Back to Journals » Infection and Drug Resistance » Volume 15
Two Cases Report of Intrathecal Tigecycline Therapy for Intracranial Infection with Acinetobacter baumannii and Review of Literatures
Authors Huang G, Lai W, Wu D , Huang Q, Zhong Q, Ye X
Received 8 February 2022
Accepted for publication 1 April 2022
Published 26 April 2022 Volume 2022:15 Pages 2211—2217
DOI https://doi.org/10.2147/IDR.S357087
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Guanlin Huang, Wentao Lai, Daxing Wu, Qianliang Huang, Qi Zhong, Xinyun Ye
Department of Neurosurgery, Ganzhou People’s Hospital, Ganzhou, Jiangxi, 341000, People’s Republic of China
Correspondence: Xinyun Ye, Department of Neurosurgery, Ganzhou People’s Hospital, Ganzhou, Jiangxi, 341000, People’s Republic of China, Email [email protected]
Abstract: Objective: To explore the treatment scheme for intracranial infection with Acinetobacter baumannii. Methods: We retrospective analyzed two cases of patients of intracranial infection with Acinetobacter baumannii. Results: The intracranial infection was controlled effectively by the scheme to intravenous“tigecycline + cefperazone-sulbactam”combined with intrathecal tigecycline injection, the two patients recover well with 21 months’ follow-up. Conclusions: Tigecycline-based drug scheme combined with intrathecal tigecycline injection can achieve the effect of controlling intracranial infection. Lumbar cisterna drainage tube plays a major role in controlling intracranial infection.
Keywords: multidrug resistant Acinetobacter baumannii, intracranial infection, tigecycline
Introduction
Acinetobacter baumannii intracranial infection has been a cause of hospital-acquired infections and resulted in high morbidity and mortality rates.1,2 Because of the presence of multiresistance and drugs poor penetration through the blood brain barrier, it’s still challengeable to treat it. Recent years, many studies revealed that it is a reliable choice to treat multiresistant Acinetobacter meningitis with the scheme of intraventricular/intrathecal and intraventricular/intrathecal colistin,3 however, considered the neurotoxicity, its application still limited. Tigecycline has a effective effect against multiresistant BA, however, tigecycline can only be used as an intravenous drug.4 And its poor penetration through blood brain barrier, it still resulting in bad effect to the intracranial infection with BA. It would be a breakthrough if tigecycline can be used in intraventricular/intrathecal. Intraventricular/intrathecal tigecycline has few been reported. Here, we described two cases infected with post-neurosurgical extensive drug resistant (XDR A) baumannii, and it was successfully cured by intrathecal tigecycline therapy.
Case Report
Case 1 A female patient, 16 years old, undergoing retrosigmoid craniotomy for resection of Vestibular Schwannomas. However, she got a high fever 39.5°C, it showed a high level of white blood cell and a low level of both glucose in the laboratory tests for cerebrospinal fluid (CSF) (Table 1) of her. Then she was diagnosed as meningitis and treated with vancomycin and meropenem. The MRI imaging was shown in Figure 1. Later, we changed the scheme to tigecycline and cefoperazone-sulbactam, because the CSF sputum culture showed she was infected with XDRA baumannii which was only sensitive to tigecycline (Table 2). The dose was 100mg/day (50mg twice a day) and cefoperazone-sulbactam (3g twice a day). Consideration the poor blood brain barrier permeability of tigecycline, intrathecal tigecycline therapy was an effective way to cure this patient. After permission from the hospital ethics committee and his family members, we carried out the therapeutic schedule of intrathecal tigecycline therapy. We injected 5mg tigecycline into Lumbar cisterna drainage tube every day, and then closed the Lumbar cisterna drainage tube about 2 hours. This patient tolerated the treatment well. After 6 days, her fever improved, but the CSF culture was still positive. We continued the schedule of intrathecal tigecycline and the CSF culture became negative after 4 weeks, with normal levels of white blood cell, glucose, the temperature decreased to 36.8°C and all symptoms disappeared. We stopped the intrathecal tigecycline therapy. In the next 3 days, the CSF cultures were all negative, in that case, the intracranial infection was effectively cured. (The appearances of CSF before and after treatment are shown in Figure 2, and the laboratory tests for CSF in the period of treatment are shown in Table 1). Intravenous antibiotics were stopped 2 weeks later, and the patient was recover well.
