Back to Journals » International Journal of Women's Health » Volume 12
Twin Reversed Arterial Perfusion Sequence: Current Treatment Options
Authors Vitucci A, Fichera A, Fratelli N, Sartori E, Prefumo F
Received 12 November 2019
Accepted for publication 14 May 2020
Published 28 May 2020 Volume 2020:12 Pages 435—443
DOI https://doi.org/10.2147/IJWH.S214254
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Everett Magann
Annachiara Vitucci, Anna Fichera, Nicola Fratelli, Enrico Sartori, Federico Prefumo
Division of Obstetrics and Gynecology, ASST Spedali Civili, Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy
Correspondence: Federico Prefumo
Division of Obstetrics and Gynecology, ASST Spedali Civili, Department of Clinical and Experimental Sciences, University of Brescia, Piazzale Spedali Civili 1, Brescia 25123, Italy
Tel +39 030 3995340
Fax +39 030 3995034
Email [email protected]
Abstract: Twin reversed arterial perfusion (TRAP) sequence is a specific and severe complication of monochorionic multiple pregnancy, characterized by vascular anastomosis and partial or complete lack of cardiac development in one twin. Despite its rarity, interest in the international literature is rising, and we aimed to review its pathogenesis, prenatal diagnostic features and treatment options. Due to the parasitic hemodynamic dependence of the acardiac twin on the pump twin, the management of these pregnancies aims to maximize the pump twin’s chances of survival. If treatment is needed, the best timing of intervention is still debated, although the latest studies encourage intervention in the first trimester of pregnancy. As for the technique of choice to interrupt the vascular supply to the acardiac twin, ultrasound-guided laser coagulation and radiofrequency ablation of the intrafetal vessels are usually the preferred approaches.
Keywords: TRAP sequence, acardiac twin, pump twin, clinical management, fetal therapy
Introduction
Twin reversed arterial perfusion (TRAP) sequence, also known as acardiac malformation, is a unique complication of monochorionic multiple pregnancy in which one of the twins lacks a complete cardiac structure (and so is called “acardiac”) while a morphologically normal co-twin (called “pump twin”) supplies both circulations. Historically the first case was described by Benedetti in 1533, and the first cases had been reported in the international literature in the 1950s;1 the first description of prenatal diagnosis of an acardiac twin was reported by Lehr and Dire in 1978.2 We aimed to review the pathogenesis, prenatal diagnostic features and treatment options of this condition.
Epidemiology, Pathogenesis and Natural History
Traditionally, the incidence of this condition has been estimated at 1:35,000 pregnancies and 1:100 monozygotic twin pregnancies. However, van Gemert et al assessed that, due to a better ultrasound diagnosis and the spread of assisted reproductive technologies in recent years, TRAP incidence is growing towards 2.6% of monozygotic twins and 1 every 9,500 to 11,000 pregnancies.3
Regarding the pathogenesis of TRAP sequence, even though still debated, two pathways have been historically proposed:4
Aberrant Placental Vascular Pattern in the Early Stages of Monochorionic Placentation
An unbalanced blood flow between the twins is elicited. The pump twin prevails thanks to its high-pressure flow, while the perfused twin receives a reversed deoxygenated blood flow, leading to compromised morphogenesis. The absence of the development of a functioning heart leads to an acardiac twin that relies on the circulation of pump twin in a parasitic fashion.
Primary Defect in Cardiac Embryogenesis
Secondary to a failure in heart formation, due to chromosome abnormality or environmental factors, the unique perfusion support for the acardiac fetus is received through anastomoses between the umbilical vessels.
Literature about the cytogenetic investigation of acardiac twins is limited. In some parasitic twins, investigators found karyotypes that were different from those of the co-twin.5 However, the etiology of cytogenetic discordancy in TRAP twins remains unclear. Monochorionic twin pregnancies in humans are either monozygotic (as a result of the late splitting of the embryo) or rarely dizygotic: the development of acardiac fetuses in dizygotic twins has been described in animals, through sharing of anastomoses in fused placentae,6 and this might also happen in humans.
Altered embryogenetic pathways have been supposed to trigger an impaired development of one of the twins and, in the presence of vascular anastomoses, the vascular support dependence of acardiac fetus on the predominant pump fetus.7,8
The acardiac twin is not viable, but during the intrauterine period is dangerous for the whole pregnancy. In fact, the well-being of the pump twin can be compromised through at least three mechanisms:9
- congestive heart failure and polyhydramnios of the pump twin, caused by a risen cardiac work due to the increased blood flow;
- preterm premature rupture of membranes (pPROM), preterm labor and preterm delivery, caused by uterine overdistension, since the acardiac twin is often bigger than pump twin and it can reach a considerable size;
- hypoxia and intrauterine growth restriction of the pump twin, caused by the deoxygenated blood that comes back to the pump twin through vascular anastomosis.
