Back to Journals » Patient Preference and Adherence » Volume 13
Two comparative assessments of intravenous immunoglobulin therapy switching patterns in the treatment of chronic inflammatory demyelinating polyneuropathy in the US
Authors Guptill JT, Runken MC, Eaddy M , Lunacsek OE , Fuldeore RM , Blanchette CM, Zacherle E, Noone JM
Received 8 September 2018
Accepted for publication 1 March 2019
Published 30 April 2019 Volume 2019:13 Pages 649—655
DOI https://doi.org/10.2147/PPA.S185852
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Jeffrey T Guptill,1 M Chris Runken,2 Michael Eaddy,3 Orsolya E Lunacsek,4 Rupali M Fuldeore,4 Christopher M Blanchette,5 Emily Zacherle,5 Joshua M Noone5
1Department of Neurology, Duke University, Durham, NC, USA; 2Grifols SSNA, Research Triangle Park, Durham, NC, USA; 3Scientific Consulting, Xcenda, Palm Harbor, FL, USA; 4Real-World Evidence, Xcenda, Palm Harbor, FL, USA; 5Department of Public Health Sciences, University of North Carolina at Charlotte, Charlotte, NC, USA
Purpose: For chronic inflammatory demyelinating polyneuropathy (CIDP) patients, each branded intravenous immunoglobulin (IVIG) treatment differs in production processes, virus elimination, formulation, and composition. Given the limited availability of real-world data comparing IVIGs for CIDP, this study evaluated switching patterns between IVIG products in 2 separate retrospective databases.
Patients and methods: Two independent analytic teams retrospectively evaluated IVIG treatment-naïve patients with an ICD diagnosis code for CIDP. Study 1 used integrated healthcare claims from IMS LifeLink PharMetrics Plus™ and Study 2 used the Truven MarketScan® Database. All analyses were descriptive, with outcomes assessed during the 2-year post-index period.
Results: One-quarter of IVIG patients switched therapies within the 2-year study period. In both studies, switching rates were lowest for IVIG-G (Gamunex®-C) (Study 1: 9.8%, Study 2: 8.9%), followed by IVIG-F (Flebogamma®) (Study 1: 25.0%, Study 2: 18.2%), and highest for IVIG-other (Octagam®/Gammaplex®) (Study 1: 50.0%, Study 2: 33.3%). When patients were switched, most switched to IVIG-G (Study 1: 51.6%, Study 2: 54.3%).
Conclusion: The small proportion of CIDP switchers in 2 independent studies suggests that IVIG therapy is generally well tolerated. However, differences existed in switch rates for different IVIG products. The reason for low switching rates could not be assessed in this study; therefore, further studies are required to detect possible relevant differences in effectiveness and tolerability.
Keywords: intravenous immunoglobulin, chronic inflammatory demyelinating polyneuropathy, treatment patterns
Introduction
Chronic inflammatory demyelinating polyneuropathy (CIDP) is an inflammatory disorder of the peripheral nervous system. Clinical features of CIDP include symmetrical weakness in proximal and distal muscles, sensory loss, imbalance, pain, and impaired ambulation, with progression for 8 weeks or more.1,2 The disease course can be relapsing, chronic, and progressive, making this disease difficult to diagnose.2
Although prevalence estimates vary widely (1.9–7.7 per 100,000), a US study examining medical records spanning 20 years, between 1982 and 2001, in Olmsted County, Minnesota estimated the prevalence of CIDP at 8.9 per 100,000 persons and the incidence of CIDP at 1.6 per 100,000/year.3 The disorder generally affects individuals aged 40–60 years and is more common in men than women.1
The primary goals of treatment for CIDP are to reduce symptoms, improve functional status, and maintain long-term remission.1 Treatment with intravenous immunoglobulin (IVIG) has been the standard of care for CIDP for over 2 decades, with 50–70% of patients responding to IVIG treatment. However, up to 50% of patients who have an initial response experience relapse within the following weeks or months after treatment benefit.1 The American Academy of Neurology Evidence-based Guidelines recommend use of IVIG for the long-term treatment of CIDP (Level A), whereas the European Federation of Neurological Societies/Peripheral Nerve Society recommend IVIG as an initial therapy (Level A) in treating different forms of CIDP.4,5 Patients with CIDP are also treated with corticosteroids and plasma exchange.5 Alternative immunosuppressive regimens may be used in patients who have not improved with these conventional treatments, who have improved but still have frequent relapses, or who have experienced intolerable side effects.1
For CIDP patients treated with IVIG, there are multiple IVIG products available that differ in terms of their production processes, formulation, and composition.6,7 Differences in the production processes of IVIG products may potentially affect safety, tolerability, and clinical outcomes in different patients.6,7 Grifols manufactures IVIG-G (Gamunex®-C) and IVIG-F (Flebogamma®); Shire manufactures IVIG-L (Gammagard® Liquid); while CLS Behring manufactures IVIG-P (Privigen®) and IVIG-C (Carimune®).