 |
Table 1 Laboratory Tests for CSF in the Period of Treatment of case 1 |
 |
Table 2 Antibiotics Susceptibility Tests for Acinetobacter baumannii in CSF of case 1 |
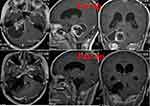 |
Figure 1 MRI imaging of case 1. |
 |
Figure 2 The appearances of CSF before and after treatment of case 1. |
Case 2 A male patient, 80 years old, accepted the operation of craniotomy removal of the frontal meningiomas. The MRI imaging was shown in Figure 3. At the first day after surgery, the patient got high fever 39°C, it showed a high level of white blood cell and a low level of both glucose in the laboratory tests for cerebrospinal fluid (CSF) (Table 3) of him. At the same time, Stagoscopy of the cerebrospinal fluid reminded gram negative bacilli, and then the CSF sputum culture showed he was infected with XDRA baumannii (Table 4), we chose the scheme of tigecycline+cefoperazone-sulbactam. We carried out the therapeutic schedule of intrathecal tigecycline therapy after permission from the hospital ethics committee and his family members. We injected 5mg tigecycline into Lumbar cisterna drainage tube and then closed the Lumbar cisterna drainage tube about 2 hours. This patient tolerated the treatment well. After 3 days, his fever improved, and his CSF culture became negative 1 weeks later, with normal levels of white blood cell, glucose. His temperature decreased to normal and all symptoms disappeared at the same time. We stopped the intrathecal tigecycline therapy. In the next 3 days, the CSF cultures were all negative, in that case, the intracranial infection was effectively cured.(The appearances of CSF before and after treatment are shown in Figure 4, and the laboratory tests for CSF in the period of treatment are shown in Table 3). Intravenous antibiotics were stopped 2 weeks later, and the patient was recover well.
 |
Table 3 Laboratory Tests for CSF in the Period of Treatment of case 2 |
 |
Table 4 Antibiotics Susceptibility Tests for Acinetobacter baumannii in CSF of case 2 |
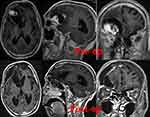 |
Figure 3 MRI imaging of case 2. |
 |
Figure 4 The appearances of CSF before and after treatment of case 2. |
Follow-Up
The two patients were recover well at 21 months of follow-up, they returned to normal life.
Discussion
BA is an opportunistic microorganism that exists widely in healthcare institutes.2 Patients with a history of ICU-stay are prone to BA infection,5 and even multiple drug-resistant BA infection,2,6 and central nervous system infections are mostly associated with craniocerebral trauma or craniocerebral surgery.7 Central system infection caused by BA accounts for 3.6%~11.2%,5 extremely poor prognosis of infection caused by BA or multi-resistant BA, with a high mortality rate. In case 1, the patient was teenagers with a history of ICU-stay. According to the Stagoscopy of the cerebrospinal fluid, we take therapeutic schedule of cefoperone sodium+tigecycline. Because of the poor blood brain barrier permeability of tigecycline, we carried out the therapeutic schedule of intrathecal tigecycline therapy. Liang et al7 summarized various combinations of antibiotics through a large number of intracranial cases of BA, they assigned patients to different groups according to whether they had intraventricular/intrathecal injection of antibiotic agents or cerebrospinal fluid (CSF) drainage therapy. They took different scheme according to the results of cerebrospinal fluid culture. Lauretti et al8 reported the scheme of intraventricular tigecycline 2 mg. It was reported that infection did not control until they changed to scheme of tigecycline 4 mg, the same to others.3,8–10 Considering the pure suppuricity of the patient’s cerebrospinal fluid, poor drainage and severe infection, we finally took intrathecal 5mg tigecycline. During the period, the patient’s Lumbar cisterna drainage was not fluent, but it can be pumped through the syringe, the drainage tube was unobstructed, it may be caused by low intracranial pressure. In case 2, the patient was an old male also with a history of ICU-stay. According to the results of cerebrospinal fluid culture, we take the same scheme, finally we controlled infection with Lumbar cisterna drainage tube. In the two cases, consolidated treatment of tiagecycline + cefoperidone continued after infection control, and with no recurrence after consolidation.
In the two cases, the laboratory tests for cerebrospinal fluid (CSF) showed a high level of white blood cell and a low level of glucose, if it happens to patients, we should be aware of the possibility of BA intracranial infection. The CSF is turbidity, Lumbar cisterna drainage was not fluent, especially in case 1, it is important to keep the drainage tube fluent. Liang et al7 reported large numbers of BA intracranial infection, they reached the conclusion that both intraventricular/intrathecal injection of antibiotic agents and CSF drainage are helpful for CSF sterilization, CSF drainage is the key to the treatment of BA intracranial infection.
To date, the most sensitive antibiotics for the treatment of BA intracranial infection are mainly polymucand and tigecycline.4,11–15 Polymytin family mainly contain polymytin B and polymytin E, but polymytin is less used in China and expensive. In the two patients, the polymytin was not accessible and was expensive, and there were no other more reasonable treatment. In that case, the teticycline was selected. Tecycline is a broad-spectrum glycine-negative bacterium and gram-positive coccus,4 which is better active for multiple drug-resistant pathogens. In the treatment of intraventricular/intrathecal tigecycline, the most common adverse reactions are digestive system symptoms, such as abdominal pain, nausea, vomiting, and there are also reports of other adverse drug reactions of intravenous teticycline. No drug effects have been reported.7–9 In case 1, when intrathecal tigecycline, the patient had obvious abdominal pain, it alleviated about 20 minutes. During the intraventricular/intrathecal tigecycline, the intraventricular/intrathecal tigecycline speed shall be as slow as possible and diluted with 10mL physiological saline, even using the micropump. The current clinical guidelines and tigecycline manual4,16 do not mention the way of intraventricular/intrathecal tigecycline. Intraventricular/intrathecal tigecycline is a hyperdrug manual, and the manual should be modified.