For these reasons, the perinatal mortality rate for the pump twin is up to 55%.9 Exceptionally, and for unknown reasons, polyhydramnios can appear in the sac of the cardiac twin.10 Some factors have been identified as markers of a pump-twin poor prognosis:
- congestive heart failure displayed by hydrops or polyhydramnios,
- delivery before 32 weeks,
- a big acardius size defined as an acardiac-to-pump twin weight ratio>70%,
- discrepancy in pump/acardiac umbilical venous diameter (UVD) ratio,
- the presence of a well-developed body and upper extremities in the acardiac twin.
In particular, an acardiac-to-pump twin weight ratio>70% has been associated with an incidence of 90% of preterm delivery, 40% of polyhydramnios and 30% of congestive heart failure.11 The pump/cardiac UVD ratio is a measure of the excess pump cardiac output12 and can be used to predict clinical outcomes of the risk prediction study performed for this parameter.13 The haemodynamic basis of the pump/cardiac UVD ratio rather than alternative non-haemodynamic parameters (eg estimated weight or abdominal circumference discrepancy) may prevent that small acardiacs with very low vascular body resistances leading to adverse outcome are missed.14
Ultrasound Diagnosis
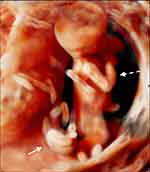
The prenatal diagnosis of an acardiac twin should be considered when at ultrasound examination a monochorionic twin pregnancy is displayed and one of the fetuses has evident morphological abnormalities.15 The typical ultrasound features are: gross differences in biometrical measurements of twins, in particular regarding abdominal circumference; absence of a morphologically normal heart in one twin associated with several other malformations in head, trunk, upper and lower extremities; presence of subcutaneous edema and fluid collections in the anomalous twin (Figure 1). At times, an irregular cardiac activity could be detected, as a result of a rudimentary heart beating or a retrograde pulsation from the pumping twin. A pathognomonic finding is the demonstration at color Doppler of a paradoxical circulation in the acardiac twin, with arterial blood flowing towards, rather than away (Figure 2) and in a caudal-to-cranial course in the abdominal aorta, which may be evident even during the first trimester.16
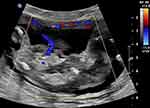 |
Figure 2 Color Doppler image of an acardiac twin at 14 weeks showing reverse perfusion through the umbilical cord. |
Intrauterine death of an abnormal monochorionic twin could resemble an acardius fetus, but maintained growth at ultrasound follow-up shows the correct diagnosis; in these cases, Doppler assessment of the monochorionic twin suspected of being demised in utero should reveal the absence of blood flow: persistent intrafetal blood flow signals should raise the suspicion of a TRAP sequence. Intra-amniotic or placental tumors, as rare differential diagnoses, can be ruled out by detection of the skeleton or the umbilical cord attachment.11
Classification
Based on the morphology of the acardiac fetus, four distinct types have been described:1,17
- Acardius acephalus: is the most common type, 60–75% of cases, characterized by a good development of pelvis and legs and an absence of cephalic pole, thoracic organs and upper extremities.
- Acardius anceps: approximately 10% of cases; is the most morphologically developed acardius, with a recognizable body shape and extremities and a rudimentary development of head and face.
- Acardius acormus: very rare, about 5% of cases; characterized by the only presence of the cephalic pole, while the body (if present) is a shriveled mass. The head can be attached to the placenta either directly or through the umbilical cord.
- Acardius amorphus: approximately 20% of cases; absence of recognizable structures; the fetus appears as a shapeless mass;
This classification allows a precise morphological description but has no prognostic value and does not provide any information about the best management option. It has been suggested that the size of the acardiac twin plays a pivotal role in the pathogenesis of complications that threaten the pump twin. The exact weight of the acardiac twin cannot be calculated using the standard formulas based on ultrasound biometry (such as Hadlock’s), because of the usual lack of anatomical structures; the following formula has been proposed to estimate the weight of acardiac fetus: weight (g) = 1.2 × (longest length in cm)2 - (1.7 × longest length in cm).18
Since the measurement of the acardiac fetus may be difficult to obtain, Wong et al focused on the use of the abdominal circumference in evaluating the acardiac fetus size and suggested a role of abdominal circumference ratio as a prognostic factor.9 They proposed a classification on the bases of prenatal ultrasound findings as acardius size and signs of impaired cardiac function of the pump twin; this classification may help in identifying the most severe cases and those that need prenatal interventions.
Acardiac anomalies are divided into:
- Type I: small or medium-sized acardiac twins, identified by an abdominal circumference ratio <50%.
- Type II: large acardiac twins, in which the abdominal circumference ratio is ≥50%.
Each type can be further divided into a “subtype a”, if pump-twin does not show signs of cardiovascular failure, or into a “subtype b”, if these markers of failure are present.
Management and Treatment
The main goals in the management of the TRAP sequence are preserving the survival of the pump twin and reaching the term for delivery. According to the classification of Wang et al discussed above, the finding of Type Ia acardiac fetus is quite reassuring about the prognosis of the pump twin and allows a conservative management of pregnancy through periodic ultrasound. This clinical conduct is associated with a good outcome in 88% of cases.19 In the presence of an acardius Type Ib, it is appropriate to repeat ultrasound in order to identify a spontaneous resolution or a worsening that requires an invasive treatment.
