Back to Journals » Clinical Ophthalmology » Volume 14
Twelve-Month Outcomes of Stand-Alone Excisional Goniotomy in Mild to Severe Glaucoma
Authors ElMallah MK, Berdahl JP , Williamson BK , Dorairaj SK , Kahook MY, Gallardo MJ , Mahootchi A , Smith SN, Rappaport LA, Diaz-Robles D, Lazcano-Gomez GS
Received 1 April 2020
Accepted for publication 7 May 2020
Published 3 July 2020 Volume 2020:14 Pages 1891—1897
DOI https://doi.org/10.2147/OPTH.S256423
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Mohammed K ElMallah,1 John P Berdahl,2 Blake K Williamson,3 Syril K Dorairaj,4 Malik Y Kahook,5 Mark J Gallardo,6 Ahad Mahootchi,7 Sanjay N Smith,8 Leonard A Rappaport,9 Daniela Diaz-Robles,10 Gabriel S Lazcano-Gomez11
1Ocala Eye, Ocala, FL, USA; 2Vance Thompson Vision, Sioux Falls, SD, USA; 3Williamson Eye Center, Baton Rouge, LA, USA; 4Mayo Clinic, Jacksonville, FL, USA; 5University of Colorado, Aurora, CO, USA; 6El Paso Eye Surgeons, El Paso, TX, USA; 7The Eye Clinic of Florida, Zephyrhills, FL, USA; 8Medical Eye Associates, South Miami, FL, USA; 9Tidewater Eye Centers, Portsmouth, VA, USA; 10Clinica Santa Lucia, Guadalajara, Mexico; 11Asociacion Para Evitar Le Ceguera (APEC), Mexico City, Mexico
Correspondence: Mohammed K ElMallah Email [email protected]
Purpose: To describe 12-month intraocular pressure (IOP) and medication use outcomes following excisional goniotomy (EG) as a stand-alone procedure in eyes with medically uncontrolled glaucoma.
Methods: This was a retrospective analysis of data from surgeons at 8 centers (6 US, 2 Mexico). Eyes with glaucoma undergoing standalone EG with a specialized instrument (Kahook Dual Blade, New World Medical, Rancho Cucamonga, CA) for IOP reduction and followed for 12 months postoperatively were included. Data were collected preoperatively, intraoperatively, and 1 day, 1 week, and 1, 3, 6, and 12 months postoperatively. The primary outcome was reduction from baseline in IOP, and key secondary outcomes included IOP-lowering medication reduction as well as adverse events.
Results: A total of 42 eyes were analyzed, of which 36 (85.7%) had mild to severe primary open-angle glaucoma (POAG). Mean (standard error) IOP at baseline was 21.6 (0.8) mmHg, and mean number of medications used at baseline was 2.6 (0.2). At 3, 6, and 12 months postoperatively, mean IOP reductions from baseline were 4.6 mmHg (22.3%), 5.6 mmHg (27.7%), and 3.9 mmHg (19.3%) (p≤ 0.001 at each time point). At the same time points, mean medications reductions of 0.7 (25.8%), 0.9 (32.6%), and 0.3 (12.5%) medications were seen (p< 0.05 at months 3 and 6, not significant at month 12). Six eyes (14.3%) underwent additional glaucoma surgery during the 12-month follow-up period.
Discussion: Standalone EG with KDB can reduce IOP, and in many cases reduce medication use, through up to 12 months in eyes with mild to severe glaucoma. Statistically significant and clinically relevant reductions in IOP were seen at every time point. While the goal of surgery was not to reduce medication burden, mean medication use was significantly reduced at all but the last time point. In the majority of eyes, the need for a bleb-based glaucoma procedure was delayed or prevented for at least 12 months.
Keywords: glaucoma, standalone, Kahook dual blade, goniotomy
Introduction
Efforts to develop a safer form of surgery for intraocular pressure (IOP) reduction in eyes with glaucoma have led to the development of a family of new devices and procedures that offer a more favorable safety profile than trabeculectomy, although with generally less efficacy.1–4 The improved safety profile of these procedures over trabeculectomy is largely attributable to their avoidance of bleb formation, a well-established source of long-term vision-threatening complications.5 With improved safety, these procedures are being utilized in a broadening array of patients, both as standalone procedures and in combination with cataract extraction, in whom glaucoma surgery might not otherwise be indicated, particularly those with mild or moderate glaucoma. In such patients, therapeutic goals may include modest IOP reductions, or reduction in the IOP-lowering medication burden. These modest goals may not justify the risks associated with traditional trabeculectomy or tube-shunt surgery but can be safely achieved in most eyes with a less invasive procedure.