More recent IVIG products have suggested improved safety and quality relative to older ones;7 however, there are limited comparative data regarding tolerability among the different brands of IVIG.6,7 In clinical practice, the attributes of an IVIG product should be selected based on patient-specific characteristics, such as risk profile, medical history, and comorbid conditions.1,6,7
Higher switching rates between IVIGs may be indicative of a lack of effectiveness or tolerability or safety concerns in real-world clinical practice. Given limited real-world data comparing available IVIGs and the importance of research assessing such differences, this study evaluated IVIG switching patterns in 2 separate retrospective databases, each analyzed independently by 2 analytic research groups.
Methods and materials
Study design
Two independent analytic teams retrospectively evaluated IVIG treatment-naïve patients with an International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis of CIDP. Study 1 required patients to receive IVIG as their first treatment after diagnosis of CIDP, excluding patients who received all other CIDP-related therapies as their initial treatment. In Study 2, patients were allowed to receive CIDP-related therapies prior to their initial IVIG therapy. These CIDP-related therapies included corticosteroids, plasma exchange, and immunosuppressants.
CIDP is difficult to diagnose, often resulting in a delay between initial diagnostic testing and clinical confirmation. Because the symptoms of CIDP are not specific and neuropathy can have many causes, individuals undergoing evaluation may receive diagnostic testing but later receive a different diagnosis. Thus, both a diagnosis of CIDP and receipt of treatment were required to reduce the likelihood of false-positive identification. The index date for patients in both studies was defined as the date of the earliest medical claim with a CIDP diagnosis. The pre-index period was defined as 12 months prior to the first CIDP diagnosis claim. All patients were followed for 2 years after the index date (post-index period).
Data sources
Study 1 used integrated healthcare claims data from the IMS LifeLink PharMetrics Plus™ Claims Database from January 1, 2009 through June 30, 2014. The database contains administrative medical and pharmacy claims, along with eligibility records, for over 103 different managed healthcare plans, encompassing over 150 million lives, about 90 million of whom have both medical and pharmacy benefits. Patients in the majority of 3-digit zip codes and in every metropolitan statistical area of the US were represented, with coverage of data from 90% of US hospitals and 80% of all US doctors. The payer type distribution is 80% commercial, 3% Medicaid, and 1.7% Medicare, with the rest being categorized as “other.”
Study 2 used the Truven MarketScan Database®, which contains commercial medical and pharmacy claims data that are sourced directly from health plans and employers between January 1, 2010 and December 31, 2013. This database represents over 50 million commercially insured individuals.
For both databases, medical claims are linked to outpatient prescription drug claims and person-level enrollment data through the use of unique patient or enrollee identifiers. Both study databases are compliant with the Health Insurance Portability and Accountability Act (HIPAA) of 1996 to protect patient privacy. The data used in these retrospective cohort studies were HIPAA-compliant, deidentified patient data, thus no institutional review board review was required.