A Type IIa acardiac fetus can be large because of subcutaneous edema or hydrops, and even if at the moment of diagnosis the pump fetus shows no signs of cardiac failure, the large size could threat the whole pregnancy due to an increased risk of preterm labor. In this case, a prenatal treatment is required. The detection of a Type IIb acardius requires a prompt intervention.
Timing of Intervention
Optimal timing of therapeutic intervention is still debated. TRAP treatment has usually been performed at 16–18 weeks, hypothesizing that the risk of miscarriage could be lower after the spontaneous obliteration of the celomic cavity. However, the spontaneous loss rate of TRAP pregnancies reported in the literature is between 35% and 50%.12,15 In the last decade, the improvement of ultrasound techniques has allowed the diagnosis of TRAP sequence in the first trimester, raising some questions about the early management of this condition: is it better to (a) wait for ultrasound signs of pump-twin impairment, avoiding an early intervention, potentially able to cause abortion and performing a preventive intervention at 16–18 weeks, or (b) plan an intervention at 12 weeks, independently of ultrasound findings, in order to preserve the pump twin from death between 12 and 16 weeks?
In 2010, a retrospective study of Lewi et al described a pump-twin loss rate of 33% (8/24) in the time elapsed from the first-trimester diagnosis and the elective intervention at 16–18 weeks,20 showing an important disadvantage of delayed procedures.
Pagani et al in a cohort study of 23 cases, 17 treated with intervention and 6 managed conservatively, reported an unexpected high spontaneous loss rate in the conservative treatment group (5/6, 83%). Data of treated cases in this study were then combined with data from ten other studies, and the analysis showed a significantly lower adverse pregnancy outcome in the group in which the treatment was undertaken before 16 weeks.21
Conversely, Roethlisberger et al in 2017 published a retrospective analysis of TRAP sequences treated before 14+0 weeks, showing that fetal loss rate is significant in cases treated in the first trimester. They also identified gestational age at intervention and discordance between crown-rump length (CRL) of the pump twin and upper pole-rump length of the TRAP twin, measured as (CRL-URL)/CRL ratio and URL/CRL ratio, as predictive factors of pregnancy outcome.22
A very recent work by Tavares de Sousa et al23 reports a live birth rate of 92% at a median gestational age of 39+6 (interquartile range 37+1 to 41+2) weeks in 12 TRAP cases treated before 14+3 weeks. These differences in outcome may possibly be explained by variation in inclusion criteria, operative technique, as well as gestational age and fetal size even in the narrow interval between 11 and 15 weeks.
Hence, a multicenter, open-label, randomized controlled trial currently ongoing (ClinicalTrails.gov:NCT02621645), named the TRAP Intervention STudy (TRAPIST), comparing treatment at 13–15 weeks vs treatment from 16 weeks, is expected to define the optimal timing of treatment.
Treatment Techniques
Historically, initial therapeutic efforts in the setting of the TRAP sequence aimed to relieve maternal or fetal complications. To counteract cardiovascular failure in the pump-twin digoxin and other inotropic agents were used.24 Indomethacin has been employed to prevent premature labor and to alleviate polyhydramnios.25 Other authors proposed serial amniodrainage in order to reduce polyhydramnios and thus prevent preterm labor.26 After the clarification of TRAP sequence pathophysiology,27 it has been established that the causal treatment is to stop the blood supply to the acardiac twin.
Robie et al reported in 1989 the first invasive intervention: they planned a hysterotomy (sectio parva) at 22 weeks with selective delivery of a 710 g acardiac twin; the healthy pump twin was subsequently delivered at 33 weeks, weighting 2130 g. This positive outcome animated other authors in North America, and between 1989 and 1992, a total of seven of these extremely invasive interventions were reported in the literature. However, the technique showed important maternal complications, including two cases of maternal pulmonary edema deriving from aggressive tocolytic therapy, and adverse pregnancy outcomes, including three cases of abruptio placentae.
In the following years, the acardius’s umbilical cord or intra-abdominal vessels were identified as the principal targets of new therapeutic techniques.
Cord Occlusion
In 1989 Hamada et al28 described the first case of percutaneous cord occlusion by coil embolization; another successful case was described 2 years later by Porreco et al.29 Later, other techniques targeting the umbilical cord were reported: ligation under fetoscopy;30 hysterotomic incision, ultrasound-guided grasping, exteriorization and ligation of the umbilical cord;31 vascular occlusion by thrombogenic agents such alcohol-soaked suture material,32 fibrin, glucose and enbucrilate gel.11
Both monopolar33,34 and bipolar35,36 thermocoagulation techniques have also been used to interrupt cord blood flow under ultrasound guidance. A supposed advantage in using bipolar coagulation is energy passage only between the two blades of the forceps, avoiding dispersion through the umbilical cord, placenta and pump-twin body.36 Nevertheless, this technique involves instruments with large diameter, raising the risk of complications such as pPROM. Furthermore, in different cases, the use of cord ligation may be challenging: edematous cords (difficulty in grasping umbilical cord), extremely short cords (accidental placental damage), complicated access to the amniotic sac of acardius (access through the pump-twin sac and disruption of dividing membrane).
