Back to Journals » Drug Design, Development and Therapy » Volume 12
Two-drug regimens for treatment of naïve HIV-1 infection and as maintenance therapy
Authors Corado KC, Caplan MR , Daar ES
Received 31 August 2018
Accepted for publication 15 October 2018
Published 1 November 2018 Volume 2018:12 Pages 3731—3740
DOI https://doi.org/10.2147/DDDT.S140767
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Katya C Corado, Margaret R Caplan, Eric S Daar
Department of Medicine, Division of HIV Medicine, David Geffen School of Medicine at UCLA, Harbor-UCLA Medical Center, Torrance, CA, USA
Abstract: As people live longer with HIV infection, there has been a resurgence of interest in challenging the use of three-drug therapy, including two nucleoside reverse transcriptase inhibitors plus a third drug, as initial treatment of HIV infection or for maintenance therapy in virologically suppressed individuals. Although initial studies showed poor efficacy and/or substantial toxicity, more recent regimens have held greater promise. The SWORD-1 and -2 studies were pivotal trials of dolutegravir plus rilpivirine as maintenance therapy in virologically suppressed patients with no history of drug resistance, leading to the US Food and Drug Administration’s approval of the regimen as a small, single tablet. More recently, the GEMINI-1 and -2 studies demonstrated that dolutegravir plus lamivudine is as safe and effective as the same regimen when combined with tenofovir disoproxil fumarate in treatment-naïve individuals. Together, these and other studies of novel two-drug regimens offer the potential for improved tolerability and simplicity, as well as a reduction in cost. We will review historical and recent trials of two-drug therapy for the treatment of HIV-1 infection.
Keywords: two-drug therapy, HIV-1 infection, treatment strategies, initial therapy, maintenance therapy
Introduction
Antiretroviral therapy (ART) for the treatment of HIV-1 infection has evolved significantly since the beginning of the epidemic. In the USA, the Food and Drug Administration (FDA) first approved zidovudine (ZDV), a nucleoside reverse transcriptase inhibitor (NRTI), in 1987 for the treatment of HIV-1-infected individuals.1 Although NRTIs have remained the mainstay of ART for the past 30 years, many lessons were learned from the initial use of ZDV as monotherapy. These lessons included the fact that the high level of viral replication and error-prone reverse transcriptase enzyme results in the rapid development of drug-resistant virus.2,3 In addition, ZDV and other early NRTIs induced mitochondrial dysfunction, which led to observed toxicities, such as hematological derangements, liver injury, lactic acidosis, myopathy, and lipodystrophy.4–6
In the decade that followed the FDA approval of ZDV, other NRTIs became available and were utilized either as monotherapy or in combination with each other.7 Unfortunately, didanosine (1991), zalcitabine (1992), and stavudine (1994), either alone or in combination, were associated with substantial toxicity or did not lead to complete viral suppression, consistently allowing for the emergence of broad NRTI resistance. In 1996, presentations at the 11th International AIDS Conference highlighted the effectiveness of combination ART, with triple-drug therapy. These data supported the recommendation of using two NRTIs, forming the “NRTI backbone,” along with a third agent, such as a protease inhibitor (PI) or a non-nucleoside reverse transcriptase inhibitor (NNRTI), as preferred first-line treatment options.8 Since that time, new ARV classes, such as integrase strand transfer inhibitors (INSTIs), have been developed and approved as components of first-line therapy. These include raltegravir (RAL) approved in 2007, elvitegravir (EVG) as part of a fixed dose combination in 2012, dolutegravir (DTG) in 2013, and bictegravir (BIC) in 2018, all of which are recommended for first-line therapy along with two NRTIs.9,10
Although newer NRTIs, such as abacavir (ABC), tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide (TAF), are better tolerated than earlier generations, they continue to have tolerability and toxicity issues, such as hypersensitivity reactions, renal and bone impairment, and possible association with cardiovascular events.11 In order to further minimize toxicity and simplify regimens, several studies have attempted to deviate from the use of the two-NRTI backbone plus a third drug for initial and maintenance therapy. This review will focus on two-drug therapy for HIV-1-infected people. Key trials from each of the following sections, ie, large, randomized, or pivotal proof-of-concept trials, are highlighted in Tables 1–4.
Treatment-naïve two-drug regimens
While the use of an NRTI backbone currently remains part of all recommended first-line regimens, an NRTI-sparing regimen or a regimen that does not include two NRTIs may be preferred in select cases.12 Before considering studies that deviate from the two-NRTI plus a third drug paradigm, it is important to review lessons learned from early studies attempting induction with standard ART and then switching to a fewer than three-drug regimen. The Trilège13 study enrolled 378 participants who were ART naïve and given 3 months of ZDV, lamivudine (3TC), and indinavir (IDV). Participants who attained a viral load of less than 500 copies/mL were then randomized into one of three maintenance groups consisting of either continued ZDV, 3TC + IDV, or ZDV + 3TC, or ZDV + IDV. Median duration of follow-up was 6 months owing to premature termination of the study by the safety monitoring board when it became clear that relapse rates where significantly higher in both two-drug therapy groups. Similar findings were seen in the ACTG 343,14 where, after a 3-month induction with ZDV, 3TC + IDV, 316 participants were randomized to either continue triple therapy, or IDV alone, or ZDV + 3TC, with greater rates of viral rebound in participants not receiving three drugs. From these studies, we learned that regimens with two NRTIs alone or those with one NRTI plus a non-pharmacologically boosted PI, such as IDV, would not be sufficient to consistently maintain viral suppression.
