Back to Journals » International Journal of General Medicine » Volume 13
Two Different Minimally Invasive Surgery Puncture Points with Thrombolysis in a Patient with Bilateral Basal Ganglia Hemorrhages
Authors Zhang D , Yu J, Wang Z, Wang Y
Received 28 October 2020
Accepted for publication 23 November 2020
Published 8 December 2020 Volume 2020:13 Pages 1435—1439
DOI https://doi.org/10.2147/IJGM.S289238
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Dongyong Zhang,1 Jiefu Yu,1 Zhenze Wang,1 Yiwei Wang2
1Department of Neurosurgery, The First Affiliated Hospital of China Medical University, Shenyang City, People’s Republic of China; 2Department of Anatomy, Shenyang Medical College, Shenyang City, People’s Republic of China
Correspondence: Dongyong Zhang
Department of Neurosurgery, The First Affiliated Hospital of China Medical University, Nanjing Bei Street, Heping District, Shenyang City 110001, People’s Republic of China
Tel +86 24 83283706
Fax +86 24 83283133
Email [email protected]
Abstract: Bilateral basal ganglia hemorrhages are extremely rare and have very poor prognosis. We describe the case of a 52-year-old woman with a history of hypertension who experienced bilateral basal ganglia hemorrhages. We performed bilateral hematoma aspiration by minimally invasive surgery via frontal and temporal puncture points. We discuss the surgical procedure and review relevant literature pertaining to the underlying causes and complications of similar cases.
Keywords: preexisting hypertension, intracranial hemorrhage, surgical procedure, hemorrhage aspiration, puncture points
Introduction
Spontaneous intracerebral hemorrhage (ICH) is relatively common and has devastating consequences including a high mortality and disability, with a 1-year survival of ~40%.1–3 ICH is frequently associated with hypertension or cerebral amyloid angiopathy.4 Most cases are treated by conservative management while in others, surgical evacuation of the hematoma is recommended. Surgical approaches include craniotomy, neuron-endoscopy, and minimally invasive surgery (MIS); however, the effectiveness of these procedures is controversial.5,6 ICH usually occurs at a single location in the brain; simultaneous bilateral basal ganglia hemorrhages are a rare event and have even higher rates of morbidity and mortality.7
The first case of bilateral basal ganglia hemorrhages was described by Miura in 1978.8 Several more have since been reported,9,10 with a comprehensive review of 56 cases recently published.11 For reasons that are unclear, there have been few cases treated by MIS in which a marker paste for computed tomography (CT) was used. Here we report the rare case of a 52-year-old woman with bilateral basal ganglia hemorrhages; we also discuss possible underlying causes based on a review of the literature as well as surgical procedures used in similar cases.
Case Presentation
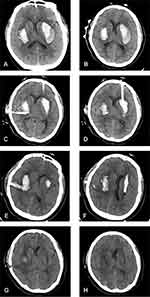
A 52-year-old woman presented at a local hospital’s emergency department after being discovered by her family with sudden-onset vomiting and loss of consciousness. Head CT showed bilateral basal ganglia hemorrhages (data not obtained). The patient was immediately transferred to our hospital. Vital signs on arrival were unremarkable except for markedly elevated blood pressure (180/100 mm Hg). The patient had a 2-year history of hypertension without any history of diabetes, trauma, surgery, or taking oral anticoagulant drugs. Her Glasgow Coma Scale (GCS) score was 6 (E1V1M4). Neurologic examinations revealed coma, quadriplegia, and bilateral positive Babinski sign. Bilateral pupils were 3 mm but sluggish to light. Head CT showed bilateral basal ganglia hemorrhages of about 18 mL on the right and 27 mL on the left side (Figure 1A). Hematoma volume was calculated using the ABC/2 method.12 Chest CT showed bilateral mild pleural effusion and infection in bilateral lungs. Laboratory tests were within normal ranges including complete blood cell counts, bleeding time, activated partial thromboplastin time, prothrombin time, liver and renal function, and blood glucose level. We decided to remove the hematomas by aspiration to reduce intracranial pressure (ICP). We pasted CT markers on the frontal and temporal areas of the patient’s head for head CT to indicate the puncture points for aspiration (Figure 1B). The patient later underwent MIS for ICH under general anesthesia. The neurosurgeon performed hematoma catheter injections using a standard sterile technique. The catheter diameter was 4.8 mm. Frontal and temporal catheters were placed, and 10 and 5 mL of blood clots were aspirated from the left and right sides, respectively. Immediate postoperative head CT confirmed the catheter track and residual hematomas (Figure 1C and D). The patient was sedated and transferred with an endotracheal tube and mechanical ventilation to the Neurosurgical Intensive Care Unit.