In 1994, Ville et al reported laser coagulation of the umbilical vessels during fetoscopy by neodymium yttrium aluminium garnet (Nd:YAG).37 In 1998, Arias et al published a systematic review including 22 cases of TRAP sequence treated invasively. Among various techniques, fetoscopic-assisted Nd:YAG laser cord occlusion appeared the best treatment if performed at or after 24 weeks.38
Umbilical cord is often an insidious target for the risk of damage to the pump twin. If the twin cords are too near, cord occlusion can harm the pump twin’s cord. An intravascular transfer of ablative material into the circulation of the pump twin is possible if the artery within the acardiac twin’s cord is erroneously inoculated. Furthermore, the same acardius’s umbilical cord can be damaged causing rupture or bleeding, because of its shortness, thinness and anomalous structure. Hydropic acardiac fetuses often present edematous cords and so laser or bipolar coagulation may be challenging.
Using fetoscopy for cord ligation or laser coagulation presents some drawbacks. A technical failure rate of 10%, an increased risk of postoperative pPROM, intra-amniotic infection and bleeding in pump twin have been reported as complications. Endoscopic cord ligation needs the use of general anesthesia and the insertion of at least two 12-gauge trocars into the amniotic cavity. Likewise, laser coagulation under endoscopy is a lengthy procedure that requires the insertion of larger instruments into the amniotic cavity.38,39 In case of anterior placentas, fetoscopic access to the umbilical cord may be hard and often additional interventions of amnioinfusion or amniotomy must be performed to reach more easily the acardiac twin’s umbilical cord. This leads to a more invasive procedure, longer operative time and complications as disruption of the intertwin membrane with subsequent risk of pseudomonoamnionicity and entanglement of the cord. Moreover, fetoscopy requires expensive instrumentation, trained operators and is available in a limited number of centers.
Intrafetal Ablation
The intrafetal approach has the goal of ablating the pelvic vessels or the abdominal aorta of the acardiac twin, which can be easily identified on color Doppler ultrasound. This procedure is not influenced by placental location, umbilical cord structure, amniotic fluid volume and position of the acardius.
In 1987, Seed et al described the first but unsuccessful attempt of intrafetal therapy: they repeatedly pierced the pulsatile tissue within the acardiac twin in order to produce tamponade.40 Sepulveda in 1995 described the first case of intrafetal ablation using a sclerosing agent (absolute alcohol), explaining the new technique and the rationale for targeting the intra-abdominal rather than the umbilical vessels.41 Various methods have been described to perform intrafetal ablation: alcohol embolization, monopolar diathermy, laser and radiofrequency under ultrasound guide.
Alcohol embolization is the easier and most available technique. It is an outpatient procedure that requires few instruments: a 20- or 22-gauge spinal needle and a 5-mL syringe loaded with absolute alcohol. Its main disadvantage is the possibility of the transfer of the sclerosant agent into the circulation of the pump twin and its subsequent intrauterine death.
Recently, several energy sources have been used to coagulate the intra-abdominal vessels and to obtain intrafetal ablation. The technique of monopolar diathermy requires an 18-gauge needle, a 1-mm diameter wire electrode insulated with polytetrafluoroethylene along most of its length except for 3 mm at the tip, a ground pad and a standard monopolar diathermy generator.33 An alternative technique was described by Sepulveda et al in 2003 with the use of a simple wire.42 Monopolar diathermy is an outpatient procedure, it can be performed under local anesthesia and also in early pregnancy. The possible risk of thermal injury to fetal and maternal tissues closer to the inserted cannula is the principal complication.
An interesting procedure is an interstitial laser, executed under local anaesthesia using an 18-gauge needle, laser fibers and an Nd:YAG or diode laser generator.43,44 The skills required are similar to any other invasive needling procedure: under ultrasound guidance, the needle is inserted close to the target vessels into the abdomen or pelvis of the acardiac twin; the laser fiber is then introduced through the needle and its tip has to be seen slightly beyond the needle. Then, pulses of laser energy are applied, increasing power if necessary, until the occlusion of the blood vessels is indicated by the hyperechogenicity of the initial vascular area.
Interstitial laser and monopolar diathermy are similar techniques, but the first one poses no risk of burns during cauterization. Like in other interstitial procedures, the risk of hemorrhage due to umbilical cord rupture is decreased by the position of laser fiber in the body of the acardius; this target also prevents unintended injury to the pump twin or to the amniotic membrane. The use of a finer gauge cannula than the one used for endoscopy or bipolar diathermy theoretically diminishes the risk of membrane rupture. Furthermore, interstitial laser can be used in early gestation: nowadays TRAP sequences are often confirmed in the first trimester and hence an early intervention could reduce the occurrence of adverse outcomes in the pump twin. The use of an operative microendoscopy before 16 weeks of gestation has been proposed, but the risk of pPROM appears higher due to larger bore cannula (2.7 mm maximal diameter).43
Radiofrequency ablation (RFA), the latest technique described in the literature, employs a radiofrequency generator and a specific 3 mm/14-gauge needle device at the end of which there are some tines that can be opened in a shape similar to an umbrella.45 The RFA needle is positioned under ultrasound guidance in the abdomen of the acardiac fetus; the tines of the RFA device are deployed; radiofrequency energy is released by the generator according to a specific calibration. When the device senses the increase in tissue impedance as a result of necrosis, the power output stops. The effectiveness of the procedure can be confirmed by Doppler ultrasound showing an absent flow to the acardius fetus.46 The possibility of injuries to the co-twin or to surrounding tissues is excluded since the energy is released from the radiofrequency device only after deployment into the target tissue.