NRTI-sparing two-drug regimens
Renewed interest in two-drug regimens occurred with the realization that pharmacologically boosted PIs had much higher barriers to resistance than other agents. The desire to pursue two-drug regimens was further fueled by increasing recognition of the long-term adverse events associated with select NRTIs. As a result, most subsequent studies of two-drug therapy focused on NRTI-sparing options. Key studies in this group are summarized in Table 1. ACTG 5142 compared three regimens for initial therapy in 757 treatment-naïve participants with HIV-1 infection; efavirenz (EFV) + two NRTIs, ritonavir-boosted lopinavir (LPV/r) + two NRTIs, or LPV/r + EFV.15 At week 96, virologic efficacy was similar in the EFV + two NRTIs and LPV/r + EFV arms (89% vs 83%). However, in cases of virologic failure, resistance occurred more frequently and there was increased toxicity in those using the NRTI-sparing regimen.
LPV/r was further studied in combination with RAL in two studies. The PROGRESS study compared this regimen to LPV/r + two NRTIs,16 and CCTG 589 study compared it to EFV + TDF + emtricitabine (FTC).17 Week 96 data from PROGRESS (n=206) showed similar proportions of responders and reported greater reductions in estimated glomerular filtration rate and bone mineral density in the two-NRTI group. A limitation of this study was that it was relatively small and had few enrolled with high baseline plasma HIV-1 RNA levels. CCTG 589 also reported no difference in viral suppression between the two arms (N=52), although there was significantly faster viral decay during the first 2 weeks in the LPV/r + RAL study group.
RAL was further studied in combination with atazanavir (ATV) as well as with boosted darunavir (DRV/r). Ninety-four participants were randomized 2:1 to receive an experimental dose of ATV, 300 mg twice daily, with RAL compared to ritonavir-boosted ATV (ATV/r) + two NRTIs.18 While week 24 data showed similar efficacy for viral load suppression in both arms, the study was stopped early owing to high rates of hyperbilirubinemia as well as the development of RAL resistance in the NRTI-sparing regimen. The NEAT study was a fully powered randomized trial which had promising results when comparing DRV/r + RAL to DRV/r + two NRTIs.19,20 This large European study randomized 805 participants into the two groups, with week 96 data showing treatment failure of 19% in the RAL vs 15% in the two-NRTI group, meeting criteria for non-inferiority. However, the RAL group was virologically inferior for those with baseline CD4 cell count of less than 200 cells/mm3, and more virologic failures and INSTI resistance were observed in those with baseline plasma HIV-1 RNA levels greater than or equal to 100,000 copies/mL. Inferior efficacy was also observed in participants with lower CD4 cell counts and higher HIV viral loads enrolled in the smaller single-arm ACTG 5262 study, where 112 individuals were given DRV/r + RAL.21
The CCR5 antagonist maraviroc (MVC) has also been examined in combination with different boosted PIs. A small study comparing LPV/r + MVC vs LPV/r + two NRTIs enrolled 50 participants; week 48 data showed 100% viral suppression in the MVC and 96% in the two-NRTI arm.22 Another small study (N=98) compared ATV/r + MVC (n=32) vs two NRTIs + one NNRTI or a PI.23 Participants in both groups experienced similar percentages of viral load suppression at week 48. However, these results did not hold true in larger studies comparing ATV/r + MVC vs ATV/r + two NRTIs (N=121), where similar viral load suppression was achieved at week 48, except in those with baseline plasma HIV-1 RNA greater than 100,000 copies/mL, where the MVC group was virologically inferior.24 In the MODERN trial, the largest trial studying a boosted PI combined with MVC, DRV/r + MVC vs DRV/r + two NRTIs, 797 participants were enrolled, and the study was closed early owing to virologic inferiority in the MVC group (viral load suppression of 77.3% for MVC vs 86.8% for the two-NRTI group).25
Single-NRTI two-drug regimens
While the studies in the previous subsection focused on NRTI-sparing regimens, two-drug regimens using a single NRTI, such as TDF or 3TC, along with a second drug of a different class have also been studied in treatment-naïve individuals. Key studies in this group are summarized in Table 2. The KALEAD study evaluated TDF + LPV/r, both given once daily.26 The study enrolled 152 participants randomized to either LPV/r + TDF or LPV/r + two NRTIs. Of note, the two-NRTI group did not contain TDF since it was not yet recommended in the Italian treatment guidelines at the time of the study. Discontinuation rates were high in both groups (41.7% and 43.8%, respectively), and at 72 weeks, the proportion of those achieving viral load suppression was 51.4% in the TDF and 52.5% in the two-NRTI arm. The investigators concluded that while results in both treatment groups were comparable, the TDF arm was not statistically non-inferior, and they ultimately acknowledged that the study was underpowered and had unexpectedly high discontinuation rates.
Because 3TC is an NRTI without major side effects, the GARDEL study compared LPV/r (400/100 mg twice daily) + 3TC (150 mg twice daily) to LPV/r (400/100 mg twice daily) + two NRTIs. The study randomized 426 participants, with 48-week data demonstrating viral suppression rates of 88.3% in the 3TC and 83.7% in the two-NRTI arm, including those with baseline plasma HIV-1 RNA greater than or equal to 100,000 copies/mL.27 It was noted that toxicity- and tolerability-related discontinuations were more common in the two-NRTI group. While this was an encouraging result, pill burden and twice-daily dosing used in this study remained a challenge. In addition, the expected gastrointestinal and metabolic disturbances with LPV/r were problematic. The first Phase of the ANDES study evaluated the better tolerated boosted PI, DRV/r + 3TC (n=75) compared to DRV/r + TDF/FTC (n=70), and demonstrated viral suppression at 48 weeks in 93% and 94%, respectively, with similar tolerability.28 The plan was to assess the results of the first Phase and, if promising, to expand the sample size to generate a fully powered clinical trial of this novel two-drug regimen.
