in uncomplicated children. If a child has experienced bilateral OME for a minimum of 3 months and has a documented hearing loss upon audiological evaluation, bilateral TT placement is recommended.1 Additionally, clinicians may provide TTs for unilateral or bilateral CSOM that lasts at least 3 months and is symptomatic, ie, vestibular issues, difficulties with school, and problems with behavior, ear pain, or reduced quality of life.
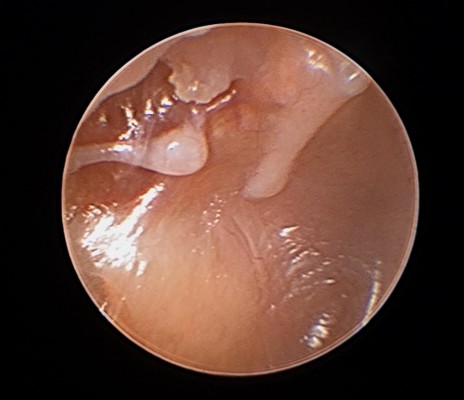
There are some populations where one may consider placement of TTs earlier than the guidelines would recommend. One such population would be children with severe speech and language delay;1 this population may benefit from earlier TT placement to maximize the benefits of speech and language therapy. Patients with craniofacial abnormalities predisposing them to CSOM and conductive hearing loss may also benefit from earlier intervention.1 Additionally, there are some patients with chronic eustachian tube dysfunction with severe tympanic membrane retraction that can result in either breakdown of the ossicles (Figure 1) or formation of cholesteatoma over time. Though fluid may not be present in the middle ear, pressure equalization with TTs to help prevent such complications may be indicated. The timing and placement of TTs in these select populations is up to the discretion of the treating practitioner.
  | Figure 1 Otoscopic image showing retraction of the tympanic membrane resulting in a breakdown of the long process of the incus. |
Practice guidelines also include treatment for rAOM. The AAO-HNS clinical practice guidelines recommend against TTs in cases of rAOM where middle-ear effusion is not currently present, whereas they recommend TT placement for children with rAOM who have a unilateral or bilateral effusion at time of assessment for surgery.1 When discussing AOM versus OME, definitions are important. The American Academy of Pediatrics (AAP) defines AOM to be diagnosed in the following clinical scenarios when middle-ear fluid is present: moderate to severe bulging of the tympanic membrane, mild bulging of the tympanic membrane with recent associated signs of otalgia, or the presence of otorrhea not secondary to otitis externa.2 Furthering this, the AAO-HNS defines rAOM as three distinct episodes of AOM over 6 months or four distinct episodes over 12 months,1 and the AAP has the stipulation of having had at least one episode in the preceding 6 months.2
Although clinical practice guidelines have clearly defined rAOM and OME, clinical trials do not always employ such specified boundaries and vary in regards to patient populations and treatments implemented. Additionally, they tend to have small sample sizes. As such, the evidence is limited for TT placement for rAOM, though a prior review by Whittemore3 found evidence that having TTs placed was better than having no surgical intervention;4 there were improvements in measures of the patient’s quality of life, caregiver concerns, hearing loss, and frequency of AOM episodes in the first 6–12 months following TTs.4 Previously, antibiotic prophylaxis was used, and was found to be superior to placebo for treatment; however, due to the risk of antibiotic resistance, it is not currently recommended unless a child is contraindicated for surgical treatment.5 Further randomized controlled studies are needed to have a clearer clinical platform from which a decision regarding the candidacy of a patient with rAOM would benefit from TTs.
Clinical practice guidelines apply only to typically developing children; children at risk for developmental delays or children with a complex medical history may also require other considerations. Children with a history of AOM who may benefit from the placement, despite not being classified as having rAOM, include children under 6 months of age who are refractory to antibiotic therapy and clinically toxic, as well as those with multiple drug allergies, persistent infection despite both enteral and parenteral antibiotics, complications from AOM such as meningitis, facial nerve paresis or paralysis, immunodeficiencies, intracranial abscess, labyrinthitis, or mastoiditis. The decision to place TTs in a complicated patient is to be considered based upon the clinician’s assessment of the patient’s particular clinical situation. The vast majority of patients, however, without the preceding clinical conditions will be well served being evaluated using evidence-based clinical practice.
Acknowledgment
I would like to acknowledge Jenna Dargie for her assistance in putting this paper together.
Disclosure
The author reports no conflicts of interest in this work.
References
Rosenfeld RM, Schwartz SR, Pynnonen MA, et al. Clinical practice guideline: tympanostomy tubes in children. Otolaryngol Head Neck Surg. 2013;149:S1–S35. | |
Lieberthal AL, Carroll AE, Chonmaitree T, et al. Clinical practice guideline: the diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964–e999. | |
Whittemore KR. What is the role of tympanostomy tubes in the treatment of recurrent acute otitis media? Laryngoscope. 2013;123:9–10. | |
Rosenfeld RM. Surgical prevention of otitis media. Vaccine. 2001;19: S134–S139. | |
Casselbrant ML, Kaleida PH, Rockette HE, et al. Efficacy of antimicrobial prophylaxis and of tympanostomy tube insertion for prevention of recurrent acute otitis media: results of a randomized clinical trial. Pediatr Infect Dis J. 1992;11:278–286. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