Although the RFA device has the largest diameter of all three methods, it appears to be relatively safe and effective in the treatment of the TRAP sequence.
Technique Comparison
As discussed before, cord occlusion procedures have several drawbacks if compared with intrafetal techniques. Tan and Sepulveda in their review of literature compared 40 cases of TRAP sequence treated by cord occlusion and 31 cases treated by intrafetal ablation. The analysis results showed a lower technical failure rate (13% vs 35%, p= 0.03), a lower rate of premature delivery or pPROM before 32 weeks (23% vs 58%, p= 0.003) and a higher rate of clinical success (77% vs 50%, p= 0.02) using intrafetal ablation.11
Several experiences about the use of RFA for a selective reduction in complex monochorionic pregnancies and, in particular, for TRAP sequence treatment have been reported in the last years. Cabassa et al published a case series and reviewed the existing literature on the subject: 6 other studies were included for a total of 88 pregnancies (monochorionic twins or triplets with a monochorionic component). The overall neonatal survival in the TRAP sequence treated by RFA was 85% (75/88). Regarding pPROM, the most common post-procedural complication, not all studies reported specific details and so the incidence of 22% (19/85) is a rough estimate.46
In the North American Fetal Therapy network registry, Lee et al found 98 TRAP cases treated by RFA, with a pump-twin survival rate of 80%.47 Chaveeva and colleagues performed a meta-analysis showing an overall survival rate of 80.8% for the pump twin after RFA treatment.48 Recently, a study by Zhang et al listed 11 cases of RFA use in the TRAP sequence. The overall survival rate of the pump twin was 70%.49
Since its first appearance in Jolly’s paper, the therapeutic use of interstitial laser in the TRAP sequence has been the subject of several studies. Pagani et al in 2013 performed a retrospective cohort study and a review of literature about the TRAP sequence treated with intrafetal laser therapy (ILF). A total of 51 pregnancies were included. The overall neonatal survival was 80% (42/51); adverse pregnancy outcome (intra-uterine death of pump twin or preterm birth before 37 weeks) was significantly lower when treatment was undertaken before 16 weeks compared with pregnancies treated at or after 16 weeks (19%, 3/16 vs 66%, 23/35, respectively; p=0.0025).21 Chaveeva et al in 2014 added their case series and updated the meta-analysis. The combined data included 104 twin pregnancies treated by ILF. The overall survival rate was 76%.48
In recent years, endoscopic procedures have been almost abandoned because of their invasiveness while both RFA and intrafetal laser are the most commonly used techniques appearing reliable and secure in the treatment of TRAP sequence.
Pagani et al revising their case series noted that, even though neonatal survival rate was comparable between the intrafetal RFA and intrafetal laser techniques (85 vs 82%, p=0.63), the incidence of pPROM before 32 weeks’ gestation was significantly higher with RFA (22 vs 7%, p=0.045).21 In a multicenter study published by Scheier et al, pump twins had a higher rate of intrauterine demise if RFA treatment was performed at 15–19 weeks compared to RFA treatment after 19 weeks (33.3 vs 10.7%).50 Chaveeva showed that the risk of death for the pump twin using intrafetal laser is lower if the therapeutic intervention is undertaken at 12–14 weeks than at later gestational ages. In any case, a real comparison among RFA and IFL has not been possible, since there is a lack of data about RFA procedures performed before 15 weeks’ gestation.48
Challenges in Monoamniotic Twins
In approximately 5% of cases of TRAP sequence the pregnancy is monoamniotic, ie the two fetuses are contained within a single amniotic cavity. Often, the umbilical cord of the acardiac twin is very short, and in this case treatment options are not different from those in the more common diamniotic pregnancies. However, if the cord of the cardiac twin is long enough, it almost necessarily becomes entangled with the umbilical cord of the pump twin (Figure 3). If a standard intrafetal technique is employed, the acardius’s cord may constrict that of the pump twin, leading to its demise.51 In monoamniotic pregnancies with entangled cords, therefore, devascularization of the acardiac twin is better achieved by coagulation of the acardiac twin cord by means of fetoscopic laser or ultrasound-guided bipolar coagulation, followed by transection of the coagulated cord performed fetoscopically with a laser fiber or miniature scissors.51–53
 |
Figure 3 Cord entanglement in monoamniotic twin reversed arterial perfusion (TRAP) sequence at 18 weeks. Long arrow: entangled cords. Short arrow: segment of free cord. Asterisk: normal twin. Reproduced with permission from Prefumo F, Fichera A, Zanardini C, Frusca T. Fetoscopic cord transection for treatment of monoamniotic twin reversed arterial perfusion sequence. Ultrasound Obstet Gynecol. 2014;43(2):234–235. Copyright © 2013 ISUOG. Published by John Wiley & Sons Ltd.52 |
Despite the rarity of this condition, the procedure appears to be safe, with a 75% survival rate of the normal twin.54
Conclusions
Management and treatment of TRAP sequence pose unresolved challenges, since both the technique of choice and the optimal timing of intervention still have to be clarified. While the current literature is rich in studies, their high heterogeneity makes the comparisons prone to bias. If treatment is needed, the best timing of intervention is still debated, although the latest studies encourage intervention in the first trimester of pregnancy. As for the technique of choice to interrupt the vascular supply to the acardiac twin, ultrasound-guided laser coagulation and radiofrequency ablation of the intrafetal vessels are usually the preferred approaches.