These novel procedures were initially designed to bypass the diseased trabecular meshwork (TM) that impedes aqueous humor outflow leading to elevated IOP.6 This was accomplished either by direct bypass of the TM—shunting aqueous into Schlemm’s canal distally—or by diverting aqueous into the supraciliary space to utilize the uveoscleral drainage pathway.1–4 More recently, procedures that deliver aqueous through a shunt to the subconjunctival space have been developed;7,8 these procedures require the formation of a filtering bleb, trading the safety associated with blebless procedures for potentially greater efficacy.
MIGS procedures may or may not involve the implantation of a permanent device in the eye to serve as a shunt for aqueous transport. The iStent and iStent Inject (Glaukos, San Clemente, CA)9,10 and Hydrus (Ivantis, Irvine, CA)11 are permanent implants that bypass the TM and deliver aqueous to Schlemm’s canal, while the CyPass (Alcon, Ft. Worth, TX; now withdrawn from the marketplace over late-appearing corneal safety issues)12,13 was a stent connecting the anterior chamber to the supraciliary space. In contrast, procedures such as incisional goniotomy, gonioscopy-assisted transluminal trabeculotomy (GATT) and ab interno trabeculotomy are performed with surgical devices that are withdrawn at the conclusion of the procedure, and excisional goniotomy is performed with a specially designed disposable blade (Kahook Dual Blade [KDB], New World Medical, Rancho Cucamonga, CA) that is also withdrawn once the procedure is complete.
Excisional goniotomy with the KDB at the time of cataract surgery has been well described.14–22 The combination procedure lowered IOP by 12–27% and medications by 21–71% 6–12 months after surgery in eyes with open-angle glaucoma.14–20 These wide ranges reflect variable indications for surgery between and within studies, with some eyes undergoing surgery primarily for IOP reduction (in which medication reduction might not be expected), and others for reduction in medication burden (in which IOP reductions might not be expected). In studies that account for patient-specific surgical goals, IOP reductions of 40% and medication reductions of 60% have been reported.15,16
Fewer studies have evaluated excisional goniotomy with the KDB as a standalone procedure. These studies have reported IOP reductions of 24–36% and medication reductions of 26–40% six23,24 or twelve20 months after surgery. In this paper, we report the extended 12-month reductions in IOP and medication use following standalone excisional goniotomy with the KDB in a cohort previously described in a 6-month analysis.23
Methods
This was a retrospective analysis of data from the medical records of patients with medically treated glaucoma undergoing standalone excisional goniotomy using the KDB. A de-identified data set was analyzed, and the study was granted a waiver of informed consent from a central ethics committee (Sterling IRB, Atlanta, GA). Data were collected from 8 surgeons at 8 centers (6 within the United States and 2 in Mexico).
Subjects included in this analysis were ≥18 years of age, phakic or pseudophakic, with any type and severity of glaucoma, with inadequate IOP control using at least 1 and up to 3 IOP-lowering medications. Indications for surgery included reduction of IOP, reduction of IOP-lowering medications, or both. Patients with a recent history (within 3 months) of IOP-lowering medication addition, laser trabeculoplasty, iridotomy, or initiation of systemic beta-blocker therapy were excluded, as were patients with previous glaucoma surgery, and those with uncontrolled systemic conditions that might confound study measurements. Both eyes of a given patient were included if both qualified.
Surgical technique was not standardized in this retrospective study. Each case was performed according to the manufacturer’s directions for use. Excisional goniotomy with the KDB has been described previously.14,23 Following entry into the anterior chamber through a peripheral corneal incision, the KDB’s distal tip pierces the TM, entering Schlemm’s canal, and is advanced along the canal, which elevates and guides the TM onto two parallel blades that excise a strip of TM,25 leaving a clear path for aqueous humor to drain into Schlemm’s canal and the distal collector channels. Standard postoperative antimicrobial and anti-inflammatory prophylaxis were administered per each surgeon’s preference.
Preoperative data collected included patient demographic information, glaucoma type and severity, past ocular history, current IOP-lowering medications, and baseline visual acuity (VA) and IOP. Intraoperative adverse events were recorded. Postoperative data included current ocular and systemic medications, VA, IOP, adverse events, and any secondary surgical interventions for IOP control at 1 day, 1 week, and 1, 3, 6, and 12 months postoperatively.