DTG has emerged as an INSTI with a high barrier to resistance, minimal drug–drug interactions, good tolerability, and ease of administration, given once daily with or without food.29 As noted, all promising two-drug regimens following Trilège and ACTG 343 have included a boosted PI as the sole high-barrier-to-resistance drug. DTG may now offer another high-barrier-to-resistance option. The PADDLE study was a first attempt to assess whether DTG could be used as part of a two-drug regimen.30 This proof-of-concept study evaluated the efficacy, safety, and tolerability of DTG + 3TC given once daily to 20 treatment-naïve individuals with plasma HIV-1 RNA of less than or equal to 100,000 copies/mL and CD4 cell counts greater than 200 cells/mm3. All participants were suppressed by week 8, and week 48 data showed that 90% (18/20) maintained viral suppression. Of the two individuals not suppressed at week 48, one died by suicide, an event not thought to be study-drug related, and the other had low-level viremia that resuppressed without change in therapy. No major tolerability or toxicity issues were observed. This study was followed by ACTG 5353, which enrolled 120 treatment-naïve participants with baseline plasma HIV-1 RNA less than 500,000 copies/mL into a single-arm study of DTG + 3TC given once daily.31 This study demonstrated high levels of virologic suppression, with similar outcomes in those with baseline plasma HIV-1 RNA levels less or equal to and greater than 100,000 copies/mL. However, there was a single individual who selected for integrase resistance and NRTI resistance mutations, which had not previously been seen in any treatment-naïve trial of DTG + two NRTIs.
As follow-up to PADDLE and ACTG 5353, two identical Phase III trials, GEMINI-1 and GEMINI-2 (NCT-02831673 and NCT-02831764), are ongoing at the time of writing this review, evaluating DTG + 3TC vs DTG + TDF/FTC for treatment-naïve individuals with plasma HIV-1 RNA levels of 1,000–500,000 copies/mL. Week 48 primary outcome data were presented at the 22nd International AIDS Conference: 1,433 participants were randomized 1:1 into the above groups, with approximately 20% having viral loads above 100,000 copies/mL and median CD4 cell counts of approximately 400 cells/mm3.32 Pooled snapshot outcomes at week 48 showed 91% viral load suppression in the DTG + 3TC and 93% in the DTG + two-NRTI arm, meeting criteria for non-inferiority, with similar results seen in those with baseline plasma HIV-1 RNA levels greater than 100,000 copies/mL. Of the six participants on the two-drug and four on the three-drug regimen who met criteria for protocol-defined virologic failure, none developed INSTI or NRTI resistance mutations. It is important to note that for pooled outcomes at week 48 for the subgroup with baseline CD4 cell counts less than 200 cells/mm3, snapshot analysis showed virologic suppression of 79% in the DTG + 3TC vs 93% in the DTG + two-NRTI group. This difference disappeared in the treatment-related discontinuation = failure analysis. The investigators noted that the difference was not driven by increased rates of virologic failure. In fact, of the 13 participants who were considered snapshot non-response in the DTG + 3TC arm, two resuppressed on the same regimen, two discontinued owing to adverse events (tuberculosis and Chagas disease), two had protocol violations, two were lost to follow-up, one withdrew consent, one withdrew to start hepatitis C therapy, and one changed ART as a result of incarceration. In addition, safety and tolerability profiles were comparable between the two groups.
Maintenance therapy in virologically suppressed individuals
Given the disappointing results of earlier induction–maintenance strategies for the treatment of HIV-1-infected people, such as in Trilège and ACTG 343, studies of dual ART strategies were abandoned for nearly a decade. A resurgence of studies assessing the safety and efficacy of two-drug regimens for maintenance therapy in virologically suppressed individuals has occurred to enhance convenience and tolerability, and potentially reduce costs. The following subsections will review studies of NRTI-sparing two-drug regimens (Table 3) and single-NRTI-containing two-drug regimens (Table 4) for maintenance of viral suppression.
NRTI-sparing two-drug regimens
Similarly to the interest in using boosted PIs + INSTIs in HIV treatment-naïve individuals, several small studies have evaluated this combination as maintenance therapy, as summarized in Table 3. The KITE Study randomized (2:1) 60 individuals without a history of virologic failure and on a stably suppressive regimen for at least 6 months to receive LPV/r + RAL or to continue on their current three-drug regimen.33 Week 48 data showed maintenance of viral load suppression in 92% of those in the RAL vs 88% in the three-drug study arm.