Abbreviations
IFL, intrafetal laser therapy; Nd:YAG, neodymium yttrium aluminium garnet; pPROM, preterm premature rupture of the membranes; RFA, radiofrequency ablation; TRAP, twin reversed arterial perfusion.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Napolitani FD, Schreiber I. The acardiac monster. A review of the world literature and presentation of 2 cases. Am J Obstet Gynecol. 1960;80(3):582–589. doi:10.1016/S0002-9378(16)36520-6
2. Lehr C, Dire J. Rare occurrence of a holoacardious acephalic monster: sonographic and pathologic findings. J Clin Ultrasound. 1978;6(4):259–261. doi:10.1002/jcu.1870060414
3. van Gemert MJC, van den Wijngaard JPHM, Vandenbussche FPHA. Twin reversed arterial perfusion sequence is more common than generally accepted. Birth Defects Res a Clin Mol Teratol. 2015;103(7):641–643. doi:10.1002/bdra.23405
4. Søgaard K, Skibsted L, Brocks V. Acardiac twins: pathophysiology, diagnosis, outcome and treatment. Six cases and review of the literature. Fetal Diagn Ther. 1999;14(1):53–59. doi:10.1159/000020889
5. Moore CA, Buehler BA, McManus BM, Harmon JP, Mirkin LD, Goldstein DJ. Acephalus-acardia in twins with aneuploidy. Am J Med Genet Suppl. 1987;3(S3):139–143. doi:10.1002/ajmg.1320280516
6. Benirschke K. The biology of the twinning process: how placentation influences outcome. Semin Perinatol. 1995;19(5):342–350. doi:10.1016/S0146-0005(05)80012-6
7. Benirschke K. The monozygotic twinning process, the twin-twin transfusion syndrome and acardiac twins. Placenta. 2009;30(11):923–928. doi:10.1016/j.placenta.2009.08.009
8. van Gemert MJC, van Den Wijngaard JPHM, Paarlberg KM, Gardiner HM, Nikkels PGJ. Acardiac twin pregnancies part IV: acardiac onset from unequal embryonic splitting simulated by a fetoplacental resistance model. Birth Defects Res. 2017;109(3):211–223. doi:10.1002/bdra.23581
9. Wong AE, Sepulveda W. Acardiac anomaly: current issues in prenatal assessment and treatment. Prenat Diagn. 2005;25(9):796–806. doi:10.1002/pd.1269
10. Marella D, Prefumo F, Valcamonico A, Donzelli CM, Frusca T, Fichera A. Polyhydramnios in sac of parasitic twin: atypical manifestation of twin reversed arterial perfusion sequence. Ultrasound Obstet Gynecol. 2015;45(6):752–753. doi:10.1002/uog.14766
11. Tan TYT, Sepulveda W. Acardiac twin: a systematic review of minimally invasive treatment modalities. Ultrasound Obstet Gynecol. 2003;22(4):409–419. doi:10.1002/uog.224
12. van Gemert MJC, Umur A, van den Wijngaard JPHM, VanBavel E, Vandenbussche FPHA, Nikkels PGJ. Increasing cardiac output and decreasing oxygenation sequence in pump twins of acardiac twin pregnancies. Phys Med Biol. 2005;50(3):N33.
13. van Gemert MJC, Pistorius LR, Benirschke K, et al. Hypothesis acardiac twin pregnancies: pathophysiology-based hypotheses suggest risk prediction by pump/acardiac umbilical venous diameter ratios. Birth Defects Res a Clin Mol Teratol. 2016;106(2):114–121. doi:10.1002/bdra.23467
14. van Lier MGJTB, Lopriore E, Vandenbussche FPHA, et al. Acardiac twinning: high resolution three-dimensional reconstruction of a low resistance case. Birth Defects Res a Clin Mol Teratol. 2016;106(3):213–217. doi:10.1002/bdra.23477
15. Sepúlveda WH, Quiroz VH, Giuliano A, Henríquez R. Prenatal ultrasonographic diagnosis of acardiac twin. J Perinat Med. 1993;21(3):241–258. doi:10.1515/jpme.1993.21.3.241
16. Sebire N, Sepulveda W, Jeanty P, Nyberg D, Nicolaides K. Multiple gestation. In: Nyberg D, McGahan J, Pretorius D, Pilu G, editors. Diagnostic Imaging of Fetal Anomalies. Philadelphia: Lippincott Williams & Wilkins; 2003:777–813.