Conclusion
Tigecycline intravenous combined with intraventricular/intrathecal tigecycline is an effective way to cure BA infection. CSF drainage is the key to treat BA intracranial infection.
Patient Consent and Ethics Statement
Both patients provided informed consent for the case details and images to be published. No ethical committee approval was required for this study as the data had been analyzed in a retrospective manner.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhan R, Zhu Y, Shen Y, et al. Post-operative central nervous system infections after cranial surgery in China: incidence, causative agents, and risk factors in 1470 patients. Eur J Clin Microbiol Infect Dis. 2014;33(5):861–866. doi:10.1007/s10096-013-2026-2
2. Ma YF, Wen L, Zhu Y. Prospective study evaluating post-operative central nervous system infections following cranial surgery. Br J Neurosurg. 2019;33(1):80–83. doi:10.1080/02688697.2018.1519112
3. Long W, Yuan J, Liu J, et al. Multidrug resistant brain abscess due to Acinetobacter baumannii ventriculitis cleared by intraventricular and intravenous tigecycline therapy: a case report and review of literature. Front Neurol. 2018;9:518. doi:10.3389/fneur.2018.00518
4. Bethesda (MD);Tigecycline. 2012. Clinical and Research Information on Drug and Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Tigecycline. [Updated 2019 Jan 23].
5. Tsimogianni A, Alexandropoulos P, Chantziara V, et al. Intrathecal or intraventricular administration of colistin, vancomycin and amikacin for central nervous system infections in neurosurgical patients in an intensive care unit. Int J Antimicrob Agents. 2017;49(3):389–390. doi:10.1016/j.ijantimicag.2017.01.002
6. Ng J, Gosbell IB, Kelly JA, Boyle MJ, Ferguson JK. Cure of multiresistant Acinetobacter baumannii central nervous system infections with intraventricular or intrathecal colistin: case series and literature review. J Antimicrob Chemother. 2006;58(5):1078–1081. doi:10.1093/jac/dkl347
7. Liang W, Yuan-Run Z, Min Y. Clinical presentations and outcomes of post-operative central nervous system infection caused by multi-drug-resistant/extensively drug-resistant Acinetobacter baumannii: a Retrospective Study. Surg Infect. 2019;20(6):460–464. doi:10.1089/sur.2018.286
8. Lauretti L, D’Alessandris QG, Fantoni M, et al. First reported case of intraventricular tigecycline for meningitis from extremely drug-resistant Acinetobacter baumannii. J Neurosurg. 2017;127(2):370–373. doi:10.3171/2016.6.JNS16352
9. Fang YQ, Zhan RC, Jia W, Zhang BQ, Wang JJ. A case report of intraventricular tigecycline therapy for intracranial infection with extremely drug resistant Acinetobacter baumannii. Medicine. 2017;96(31):e7703. doi:10.1097/MD.0000000000007703
10. Chusri S, Sakarunchai I, Kositpantawong N, et al. Outcomes of adjunctive therapy with intrathecal or intraventricular administration of colistin for post-neurosurgical meningitis and ventriculitis due to carbapenem-resistant acinetobacter baumannii. Int J Antimicrob Agents. 2018;51(4):646–650. doi:10.1016/j.ijantimicag.2017.12.002
11. Barberán J, Salso S, Alhambra A. [Tigecycline: 10 years of history and still in full force]. Rev Esp Quimioter. 2015;28(2):61–78. Spanish.
12. Tsolaki V, Karvouniaris M, Manoulakas E, et al. Intraventricular CNS treatment with Colistin-Tigecycline combination: a case series. J Crit Care. 2018;47:338–341. doi:10.1016/j.jcrc.2018.07.025
13. Karaiskos I, Galani L, Baziaka F, Giamarellou H. Intraventricular and intrathecal colistin as the last therapeutic resort for the treatment of multidrug-resistant and extensively drug-resistant Acinetobacter baumannii ventriculitis and meningitis: a literature review. Int J Antimicrob Agents. 2013;41(6):499–508. doi:10.1016/j.ijantimicag.2013.02.006
14. Imberti R, Iotti GA, Regazzi M. Intraventricular or intrathecal colistin for the treatment of central nervous system infections caused by multidrug-resistant Gram-negative bacteria. Expert Rev Anti Infect Ther. 2014;12(4):471–478. doi:10.1586/14787210.2014.896740
15. Bargiacchi O, De Rosa FG. Intrathecal or intraventricular colistin: a review. Infez Med. 2016;24(1):3–11.
16. Pankey GA. Tigecycline. J Antimicrob Chemother. 2005;56(3):470–480. doi:10.1093/jac/dki248
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