In contrast, ATV studies did not yield the same results as the LPV/r-based regimens. A retrospective study of the Dat’AIDS cohort followed patients who switched to ATV + RAL or ATV/r + RAL between 2008 and 2014 for up to 96 weeks.34 The cohort (N=283) was not an optimal study population, with 45% having a history of virologic failure, 20% having detectable viral load at the time of starting two-drug therapy, and 47% switching to two-drug therapy owing to ART-related adverse effects. While no difference was found between the boosted and unboosted ATV groups, cumulative percentages of participants remaining free of therapeutic failure at week 48 (65.4%) and week 96 (53.4%) were low. Another retrospective trial with unboosted ATV + RAL showed similarly concerning results (N=102, 18.6% failure at 123 weeks).35 Both of these trials reported frequent selection for RAL resistance. HARNESS was a prospective trial comparing ATV/r + RAL vs ATV/r + two NRTIs for maintenance therapy in individuals who did not have a history of virologic failure or exposure to ATV or RAL.36 Results at 48 weeks supported conclusions of the retrospective trials, with 69.4% in the RAL group maintaining virologic suppression vs 86.5% of those in the two-NRTI group. In a more promising small Japanese study of boosted DRV,37 28 stably suppressed individuals without a history of virologic failure switched from LPV/r + two NRTIs to DRV/r + RAL, with 100% maintaining virologic suppression at week 48.
The novel combination of RAL and an NNRTI has been evaluated for maintenance therapy in small studies. RAL + etravirine (ETR) was examined in a small retrospective (N=18),38 an observational (N=25),39 and a prospective trial (N=38).40 These studies showed rates of viral suppression at week 48 to often be higher than 80%; however, they were small and not randomized, and when virologic failure was seen, resistance was often documented to RAL and/or ETR.
As in the ART-naïve trials with dual therapy, MVC did not perform well in two-drug maintenance regimens. The MARCH study took individuals who were virologically suppressed on a boosted PI + two NRTIs and placed them into one of three study arms: continue current therapy, replace the boosted PI with MVC, or replace the two-NRTI with MVC (MVC + boosted PI).41 Results at 48 weeks showed ongoing virologic suppression of 97.6%, 93.6%, and 84.1%, respectively, demonstrating inferiority of the NRTI-sparing regimen. Similarly, high rates of virologic failure in the MVC + DRV/r arm of the GUSTA trial led to early study termination.42
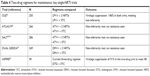
Perhaps the most promising NRTI-sparing maintenance studies utilized rilpivirine (RPV) with a boosted PI or INSTI. In a proof-of-concept study, 60 participants with no previous resistance to study drugs who were stably suppressed on ART were continued on a boosted PI + two NRTIs or switched to DRV/r + RPV.43 At 48 weeks, 93.4% and 96.7% of participants continued to be virologically suppressed, respectively. In addition, there has been much enthusiasm about the results of newer INSTI medications combined with RPV. Cabotegravir (CAB) is a new INSTI that is a structural analog of DTG, having a half-life of 40 hours with oral dosing and minimal drug interactions.44 It has been studied in oral form in combination with oral RPV in the LATTE study and in a long-acting (LA) injectable form along with injectable LA RPV in LATTE-2.45,46 In LATTE, 243 participants were assigned 1:1:1:1 to receive oral CAB (10 mg, 30 mg, 60 mg) or EFV 600 mg along with two NRTIs for the first 24-week induction period. Those virologically suppressed at week 24 continued to the maintenance Phase (86% CAB group, 74% EFV group), with those on CAB having NRTIs switched to oral RPV 25 mg, with the others remaining on EFV + two NRTIs for 72 weeks. Week 48 data demonstrated that 82% in the CAB group and 71% in the EFV group were virologically suppressed, and at week 96, 76% and 63% had undetectable levels of plasma HIV-1 RNA, respectively. Combined efficacy and safety results showed that a CAB dose of 30 mg was optimal. In LATTE-2, treatment-naïve individuals were given a 20-week induction with oral CAB 30 mg + ABC/3TC, and once virologically suppressed, randomized to intramuscular LA CAB + LA RPV at 4-week (400 and 600 mg, respectively) or 8-week (600 and 800 mg, respectively) intervals, or to continue oral therapy. Of the 286 participants followed during the maintenance period, viral suppression was observed at 32 weeks in 91%, 94%, and 95%, respectively. Week 96 data demonstrated viral suppression of 84%, 87%, and 94%, respectively. Injectable formulations were found to be well accepted and tolerated. Fully powered Phase III registrational maintenance trials are fully enrolled and in follow-up and will compare two NRTIs with INSTI given once daily to dosing every 4 weeks with LA CAB + LA RPV (ATLAS, NCT02951052 and FLAIR, NCT02938520) and dosing every 4 weeks vs every 8 weeks with the LA preparations (ATLAS-2M, NCT03299049).
Promising findings were also seen with the use of DTG + RPV as maintenance therapy. An Italian observational cohort study followed 132 patients who were switched by their providers for clinical reasons to DTG + RPV.47 Forty-three percent had at least one failure with previous ART, 45% had reverse transcriptase mutations, including to RPV, and one had INSTI resistance. Despite this, viral suppression was maintained in 81% at week 24 and in 90.9% at week 48, with similar findings seen at week 96.48 SWORD-1 and SWORD-2 are recently published Phase III studies.49 These identical studies randomized 1,024 individuals, who were on their first or second ART regimen without history of virologic failure, to switch to DTG + RPV or continue their current regimen. At week 52, those who had continued on their current regimen were also switched to DTG + RPV. At the time of enrollment, study subjects had been on a stable regimen for an average of over 4 years, with approximately 70% receiving TDF and FTC as their NRTI backbone. Week 48 data showed that 95% of SWORD-1 and 94% of SWORD-2 participants on DTG + RPV had viral loads less than 50 copies/mL compared to 96% of SWORD-1 and 94% of SWORD-2 subjects who remained on their original ART. In pooled analysis of the intention-to-treat population, 95% maintained viral loads less than 50 copies/mL in both treatment groups, meeting non-inferiority criteria. In 2017, the FDA approved the combination DTG + RPV as a single-pill form.1
Single-NRTI two-drug regimens
Several studies have assessed the safety and efficacy of a single NRTI plus a second drug for maintenance of viral suppression. Key studies in this group are summarized in Table 4. The COOL study enrolled 143 participants who were suppressed on EFV, 3TC + TDF for at least 6 months and randomized them to receive EFV + TDF or remain on triple therapy.50 Viral suppression at 48 weeks was maintained in 81.7% and 97.2%, respectively, with the two-drug regimen not meeting prespecified non-inferiority criteria. In addition, three individuals from the two-drug regimen group developed EFV resistance. Once again, the COOL study appears to confirm that a two-drug regimen needs at least one active agent with a high barrier to resistance.