Urokinase injection and clot aspiration were performed for the residual hematomas. On postoperative day 1, the 2 catheters were clamped after infusion of urokinase (20,000 U dissolved in 3 mL normal saline) for 2 h. However, at 1.5 h, the patient exhibited the Cushing reflex and bilateral pupils were 5 mm and unresponsive to light. We opened the catheter valve, which alleviated the patient’s signs and symptoms. GCS score improved, and the patient started responding to commands starting from postoperative day 2. We infused urokinase every 12 h up to 4 doses instead of performing a single injection, and the patient was stable during the process. On postoperative day 3, head CT showed a residual clot (10 mL on the right and 2 mL on the left side) and the patient was extubated (Figure 1E and F). Human albumin and furosemide were administered to control ICP and cerebral edema. The patient’s pneumonia continued to worsen after the operation, requiring assisted ventilation. She underwent tracheotomy by general anesthesia on postoperative day 10. On postoperative day 19, the ventilator was removed and head CT revealed absorption of the intracranial hematoma (Figure 1G and H). Over the 2 years of follow-up, there was no recurrence of ICH. The non-fluent aphasia improved but speech did not return to normal. The patient suffered mild disorder of the left and right limb muscles, but she was able to walk unassisted. The patient’s modified Rankin Scale score was 3 on postoperative day 90 and 2 at the 2-year follow-up. This case was approved by the medical ethics committee of the First Affiliated Hospital of China Medical University to publish the case details. Written, informed consent was obtained from the patient for publication of case details and accompanying images.
Discussion
Basal ganglia hemorrhage is a common type of hypertensive hemorrhage and is in most cases solitary. Bilateral basal ganglia hemorrhages are rare and have worse prognosis than single ICH, even for a small hemorrhage volume.11 The indication for surgery for bilateral basal ganglia hemorrhages remains controversial; the aim is to remove the hematoma, reduce its place-occupying effect, and prevent secondary damage. While many surgical approaches are available for hypertensive intracranial hemorrhage including craniotomy, neuron-endoscopy operation, and MIS, an individualized method should be selected. For bilateral basal ganglia hemorrhages, MIS may be a better choice than craniotomy.
MIS is commonly used to treat basal ganglia basal hemorrhages in China. The procedure involves making small incisions and creating a bony skull opening; the surgeon inserts either a catheter or small tube into the clot for drainage or direct evacuation, respectively. There are 2 common surgical protocols for basal ganglia ICH employed at primary hospitals in China—namely, frontal and temporal approaches. Most neurosurgeons select the latter, which has the advantage of an entry point close to the hematoma, making it easier to position the tube so that its trajectory is along the clot. Chi et al often organize classes to teach other neurosurgeons how to apply this technique;13 however, it cannot be adapted to the long axis of an oval-shaped hematoma, which is not conducive to clot drainage. The frontal approach is more suitable for oval-shaped hematomas14 as it has a longer puncture path than the temporal approach. On the other hand, without neuronavigation or stereotactic equipment, it is easy to deviate from the hematoma, causing the puncture to fail.