Study population
Patients with at least 1 diagnosis code for CIDP (ICD-9-CM code: 357.81) and evidence of starting IVIG therapy (identified with Healthcare Common Procedure Coding System codes) as the initial therapy were included in Study 1. Study 2 required patients to have 2 diagnoses of CIDP at least 90 days apart.
Patients in both studies were required to be 18 years of age or older at the first CIDP diagnosis, have no CIDP diagnosis codes or CIDP-related therapies (Study 1) or IVIG therapies (Study 2) in the 12-month pre-index period, and have continuous health plan enrollment with medical and pharmacy benefits during the 12-month pre-index period and 2-year post-index period following their initial CIDP diagnosis. Study 1 excluded patients with other diagnoses where IVIG was recommended as a therapeutic option (Table 1); this criterion was not applied in Study 2.
 | Table 1 Exclusionary conditions (conditions other than CIDP where IVIG treatment is used) |
Study outcomes
Patients were placed into cohorts based on the initial IVIG given after CIDP diagnosis. The study cohorts were characterized using available demographic characteristics and the Charlson comorbidity index (CCI). The primary study outcome was the rate of switching to another IVIG product. Due to small sample sizes, 2 IVIG products were combined and are referred to as IVIG-other (Octagam®/Gammaplex®), manufactured by Octapharma and BPL, respectively. Thus the cohorts for the analyses are defined as IVIG-G, IVIG-F, IVIG-L, IVIG-P, IVIG-C, and IVIG-other.
Statistical analyses
As the purpose of this study was to assess the proportion of patients who switched and which IVIG product patients were switched to, all analyses were descriptive in nature. Frequencies and percentages were reported for categorical variables, and means with standard deviations (SDs) were reported for continuous variables. The baseline characteristics were measured in the 12-month pre-index period prior to IVIG initiation and outcomes were assessed in the 2-year post-index period. All analyses were conducted using SAS version 9.3 (SAS Institute; Cary, NC, USA).
Results
Baseline characteristics
There were 126 patients who met all study inclusion criteria in Study 1, and 151 patients met all criteria in Study 2. Study attrition and exclusion criteria are shown in Table 2. Patients were grouped into cohorts based on their index IVIG product; baseline demographic and clinical characteristics by index IVIG product are shown in Table 3. In both studies, the most commonly prescribed index IVIG products were IVIG-L (Study 1: 38.9%, Study 2: 33.1%) and IVIG-G (Study 1: 32.5%, Study 2: 37.1%).
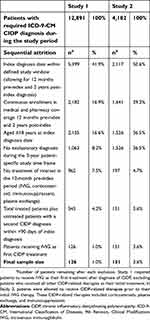 | Table 2 Identification of final study population |
 | Table 3 Cohort distribution and demographic characteristics |
The average age of patients in the IVIG cohorts in Study 1 was consistently older than those in Study 2 (Table 3). The gender distribution across both studies was relatively similar among patients with IVIG-G, IVIG-other, and IVIG-F, whereas the percentage of males in all other IVIG cohorts was at least 20% points higher in Study 1.
IVIG treatment switching
Approximately one-quarter of all IVIG patients switched therapies in each study (Table 4). In both studies, switching rates were lowest for IVIG-G (Study 1: 9.8%, Study 2: 8.9%), followed by IVIG-F (Study 1: 25.0%, Study 2: 18.2%). The highest rate of switching was observed in patients who initiated on IVIG-other (Study 1: 50.0%, Study 2: 33.3%).
 | Table 4 Index IVIG switch rates |
When patients were switched to a second IVIG, most were switched to IVIG-G (Study 1: 51.6%, Study 2: 54.3%). Other common products patients were switched to for their second IVIG included IVIG-L (Study 1: 16.1%, Study 2: 14.3%), IVIG-P (Study 1: 12.9%, Study 2: 14.3%), and IVIG-other (Study 1: 16.1%, Study 2: 14.3%) (Figure 1A and B).