17. Pepe F, Teodoro MC, Luca C, Privitera F. Conservative management in a case of uncomplicated trap sequence: a case report and brief literature review. J Prenat Med. 2015;9(3–4):29–34. doi:10.11138/jpm/2015.9.3.029
18. Moore TR, Gale S, Benirschke K. Perinatal outcome of forty-nine pregnancies complicated by acardiac twinning. Am J Obstet Gynecol. 1990;163(3):907–912. doi:10.1016/0002-9378(90)91094-S
19. Jelin E, Hirose S, Rand L, et al. Perinatal outcome of conservative management versus fetal intervention for twin reversed arterial perfusion sequence with a small acardiac twin. Fetal Diagn Ther. 2010;27(3):138–141. doi:10.1159/000295176
20. Lewi L, Valencia C, Gonzalez E, Deprest J, Nicolaides KH. The outcome of twin reversed arterial perfusion sequence diagnosed in the first trimester. Am J Obstet Gynecol. 2010;203(3):
21. Pagani G, D’Antonio F, Khalil A, Papageorghiou A, Bhide A, Thilaganathan B. Intrafetal laser treatment for twin reversed arterial perfusion sequence: cohort study and meta-analysis. Ultrasound Obstet Gynecol. 2013;42(1):6–14. doi:10.1002/uog.12495
22. Roethlisberger M, Strizek B, Gottschalk I, et al. First-trimester intervention in twin reversed arterial perfusion sequence: does size matter? Ultrasound Obstet Gynecol. 2017;50(1):40–44. doi:10.1002/uog.16013
23. Tavares de Sousa M, Glosemeyer P, Diemert A, Bamberg C, Hecher K. First-trimester intervention in twin reversed arterial perfusion sequence. Ultrasound Obstet Gynecol. 2020;55(1):47–49. doi:10.1002/uog.20860
24. Simpson PC, Trudinger BJ, Walker A, Baird PJ. The intrauterine treatment of fetal cardiac failure in a twin pregnancy with an acardiac, acephalic monster. Am J Obstet Gynecol. 1983;147(7):842–844. doi:10.1016/0002-9378(83)90056-X
25. Ash K, Harman CR, Critter H. TRAP sequence—Successful outcome with indomethacin treatment. Obstet Gynecol. 1990;76(5 Pt 2):960–962.
26. Platt LD, DeVore GR, Bieniarz A, Benner P, Rao R. Antenatal diagnosis of acephalus acardia: a proposed management scheme. Am J Obstet Gynecol. 1983;146(7):857–859. doi:10.1016/0002-9378(83)91090-6
27. Van Allen MI, Smith DW, Shepard TH. Twin reversed arterial perfusion (TRAP) sequence: a study of 14 twin pregnancies with acardius. Semin Perinatol. 1983;7(4):285–293.
28. Hamada H, Okane M, Koresawa M, Kubo T, Iwasaki H. [Fetal therapy in utero by blockage of the umbilical blood flow of acardiac monster in twin pregnancy]. Acta Obstet Gynaecol Jpn. 1989;41(11):1803–1809. Japanese.
29. Porreco RP, Barton SM, Haverkamp AD. Occlusion of umbilical artery in acardiac, acephalic twin. Lancet. 1991;337(8737):326–327. doi:10.1016/0140-6736(91)90946-M
30. McCurdy CM, Childers JM, Seeds JW. Ligation of the umbilical cord of an acardiac-acephalus twin with an endoscopic intrauterine technique. Obstet Gynecol. 1993;82(4 Pt 2 Suppl):708–711.
31. Foley MR, Clewell WH, Finberg HJ, Mills MD. Use of the Foley Cordostat grasping device for selective ligation of the umbilical cord of an acardiac twin: a case report. Am J Obstet Gynecol. 1995;172(1 Pt 1):212–214. doi:10.1016/0002-9378(95)90117-5
32. Holzgreve W, Tercanli S, Krings W, Schuierer G, Quintero RA. A simpler technique for umbilical-cord blockade of an acardiac twin. N Engl J Med. 1994;331(1):56–57.
33. Rodeck C, Deans A, Jauniaux E. Thermocoagulation for the early treatment of pregnancy with an acardiac twin. N Engl J Med. 1998;339(18):1293–1295. doi:10.1056/NEJM199810293391805
34. Holmes A, Jauniaux E, Rodeck C. Monopolar thermocoagulation in acardiac twinning. Br J Obstet Gynaecol. 2001;108(9):1000–1002.