The head CT of our patient showed that the left hematoma was kidney-shaped with low-density surroundings. In our experience, such hematomas can easily be drained through puncture. The left puncture point was marked on the patient’s forehead and the tube traversed the maximal long axis of the hematoma. During the operation, the puncture point and plane were marked on the scalp based on the head CT image (Figure 1B). These points and lines helped neurosurgeons establish the puncture direction, making the operation quick and easy. The low-density area around the clot was drained, and postoperative CT confirmed a decrease in the volume (Figure 1C and D). In order to avoid the occurrence of re-bleeding, the volume of the first aspiration should not be too large, aspiration speed should not be too fast, and the negative pressure should not be too great. After 3 days of drainage, the left hematoma was almost completely evacuated (Figure 1E and F). The right hematoma was smaller than the one on the left and had an oval shape. We decided on a puncture point on the temporal side based on the length of the patient’s forehead and large size of the lesion (Figure 1B). However, the tube could not cross the maximal long axis and because of the high density of the hematoma, there was a large residual volume after 3 days of drainage (Figure 1E and F). Catheters with more extensive contact with hemorrhagic clots can remove more blood.15 Through the patient’s self-control, we found that in ICH MIS, a low-density hematoma with a puncture route through its maximal long axis leads to a small residual hematoma volume.
The cause of bilateral basal ganglia hemorrhages is unclear. The 2 major risk factors for spontaneous intracranial hemorrhage are hypertension and cerebral amyloid angiopathy.4 In most cases of bilateral basal ganglia hemorrhages, there is a history of hypertension.11,16 The putamen and thalamus are common locations for multiple concurrent ICHs.16 Simultaneous rupture of bilateral hypertension-induced microaneurysms on perforating arteries can cause bilateral basal ganglia hemorrhages;17 it was also suggested that simultaneous or subsequent rupture of bilateral small vessels by chance could induce bilateral basal ganglia hemorrhages.10 Sustained high blood pressure during hemorrhagic stroke can trigger another bleeding site as a result of acute vascular changes in perforating arteries that affect previously weakened intimal and medial layers.18,19 Hematoma volume was also found to be a risk factor for multiple concurrent ICHs, suggesting that large hematomas can induce smaller ones.19
MIS is relatively safe, although there are occasional complications such as bleeding, infection, and elevated ICP. We applied a stability protocol for MIS to prevent bleeding that included a time from onset to operation >6 h; repeated CT ≥6 h after the diagnostic CT showed that the hemorrhage had not expanded by >5 mL; slow and gentle aspiration during the operation; aspiration of not more than 70% of the hematoma volume; and stabilization of blood pressure.6,14 Intracranial infection is a complication of neurosurgery. To avoid adverse events, we kept the surgery time short, performed the operation and urokinase injection using standard sterile technique, tunneled subcutaneously more than 3 cm away from the incision, and withdrew the drainage tube not more than 4 days after operation. The pathophysiology of ICH includes primary and secondary brain damage. The hemorrhage’s mass can increase ICP, subsequently compressing brain tissue and affecting blood flow and leading to brain herniation. We performed hematoma evacuation by MIS to remove the clots, which has many advantages over craniotomy such as reduced brain tissue damage, shorter surgery time, and lower cost. Evacuation also decreased the physiologic response to the hematoma and removed clot components that could cause secondary brain damage.
Conclusion
Bilateral basal ganglia hemorrhages are rare and have very poor prognosis. Our report describes the unique case of a patient who developed bilateral basal ganglia hemorrhages and was treated by MIS via frontal and temporal puncture points, and had a good outcome. Based on our experience, MIS may be a superior surgical method to craniotomy for the treatment of bilateral basal ganglia hemorrhages.
Informed Consent
This case was reported with consent from the patient’s family.