The primary efficacy outcome of this study was IOP reduction from baseline. Paired t-tests were used to compare mean IOP at each time point to baseline. The change from baseline in the number of IOP-lowering medications was a secondary efficacy endpoint and was analyzed similarly to IOP. Means are reported ± standard error (SE). Safety analysis consisted of the incidence of intraoperative and postoperative adverse events. As the study endpoints are descriptive rather than designed to test a specific hypothesis, formal power and sample size calculations were not performed.
Results
A total of 42 eyes of 35 subjects were included in this analysis. Baseline demographic and glaucoma status are given in Table 1. The majority of subjects were Caucasian, female, and had mild to severe primary open-angle glaucoma (POAG) as described in the International Classification of Diseases-10.
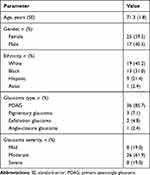 |
Table 1 Demographic and Baseline Glaucoma Status Data |
Mean (standard error) IOP at baseline was 21.6 (0.8) mmHg in study eyes and from Day 1 through 12 months of postoperative follow-up ranged from 14.7 (0.7) to 16.5 (0.8) mmHg, representing reductions of 3.9–6.1 mmHg (14.9–25.6%, p≤0.001 at each time point versus baseline) (Table 2 and Figure 1). Significant reductions in IOP were evident as soon as Day 1 postoperatively and remained significant throughout follow-up. At Month 12, 59.5% (25/42) of patients had achieved IOP ≤18 mmHg and 42.9% (18/42) had achieved a ≥20% IOP reduction from baseline.
 |
Table 2 Mean IOP and Medication Data at Each Time Point |
 |
Figure 1 Mean intraocular pressure (IOP) at each time point, n=42 eyes (error bars represent standard error). |
At the time of surgery, study eyes required a mean of 2.6 (0.2) IOP-lowering medications (Table 2). Through 12 months of follow-up, mean medication reductions of 0.3 to 0.9 were seen; these medication reductions were significant (p≤0.028) at all time points except Month 12 (mean 2.2 medications, 0.3 medication reduction [14.6%], p=0.18). By Month 12, 40.5% (17/42) of eyes were able to discontinue the use of 1 or more IOP-lowering medications.
Goniotomy using the KDB was safe and well tolerated. Intraoperative and early postoperative complications in this cohort were reported previously and included a single intraoperative Descemet’s tear and three cases of postoperative IOP elevation, one of which resolved with medical therapy and two of which necessitated additional surgery.23 No new adverse events were noted between 6 and 12 months postoperatively. Overall, through 12 months of follow-up, 6 eyes required additional glaucoma surgery (Table 3), including 4 trabeculectomies (2 with Ex-Press mini-shunt [Alcon Laboratories, Fort Worth, TX] and 2 Ahmed valve implantations (New World Medical, Rancho Cucamonga, CA)).
 |
Table 3 Clinical Summary of Eyes Requiring Additional Surgery |
Discussion
In eyes with medically uncontrolled mild to severe glaucoma, excisional goniotomy with the Kahook Dual Blade provided significant and sustained IOP reductions averaging 4–6 mmHg through 12 months of follow-up. In these eyes undergoing surgery primarily for IOP reduction, significant medication reductions of 19–28% were achieved through 6 months of follow-up, although medication reductions were not significant at Month 12. Overall, 85.7% of eyes (36/42) were able to avoid more invasive glaucoma surgery during the 12-month postoperative period.
Two prior studies of excisional goniotomy with the KDB, both retrospective and varying from 6 to 12 months in duration, have evaluated the procedure’s efficacy when performed as a standalone procedure.20,24 These studies reported mean IOP reductions ranging from 24% to 31% and mean medication reductions ranging from 26% to 37%. A 6-month analysis of subjects included in the current 12-month analysis reported mean IOP reductions of 30–44% and mean medication reductions of 40% at Month 6.23 The 6-month analysis included a stratified subgroup analysis of patients with high versus low baseline IOP (above versus below the sample median) under the assumption that surgical goals in high IOP eyes are IOP reduction and in low IOP eyes are medication reductions; in this analysis, IOP reduction in high IOP eyes was 46% and medication reduction in low IOP eyes was 36%.23 The results of the current study are generally consistent with these prior reports; of note, the baseline IOP in the current study (21.6 mmHg) was higher than in either of the prior 2 studies (18.4–20.4 mmHg).20,24
These results also compare favorably to outcomes with other incisional and excisional trabecular meshwork-based procedures. Standalone incisional goniotomy with the Trabectome has been reported to produce mean IOP reductions of 23–39%26,27 and medication reductions of 7-43%.27–31 Gonioscopy-assisted transluminal trabeculotomy can lower IOP 28–44% and medications 28–70%,32–37 while ab interno trabeculotomy produces IOP reductions of 31–32% and medication reductions of 35–82%.38,39
With so many novel glaucoma surgeries available, the significance of glaucoma severity on the choice of surgical procedures to perform is incompletely characterized. Some procedures—such as the trabecular microbypass40 and the Schlemm’s canal stent41 are indicated per their label in eyes with mild to moderate open-angle glaucoma. The KDB’s label is not restricted by the severity or type of glaucoma which it can be used to treat. Standalone excisional goniotomy with the KDB has been shown to produce mean IOP reductions of 24% and medication reductions of 44% in a prior study.24 In the current study, six eyes (1 mild, 4 moderate, and 1 severe) required additional glaucoma surgery; corresponding failure rates by severity were thusly 12.5% for mild cases, 15.4% for moderate cases, and 12.5% for severe cases.