Several studies evaluated a boosted PI + 3TC for maintenance. In a non-inferiority trial, 250 virologically suppressed individuals on LPV/r + either 3TC or FTC + a second NRTI for at least 6 months and no history of virologic failure were randomized to continue triple therapy or switch to LPV/r + 3TC.51 At 48 weeks, 86.6% and 87.8% maintained viral suppression, respectively, meeting non-inferiority criteria. One person in the two-drug arm with virologic failure developed 3TC resistance. ATLAS-M52 and SALT53,54 were large non-inferiority trials studying ATV/r + 3TC for maintenance. In ALTAS-M, 266 participants were on ATV/r + two NRTIs with undetectable viral loads and no history of virologic failure and were randomized to either continue their current regimen or switch to ATV/r + 3TC. Non-inferiority was met when 79.7% and 89.5% of participants, respectively, remained virologically suppressed. In fact, the two-drug arm was found to be superior. The SALT trial enrolled 286 individuals with no history of drug resistance or treatment failure and an undetectable viral load for at least 6 months to receive ATV/r + two NRTIs or ATV/r + 3TC. Sixty-five percent of individuals had already been on a PI-based regimen, 33% were on an NNRTI-based regimen, and 82% had experience with TDF. At 48 weeks, virologic suppression was maintained in 78% in the two-NRTI arm and 84% in the 3TC arm, meeting non-inferiority, which was upheld at 96 weeks with virologic suppression in 73.9% and 74.4% of participants, respectively. Of note, neither ATLAS-M nor SALT demonstrated selection of drug resistance in those randomized to the two-drug study arm.
DRV/r has also been studied in combination with 3TC in the DUAL GESIDA, a non-inferiority trial in Spain where 249 participants were on DRV/r + two NRTIs (either TDF/FTC or ABC/3TC) with no history of resistance and an undetectable viral load for at least 6 months. They were randomized to continue the current regimen or switch to DRV/r + 3TC.55 After 48 weeks of study, the viral load in 92.7% and 88.9% of participants remained undetectable, respectively, meeting non-inferiority, and as in the prior trials, no 3TC resistance was selected for in those experiencing virologic failure.
DTG + 3TC has also been explored for maintenance therapy in several studies. The ASPIRE study randomized in an open-label fashion those virologically suppressed with no history of treatment failure to either continue a standard three-drug regimen (n=45) or to receive DTG + 3TC (n=45).56 At 48 weeks, virologic suppression was maintained in 91% of those assigned to the two-drug and 89% of those assigned to the three-drug regimen. Two small studies compared remaining on a three-drug regimen to switching to DTG + 3TC or DTG monotherapy, both of which provided promising results from the two-drug regimen but high failure rates in the monotherapy study arms.57,58 A fully powered Phase III trial of maintenance therapy with single-tablet DTG/3TC is fully enrolled and in follow-up at the time of writing (TANGO, NCT03446573).
Discussion
This review of two-drug therapies for initial and maintenance treatment of HIV-1-infected people has covered trials from the late 1990s to the present. There are many reasons to study a regimen that contains fewer than three drugs, including the potential for improved tolerability, less toxicity, simpler regimens, and, while not the focus of this review, cost savings, which can be substantial.59 A major lesson learned from the earlier trials was that for both initial and maintenance therapy at least one of the components of the two-drug regimen must have a relatively high barrier to resistance. When this was not the case, virologic failure was more common and often associated with the emergence of drug-resistance mutations. While there were mixed results from many studies of two-drug regimens for both initial and maintenance strategies, it was frequently found that the use of MVC was often associated with poor virologic responses, even when used with a boosted PI.24,25,41,42
In general, trials of maintenance strategies with two-drug regimens have been more successful than studies initiating treatment-naïve patients on such therapy. The strongest data supporting the use of two-drug regimens for maintenance therapy include a boosted PI + 3TC, the very large SWORD-1 and -2 studies demonstrating high rates of suppression with DTG + RPV,49 and the very promising early results with LA CAB + LA RPV.46 Clinicians must remember that most of these studies enrolled stably suppressed subjects without hepatitis B infection since these regimens do not include two active drugs against this virus, and those having no history of treatment failure.
For treatment-naïve patients, the strongest data for two-drug therapy come from the recent results with DTG + 3TC. While longer follow-up for this novel regimen will be important, the data from ACTG 5353,31 PADDLE,30 and the GEMINI-1 and -2 trials32 make a strong case for this combination in the setting where resistance data are available, plasma HIV-1 RNA is up to 500,000 copies/mL, and there is no evidence of chronic hepatitis B infection. There are also strong and emerging data on the use of a boosted PI with 3TC,27,28 with limited and somewhat weaker data on boosted PIs plus INSTIs.16,19,20
Conclusion
A review of the most significant studies including two-drug regiments for the initial treatment of HIV-1 or to maintain virologic suppression has demonstrated that, after more than two decades of three-drug regimens, a paradigm shift may be on the near horizon. For certain populations where drug toxicity needs to be minimized, where there is intolerance of medications, or if a simpler regimen is desired, using two-drug therapy may be considered a safe and cost-effective alternative.