Acknowledgments
The authors thank the patient for participating in this case report.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Broderick JP, Adams HP
2. Sacco S, Marini C, Toni D, Olivieri L, Carolei A. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke. 2009;40(2):394–399. doi:10.1161/STROKEAHA.108.523209
3. van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9(2):167–176. doi:10.1016/S1474-4422(09)70340-0
4. Gross BA, Jankowitz BT, Friedlander RM. Cerebral intraparenchymal hemorrhage: a review. JAMA. 2019;321(13):1295–1303. doi:10.1001/jama.2019.2413
5. Mendelow AD, Gregson BA, Rowan EN, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382(9890):397–408. doi:10.1016/S0140-6736(13)60986-1
6. Hanley DF, Thompson RE, Rosenblum M, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint Phase 3 trial. Lancet. 2019;393(10175):1021–1032. doi:10.1016/S0140-6736(19)30195-3
7. Renard D, Castelnovo G, Ion I, Guillamo JS, Thouvenot E. Single and simultaneous multiple intracerebral hemorrhages: a radiological review. Acta Neurol Belg. 2020;120(4):819–829. doi:10.1007/s13760-020-01385-4
8. Miura N, Nakahara A, Kagawa M, Kitamura K, Kobayashi N. A study of hypertensive intracerebral hemorrhage. (II)–Sequential CT examination and classification (author’s transl). No Shinkei Geka. 1978;6:635–645.
9. Zhao J, Chen Z, Wang Z, Yu Q, Yang W. Simultaneous bilateral hypertensive basal ganglia hemorrhage. Neurol Neurochir Pol. 2016;50(4):275–279. doi:10.1016/j.pjnns.2016.03.003
10. Xu F, Lian L, Liang Q, et al. Good functional outcome following bilateral external capsule hemorrhage mimicking bilateral basal ganglia hemorrhage in a coma patient. Br J Neurosurg. 2019;29:1–3. doi:10.1080/02688697.2018.1559276
11. Yang Z, Chen J, Mu J. Simultaneous bilateral basal ganglia hemorrhage. Curr Drug Deliv. 2017;14:807–815. doi:10.2174/1567201813666160607202811
12. Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi:10.1161/01.STR.27.8.1304
13. Chi FL, Lang TC, Sun SJ, et al. Relationship between different surgical methods, hemorrhage position, hemorrhage volume, surgical timing, and treatment outcome of hypertensive intracerebral hemorrhage. World J Emerg Med. 2014;5:203–208. doi:10.5847/wjem.j.issn.1920-8642.2014.03.008
14. Liang KS, Ding J, Yin CB, et al. Clinical study on minimally invasive liquefaction and drainage of intracerebral hematoma in the treatment of hypertensive putamen hemorrhage. Technol Health Care. 2017;25:1061–1071. doi:10.3233/THC-170950
15. Hanley DF, Thompson RE, Muschelli J, et al. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, Phase 2 trial. Lancet Neurol. 2016;15(12):1228–1237. doi:10.1016/S1474-4422(16)30234-4
16. Takeuchi S, Takasato Y, Masaoka H, Hayakawa T, Yatsushige H, Sugawara T. Simultaneous multiple hypertensive intracranial hemorrhages. J Clin Neurosci. 2011;18(9):1215–1218. doi:10.1016/j.jocn.2011.01.020
17. Yen CP, Lin CL, Kwan AL, et al. Simultaneous multiple hypertensive intracerebral haemorrhages. Acta Neurochir (Wien). 2005;147(4):
18. Maurino J, Saposnik G, Lepera S, Rey RC, Sica RE. Multiple simultaneous intracerebral hemorrhages: clinical features and outcome. Arch Neurol. 2001;58(4):629–632. doi:10.1001/archneur.58.4.629
19. Yamaguchi Y, Takeda R, Kikkawa Y, et al. Multiple simultaneous intracerebral hemorrhages: clinical presentations and risk factors. J Neurol Sci. 2017;383:35–38. doi:10.1016/j.jns.2017.10.005
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.