35. Hecher K, Lewi L, Gratacos E, Huber A, Ville Y, Deprest J. Twin reversed arterial perfusion: fetoscopic laser coagulation of placental anastomoses or the umbilical cord. Ultrasound Obstet Gynecol. 2006;28(5):688–691. doi:10.1002/uog.3816
36. Nicolini U, Poblete A, Boschetto C, Bonati F, Roberts A. Complicated monochorionic twin pregnancies: experience with bipolar cord coagulation. Am J Obstet Gynecol. 2001;185(3):703–707. doi:10.1067/mob.2001.117190
37. Ville Y, Hyett JA, Vandenbussche FPHA, Nicolaides KH. Endoscopic laser coagulation of umbilical cord vessels in twin reversed arterial perfusion sequence. Ultrasound Obstet Gynecol. 1994;4(5):396–398. doi:10.1046/j.1469-0705.1994.04050396.x
38. Arias F, Sunderji S, Gimpelson R, Colton E. Treatment of acardiac twinning. Obstet Gynecol. 1998;91(5 Pt 2):818–821. doi:10.1016/s0029-7844(97)00708-4
39. Quintero RA, Muñoz H, Pommer R, Diaz C, Bornick PW, Allen MH. Operative fetoscopy via telesurgery. Ultrasound Obstet Gynecol. 2002;20(4):390–391. doi:10.1046/j.1469-0705.2002.00809.x
40. Seeds JW, Herbert WNP, Richards DS. Prenatal sonographic diagnosis and management of a twin pregnancy with placenta previa and hemicardia. Am J Perinatol. 1987;4(4):313–316. doi:10.1055/s-2007-999798
41. Sepulveda W, Bower S, Hassan J, Fisk NM. Ablation of acardiac twin by alcohol injection into the intra-abdominal umbilical artery. Obstet Gynecol. 1995;86(4 Pt 2):680–681. doi:10.1016/0029-7844(95)00171-M
42. Sepulveda W, Corral E, Gutierrez J. A simple device for vascular occlusion of acardiac twins. Ultrasound Obstet Gynecol. 2003;21(4):386–388. doi:10.1002/uog.96
43. Jolly M, Taylor M, Rose G, Govender L, Fisk NM. Interstitial laser: a new surgical technique for twin reversed arterial perfusion sequence in early pregnancy. Br J Obstet Gynaecol. 2001;108(10):1098–1102.
44. Sepulveda W, Hasbun J, Dezerega V, Devoto JC, Alcalde JL. Successful sonographically guided laser ablation of a large acardiac twin at 26 weeks’ gestation. J Ultrasound Med. 2004;23(12):1663–1666. doi:10.7863/jum.2004.23.12.1663
45. Tsao KJ, Feldstein VA, Albanese CT, et al. Selective reduction of acardiac twin by radiofrequency ablation. Am J Obstet Gynecol. 2002;187(3):635–640. doi:10.1067/mob.2002.125242
46. Cabassa P, Fichera A, Prefumo F, et al. The use of radiofrequency in the treatment of twin reversed arterial perfusion sequence: a case series and review of the literature. Eur J Obstet Gynecol Reprod Biol. 2013;166(2):127–132. doi:10.1016/j.ejogrb.2012.10.009
47. Lee H, Bebbington M, Crombleholme TM. The North American fetal therapy network registry data on outcomes of radiofrequency ablation for twin-reversed arterial perfusion sequence. Fetal Diagn Ther. 2013;33(4):224–229. doi:10.1159/000343223
48. Chaveeva P, Poon LC, Sotiriadis A, Kosinski P, Nicolaides KH. Optimal method and timing of intrauterine intervention in twin reversed arterial perfusion sequence: case study and meta-analysis. Fetal Diagn Ther. 2014;35(4):267–279. doi:10.1159/000358593
49. Zhang ZT, Yang T, Liu CX, Li N. Treatment of twin reversed arterial perfusion sequence with radiofrequency ablation and expectant management: a single center study in China. Eur J Obstet Gynecol Reprod Biol. 2018;225:9–12. doi:10.1016/j.ejogrb.2018.03.046
50. Scheier M, Molina FS. Outcome of twin reversed arterial perfusion sequence following treatment with interstitial laser: a retrospective study. Fetal Diagn Ther. 2012;31(1):35–41. doi:10.1159/000334156
51. Valsky DV, Martinez-Serrano MJ, Sanz M, et al. Cord occlusion followed by laser cord transection in monochorionic monoamniotic discordant twins. Ultrasound Obstet Gynecol. 2011;37(6):684–688. doi:10.1002/uog.8924
52. Prefumo F, Fichera A, Zanardini C, Frusca T. Fetoscopic cord transection for treatment of monoamniotic twin reversed arterial perfusion sequence. Ultrasound Obstet Gynecol. 2014;43(2):234–235.
53. Tonni G, Grisolia G, Zampriolo P, et al. TRAP Sequence in Monochorionic/Monoamniotic (MC/MA) discordant twins: two cases treated with fetoscopic laser surgery. Fetal Pediatr Pathol. 2018;37(6):433–447. doi:10.1080/15513815.2018.1526240
54. Peeters SHP, Devlieger R, Middeldorp JM, et al. Fetal surgery in complicated monoamniotic pregnancies: case series and systematic review of the literature. Prenat Diagn. 2014;34(6):586–591. doi:10.1002/pd.4353
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.