Disclosure
KC Corado has received research support from Gilead Sciences. MR Caplan has received research support from Gilead Sciences. ES Daar has received research support from Gilead Sciences, Merck & Co., and ViiV Healthcare. He has also acted as a consultant for Gilead Sciences, Merck & Co., ViiV Healthcare, and Theratechnologies. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
US Department of Health and Human Services [webpage on the Internet]. FDA approval of HIV medicines; 2018. Available from: https://aidsinfo.nih.gov/understanding-hiv-aids/infographics/25/fda-approval-of-hiv-medicines. Accessed July 18, 2018. | ||
Antonelli G, Turriziani O, Verri A, et al. Long-term exposure to zidovudine affects in vitro and in vivo the efficiency of phosphorylation of thymidine kinase. AIDS Res Hum Retroviruses. 1996;12(3):223–228. | ||
Gröschel B, Cinatl J, Cinatl J. Viral and cellular factors for resistance against antiretroviral agents. Intervirology. 1997;40(5–6):400–407. | ||
Chariot P, Drogou I, de Lacroix-Szmania I, et al. Zidovudine-induced mitochondrial disorder with massive liver steatosis, myopathy, lactic acidosis, and mitochondrial DNA depletion. J Hepatol. 1999;30(1):156–160. | ||
Chiao SK, Romero DL, Johnson DE. Current HIV therapeutics: mechanistic and chemical determinants of toxicity. Curr Opin Drug Discov Devel. 2009;12(1):53–60. | ||
Pan-Zhou XR, Cui L, Zhou XJ, Sommadossi JP, Darley-Usmar VM. Differential effects of antiretroviral nucleoside analogs on mitochondrial function in HepG2 cells. Antimicrob Agents Chemother. 2000;44(3):496–503. | ||
Carpenter CC, Fischl MA, Hammer SM, et al. Antiretroviral therapy for HIV infection in 1996. Recommendations of an international panel. International AIDS Society-USA. JAMA. 1996;276(2):146–154. | ||
Carpenter CC, Fischl MA, Hammer SM, et al. Antiretroviral therapy for HIV infection in 1997. Updated recommendations of the International AIDS Society-USA panel. JAMA. 1997;277(24):1962–1969. | ||
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. US Department of Health and Human Services; 2018. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf | ||
Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2018 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320(4):379–396. | ||
Corado KC, Daar ES. Emtricitabine + tenofovir alafenamide for the treatment of HIV. Expert Opin Pharmacother. 2017;18(4):427–432. | ||
Pasquau J, Hidalgo-Tenorio C. Nuke-Sparing Regimens for the Long-Term Care of HIV Infection. AIDS Rev. 2015;17(4):220–230. | ||
Pialoux G, Raffi F, Brun-Vezinet F, et al. A randomized trial of three maintenance regimens given after three months of induction therapy with zidovudine, lamivudine, and indinavir in previously untreated HIV-1-infected patients. Trilège (Agence Nationale de Recherches sur le SIDA 072) Study Team. N Engl J Med. 1998;339(18):1269–1276. | ||
Havlir DV, Marschner IC, Hirsch MS, et al. Maintenance antiretroviral therapies in HIV-infected subjects with undetectable plasma HIV RNA after triple-drug therapy. AIDS Clinical Trials Group Study 343 Team. N Engl J Med. 1998;339(18):1261–1268. | ||
Riddler SA, Haubrich R, Dirienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358(20):2095–2106. | ||
Reynes J, Trinh R, Pulido F, et al. Lopinavir/ritonavir combined with raltegravir or tenofovir/emtricitabine in antiretroviral-naive subjects: 96-week results of the PROGRESS study. AIDS Res Hum Retroviruses. 2013;29(2):256–265. | ||
Karris MY, Jain S, Bowman VQ, et al. Nucleoside-Sparing Regimens With Raltegravir and a Boosted Protease Inhibitor: An Unsettled Issue. J Acquir Immune Defic Syndr. 2016;72(2):e48–e50. | ||
Kozal MJ, Lupo S, Dejesus E, et al. A nucleoside- and ritonavir-sparing regimen containing atazanavir plus raltegravir in antiretroviral treatment-naïve HIV-infected patients: SPARTAN study results. HIV Clin Trials. 2012;13(3):119–130. | ||
Lambert-Niclot S, George EC, Pozniak A, et al. Antiretroviral resistance at virological failure in the NEAT 001/ANRS 143 trial: raltegravir plus darunavir/ritonavir or tenofovir/emtricitabine plus darunavir/ritonavir as first-line ART. J Antimicrob Chemother. 2016;71(4):1056–1062. | ||
Raffi F, Babiker AG, Richert L, et al. Ritonavir-boosted darunavir combined with raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults infected with HIV-1: 96 week results from the NEAT001/ANRS143 randomised non-inferiority trial. Lancet. 2014;384(9958):1942–1951. | ||
Taiwo B, Zheng L, Gallien S, et al. Efficacy of a nucleoside-sparing regimen of darunavir/ritonavir plus raltegravir in treatment-naive HIV-1-infected patients (ACTG A5262). AIDS. 2011;25(17):2113–2122. | ||