Discussion
To our knowledge, these US retrospective database studies are the first to evaluate IVIG product utilization patterns among CIDP patients initiating IVIG therapy in a real-world setting. Differences between branded IVIG therapies available for the treatment of CIDP may differ in aspects, such as the production process, formulation, and composition;6,7 this, in turn, may affect safety, tolerability, and clinical outcomes in patients.6,7 These studies examined switching patterns among insured US ICD-9-CM-diagnosed and treated populations, which may provide some insight into the inherent differences among IVIG products.
Results from these 2 studies conducted independently by 2 different research organizations in 2 large US claims databases found consistent results despite key differences in the populations of interest. Overall, approximately 1 in 4 CIDP patients switched from their index IVIG during the study periods, suggesting that IVIG therapy, as a whole, is generally efficacious, safe, and well tolerated. There were, however, differences in the switch rates for different IVIG products seen in both studies. Results showed that treatment switching rates were lowest for IVIG-G (8.9–9.8%) and that when patients switched IVIGs, the most common IVIG therapy they were switched to was IVIG-G (51.6–54.3% of patients who switched IVIG). Relative to other IVIG products, it is reported that IVIG-G requires fewer steps and has a shorter processing time, thereby yielding 50% more immunoglobulin G and producing a purer final product than the IVIG solvent and detergent processes.8 These manufacturing differences, among other differences in product features (eg, product formulation, sugar content, sodium content, osmolality, immunoglobulin A, and pH), may affect safety, tolerability, and clinical efficacy.6,7 The low switching rates for IVIG-G seen in both of these studies are supported by clinical trial efficacy and safety measures showing improvement in measures of functional disability and grip strength maintained over 24 weeks.8
Limitations
These analyses were conducted using claims-based datasets with data collected as part of the administration of healthcare. The use of administrative claims in a retrospective study is associated with inherent limitations, including a lack of data on patients’ clinical presentation, severity of disease, and reasons for switching. Despite these potential limitations, both studies—conducted by independent research firms using 2 separate data sources and applying non-identical exclusion/inclusion criteria—resulted in similar switching patterns, strongly supporting the results described in this study.
CIDP is a rare disease, and therefore the sample sizes within treatment cohorts for both studies were small, which may limit any conclusion of IVIG treatment patterns across cohorts. It should be noted that CIDP is difficult to diagnose, and strict inclusion criteria were applied to these studies to ensure that relevant populations were identified. Patients were required to have at least 2 years of eligibility following their initial CIDP diagnosis in order to reduce false positives and to allow for sufficient follow-up to evaluate real-world treatment patterns, particularly rates of switching.
The reasons for IVIG selection and switching could not be assessed in these claims database studies. Changes in the use of IVIG products due to insurance coverage may not be captured within an administrative claims database. For CIDP patients, there may be different reasons for switching among IVIG therapies. As noted, differences among IVIG products and patient risk profiles/medical histories may influence a product’s efficacy, safety, or tolerability across the CIDP patient population.1,7 Therefore, clinical practice recommendations are to switch to another IVIG brand should a particular IVIG formulation produce intolerable side effects or an inadequate clinical response.1,5 Additionally, the high cost of IVIG treatment may affect access and reimbursement, potentially influencing the selection of an IVIG product and switching patterns. Given the importance of long-term use of IVIG therapies in controlling relapses and progression of CIDP, this is an important area for future research. Further research is also needed to identify and confirm patterns of response and switching among IVIG products and to compare their long-term benefits, safety, and cost consequences.
As these studies were observational in nature, causality should not be ascribed to any associations seen between type of IVIG used and the outcomes studied. Optimal study designs using clinically confirmed CIDP patients include prospective observational studies to assess comparative effectiveness or pragmatic clinical trials, where patients are randomized to different IVIG therapies and followed prospectively. Results of this study are generalizable to an insured population of CIDP patients in the US and may not reflect treatment patterns outside of these studied populations.