Nozza S, Galli L, Antinori A, et al. Maraviroc 150 mg daily plus lopinavir/ritonavir, a nucleoside/nucleotide reverse transcriptase inhibitor-sparing regimen for HIV-infected naive patients: 48-week final results of VEMAN study. Clin Microbiol Infect. 2015;21(5):510.e1–e9. | ||
Pulido I, Genebat M, Alvarez-Rios AI, et al. Immunovirological Efficacy of Once-Daily Maraviroc Plus Ritonavir-Boosted Atazanavir After 48 Weeks in Naive HIV-Infected Patients. Viral Immunol. 2016;29(8):471–477. | ||
Mills A, Mildvan D, Podzamczer D, et al. Maraviroc once-daily nucleoside analog-sparing regimen in treatment-naive patients: randomized, open-label pilot study. J Acquir Immune Defic Syndr. 2013;62(2):164–170. | ||
Stellbrink HJ, Le Fevre E, Carr A, et al. Once-daily maraviroc versus tenofovir/emtricitabine each combined with darunavir/ritonavir for initial HIV-1 treatment. AIDS. 2016;30(8):1229–1238. | ||
Pinola M, Lazzarin A, Antinori A, et al. Lopinavir/ritonavir + tenofovir Dual Therapy versus Lopinavir/ritonavir-Based Triple Therapy in HIV-Infected Antiretroviral Naive Subjects: The Kalead Study. J Antivir Antiretrovir. 2010;2:56–62. | ||
Cahn P, Andrade-Villanueva J, Arribas JR, et al. Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect Dis. 2014;14(7):572–580. | ||
Figueroa MI, Sued OG, Gun AM, et al. DRV/R/3TC FDC for HIV-1 treament naïve patients: week 48 results of the ANDES Study. In: Conference on Retroviruses and Opportunistic Infections; March 4–7, 2018; Boston, MA, USA. Abstract No. 489. | ||
Curtis L, Nichols G, Stainsby C, et al. Dolutegravir: clinical and laboratory safety in integrase inhibitor-naive patients. HIV Clin Trials. 2014;15(5):199–208. | ||
Cahn P, Rolón MJ, Figueroa MI, Gun A, Patterson P, Sued O. Dolutegravir-lamivudine as initial therapy in HIV-1 infected, ARV-naive patients, 48-week results of the PADDLE (Pilot Antiretroviral Design with Dolutegravir LamivudinE) study. J Int AIDS Soc. 2017;20(1):21678. | ||
Taiwo BO, Zheng L, Stefanescu A, et al. ACTG A5353: A Pilot Study of Dolutegravir Plus Lamivudine for Initial Treatment of Human Immunodeficiency Virus-1 (HIV-1)-infected Participants With HIV-1 RNA <500000 Copies/mL. Clin Infect Dis. 2018;66(11):1689–1697. | ||
Cahn P, Madero JS, Arribas J, et al. Non-inferior efficacy of dolutegravir (DTG) plus lamivudine (3TC) versus DTG plus tenofovir/emtricitabine (TDF/FTC) fixed-dose combination in antiretroviral treatment-naïve adults with HIV-1 infection – 48-week results from the GEMINI studies. In: 22nd International AIDS Conference; July 23–27, 2018; Amsterdam, The Netherlands. | ||
Ofotokun I, Sheth AN, Sanford SE, et al. A switch in therapy to a reverse transcriptase inhibitor sparing combination of lopinavir/ritonavir and raltegravir in virologically suppressed HIV-infected patients: a pilot randomized trial to assess efficacy and safety profile: the KITE study. AIDS Res Hum Retroviruses. 2012;28(10):1196–1206. | ||
Gantner P, Bani-Sadr F, Garraffo R, et al. Switch to Ritonavir-Boosted versus Unboosted Atazanavir plus Raltegravir Dual-Drug Therapy Leads to Similar Efficacy and Safety Outcomes in Clinical Practice. PLoS One. 2016;11(10):e0164240. | ||
Marinaro L, Calcagno A, Ripamonti D, et al. Efficacy, safety and pharmacokinetics of atazanavir (200mg twice daily) plus raltegravir (400mg twice daily) dual regimen in the clinical setting. J Clin Virol. 2017;87:30–36. | ||
van Lunzen J, Pozniak A, Gatell JM, et al. Brief Report: Switch to Ritonavir-Boosted Atazanavir Plus Raltegravir in Virologically Suppressed Patients With HIV-1 Infection: A Randomized Pilot Study. J Acquir Immune Defic Syndr. 2016;71(5):538–543. | ||
Nishijima T, Gatanaga H, Shimbo T, et al. Switching tenofovir/emtricitabine plus lopinavir/r to raltegravir plus Darunavir/r in patients with suppressed viral load did not result in improvement of renal function but could sustain viral suppression: a randomized multicenter trial. PLoS One. 2013;8(8):e73639. | ||
Calin R, Paris L, Simon A, et al. Dual raltegravir/etravirine combination in virologically suppressed HIV-1-infected patients on antiretroviral therapy. Antivir Ther. 2012;17(8):1601–1604. | ||
Casado JL, Bañón S, Rodriguez MA, Moreno A, Moreno S. Efficacy and pharmacokinetics of the combination of etravirine plus raltegravir as novel dual antiretroviral maintenance regimen in HIV-infected patients. Antiviral Res. 2015;113:103–106. | ||
Calza L, Magistrelli E, Colangeli V, et al. Dual Raltegravir-Etravirine Combination as Maintenance Regimen in Virologically Suppressed HIV-1-Infected Patients. AIDS Res Hum Retroviruses. 2017;33(7):632–638. | ||
Pett SL, Amin J, Horban A, et al. Maraviroc, as a Switch Option, in HIV-1-infected Individuals With Stable, Well-controlled HIV Replication and R5-tropic Virus on Their First Nucleoside/Nucleotide Reverse Transcriptase Inhibitor Plus Ritonavir-boosted Protease Inhibitor Regimen: Week 48 Results of the Randomized, Multicenter MARCH Study. Clin Infect Dis. 2016;63(1):122–132. | ||
Rossetti B, Gagliardini R, Meini G, et al. Switch to maraviroc with darunavir/r, both QD, in patients with suppressed HIV-1 was well tolerated but virologically inferior to standard antiretroviral therapy: 48-week results of a randomized trial. PLoS One. 2017;12(11):e0187393. | ||
Maggiolo F, di Filippo E, Valenti D, Serna Ortega PA, Callegaro A. NRTI Sparing Therapy in Virologically Controlled HIV-1 Infected Subjects: Results of a Controlled, Randomized Trial (Probe). J Acquir Immune Defic Syndr. 2016;72(1):46–51. | ||
Whitfield T, Torkington A, van Halsema C. Profile of cabotegravir and its potential in the treatment and prevention of HIV-1 infection: evidence to date. HIV AIDS (Auckl). 2016;8:157–164. | ||
Margolis DA, Brinson CC, Smith GHR, et al. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis. 2015;15(10):1145–1155. | ||
Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390(10101):1499–1510. | ||
Capetti AF, Sterrantino G, Cossu MV, et al. Switch to Dolutegravir plus Rilpivirine Dual Therapy in cART-Experienced Subjects: An Observational Cohort. PLoS One. 2016;11(10):e0164753. | ||
Capetti AF, Cossu MV, Sterrantino G, et al. Dolutegravir Plus Rilpivirine as a Switch Option in cART-Experienced Patients: 96-Week Data. Ann Pharmacother. 2018;52(8):740–746. | ||
Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391(10123):839–849. | ||
Girard PM, Cabié A, Michelet C, et al. A randomized trial of two-drug versus three-drug tenofovir-containing maintenance regimens in virologically controlled HIV-1 patients. J Antimicrob Chemother. 2009;64(1):126–134. | ||
Arribas JR, Girard PM, Landman R, et al. Dual treatment with lopinavir-ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a randomised, open-label, non-inferiority trial. Lancet Infect Dis. 2015;15(7):785–792. | ||
Di Giambenedetto S, Fabbiani M, Quiros Roldan E, et al. Treatment simplification to atazanavir/ritonavir + lamivudine versus maintenance of atazanavir/ritonavir + two NRTIs in virologically suppressed HIV-1-infected patients: 48 week results from a randomized trial (ATLAS-M). J Antimicrob Chemother. 2017;72(4):1163–1171. | ||
Perez-Molina JA, Rubio R, Rivero A, et al. Dual treatment with atazanavir-ritonavir plus lamivudine versus triple treatment with atazanavir-ritonavir plus two nucleos(t)ides in virologically stable patients with HIV-1 (SALT): 48 week results from a randomised, open-label, non-inferiority trial. Lancet Infect Dis. 2015;15(7):775–784. | ||
Perez-Molina JA, Rubio R, Rivero A, et al. Simplification to dual therapy (atazanavir/ritonavir + lamivudine) versus standard triple therapy [atazanavir/ritonavir + two nucleos(t)ides] in virologically stable patients on antiretroviral therapy: 96 week results from an open-label, non-inferiority, randomized clinical trial (SALT study). J Antimicrob Chemother. 2017;72(1):246–253. | ||
Pulido F, Ribera E, Lagarde M, et al. Dual Therapy With Darunavir and Ritonavir Plus Lamivudine vs Triple Therapy With Darunavir and Ritonavir Plus Tenofovir Disoproxil Fumarate and Emtricitabine or Abacavir and Lamivudine for Maintenance of Human Immunodeficiency Virus Type 1 Viral Suppression: Randomized, Open-Label, Noninferiority DUAL-GESIDA 8014-RIS-EST45 Trial. Clin Infect Dis. 2017;65(12):2112–2118. | ||
Taiwo BO, Marconi VC, Berzins B, et al. Dolutegravir Plus Lamivudine Maintains Human Immunodeficiency Virus-1 Suppression Through Week 48 in a Pilot Randomized Trial. Clin Infect Dis. 2018;66(11):1794–1797. | ||
Joly V, Burdet C, Landman R, et al. Promising results of dolutegravir + lamivudine maintenance in ANRS 167 LAMIDOL trial. In: Conference on Retroviruses and Opportunistic Infections; February 13–16, 2017; Seattle, WA, USA. Abstract No. 458. | ||
Blanco JL, Rojas J, Paredes R, et al. Dolutegravir-based maintenance monotherapy versus dual therapy with lamivudine: a planned 24 week analysis of the DOLAM randomized clinical trial. J Antimicrob Chemother. 2018:1965–1971. | ||
Girouard MP, Sax PE, Parker RA, et al. The Cost-effectiveness and Budget Impact of 2-Drug Dolutegravir-Lamivudine Regimens for the Treatment of HIV Infection in the United States. Clin Infect Dis. 2016;62(6):784–791. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.