Lastly, the authors acknowledge funding for this study has been provided by Grifols, whose IVIG preparation showed positive results in both independent studies. However, 2 independent research organizations conducted separate studies using different inclusion criteria to identify CIDP patient populations in 2 separate claims databases and found consistent results.
Conclusion
This real-world comparison of IVIG utilization in ICD-9-CM-diagnosed CIDP patients used 2 separate database analyses conducted by independent analytic research teams to determine if substantial differences in IVIG switch rates exist across commercially available IVIG products. Both studies used different approaches to patient selection, yet showed similar results indicated by similar switching rates, with the lowest incidence of switching found in patients initiating IVIG-G. A better understanding of factors that affect efficacy and safety/tolerability may lead to better IVIG products with more favorable properties and improved patient outcomes.
Acknowledgments
The authors would like to acknowledge Ann Cameron for her assistance in writing the manuscript. This study was funded by Grifols SSNA. The study design, analysis, and reporting were conducted and led by investigators from each of the 2 independent analytic teams. The sponsor provided clinical support and interpretation during study design, analysis, and reporting. The initial draft of the manuscript was provided by Xcenda with critical review and input from all coauthors.
Disclosure
Dr. Guptill received grant support from the Grifols Foundation for an unrelated project and is supported by K23NS085049. Dr. Runken is an employee of Grifols SSNA. Dr. Eaddy, Dr. Lunacsek, and Ms. Fuldeore are employees of Xcenda, which was paid by Grifols SSNA to complete this study. Dr. Blanchette, Mrs. Zacherle, and Dr. Noone are employees of University of North Carolina, Charlotte and were paid by Grifols SSNA to complete this study. Dr Guptill also reports grants from Jose Antonio Grifols Lucas Foundation, personal fees from Grifols, Inc, outside the submitted work; Dr Eaddy reports fees from Xcenda, during the conduct of the study. Dr Blanchette reports personal fees from Grifols, during the conduct of the study; personal fees from United Therapeutics, and Ipsos, outside the submitted work; Mrs Zacherle reports personal fees from Blanchette Research LLC, during the conduct of the study; Dr Noone reports personal fees from Grifols, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Gorson KC. An update on the management of chronic inflammatory demyelinating polyneuropathy. Ther Adv Neurol Disord. 2012;5(6):357–359. doi: 10.1177/1756285612457215.
2. Köller H, Kieseier BC, Jander S, Hartung HP. Chronic inflammatory demyelinating polyneuropathy. N Engl J Med. 2005;352(13):1343–1356. doi: 10.1056/NEJMra041347
3. Laughlin RS, Dyck PJ, Melton LJ, Leibson C, Ransom J, Dyck PJB. Incidence and prevalence of CIDP and the association of diabetes mellitus. Neurology. 2009;73(1):39–45. doi: 10.1212/WNL.0b013e3181aaea47.
4. Patwa HS, Chaudhry V, Katzberg H, Rae-Grant AD, So YT. Evidence-based guideline: intravenous immunoglobulin in the treatment of neuromuscular disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2012;78(13):1009–1015. doi: 10.1212/WNL.0b013e31824de293.
5.
6. Abolhassani H, Asgardoon MH, Rezaei N, Hammarstrom L, Aghamohammadi A. Different brands of intravenous immunoglobulin for primary immunodeficiencies: how to choose the best option for the patient? Expert Rev Clin Immunol. 2015;11(11):1229–1243. doi:10.1586/1744666X.2015.1079485
7. Gelfand EW. Differences between IGIV products: impact on clinical outcome. Int Immunopharmacol. 2006;6(4):592–599. doi:10.1016/j.intimp.2005.11.003
8. Hughes R AC, Donofrio P, Bri Vl, et al.;
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