Limitations of the current analysis include its retrospective nature and the lack of a control group. Absent an active control, we have attempted above to benchmark the findings of this study with those of other procedures as reported in the literature. The standalone nature of the procedure is a strength of this analysis, as there are few studies evaluating the effects of new glaucoma procedures performed on their own, without the confounding effect that cataract surgery has on both IOP and medication use in glaucomatous eyes.42 Extending follow-up through 12 months builds on a prior report of 6-month outcomes in this data set23 and provides longer-term data to guide surgical decision-making for this chronic disease. Also, this data set was derived from multiple surgeons; while this can be considered a limitation in that procedures may not have been robustly standardized, there is also value in assessing the outcome of the procedure in a multi-surgeon, real-world setting where such standardization would be artificial.
In summary, excisional goniotomy with the KDB as a standalone procedure can reduce IOP, and in many cases reduce medication use, through up to 12 months in eyes with mild to severe glaucoma. Statistically significant and clinically relevant reductions in IOP were seen at every time point. While the goal of surgery was not to reduce medication burden, mean medication use was significantly reduced at all but the last time point. In the majority of eyes, the need for a bleb-based glaucoma procedure (and its attendant long-term risks) was delayed or prevented for at least 12 months.
Disclosure
Dr Mohammed K. ElMallah provided research support for New World Medical, during the conduct of the study; research support for Glaukos and Ivantis, outside the submitted work. Dr John P Berdahl reports personal fees from New World Medical, during the conduct of the study. Dr Malik Y. Kahook reports patent royalties from New World Medical, during the conduct of the study; In addition, Dr Malik Y. Kahook has a patent US 2016/0354248 A1 issued to New World Medical. Dr Mark Gallardo reports non-financial support from New World Medical for data analysis, outside the submitted work. Dr Ahad Mahootchi provided research support for New World Medical, during the conduct of the study; grants, personal fees from Allergan, personal fees from Iridex and Ivantis, outside the submitted work. Dr Leonard A. Rappaport reports personal fees from New World Medical, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Richter GM, Coleman AL. minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189–206.
2. Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM. Minimally-invasive glaucoma surgeries (Migs) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12:e0183142.
3. Dick HB, Schultz T, Gerste RD. Miniaturization in glaucoma monitoring and treatment: a review of new technologies that require a minimal surgical approach. Ophthalmol Ther. 2019;8:19–30.
4. Agrawal P, Bradshaw SE. Systematic literature review of clinical and economic outcomes of micro-invasive glaucoma surgery (Migs) in primary open-angle glaucoma. Ophthalmol Ther. 2018;7:49–73.
5. Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC. Postoperative Complications in the tube versus trabeculectomy (Tvt) study during five years of follow-up. Am J Ophthalmol. 2012;153:804–814.
6. Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23:96–104.
7. Grover DS, Flynn WJ, Bashford KP, et al. Performance and safety of a new ab interno gelatin stent in refractory glaucoma at 12 months. Am J Ophthalmol. 2017;183:25–36.
8. Batlle JF, Fantes F, Riss I, et al. Three-year follow-up of a novel aqueous humor microshunt. J Glaucoma. 2016;25:e58–65.
9. Samuelson TW, Sarkisian SR
10. Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118:459–467.
11. Samuelson TW, Chang DF, Marquis R, et al. A schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: the horizon study. Ophthalmology. 2018.
12. US Food and Drug Administration. Update: potential eye damage from alcon cypass micro-stent used to treat open-angle glaucoma: FDA safety communication; 2018. Available from: https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm624283.htm.
13. Vold S, Ahmed II, Craven ER, et al. Two-year compass trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology. 2016;123:2103–2112.
14. Greenwood MD, Seibold LK, Radcliffe NM, et al. Goniotomy with a single-use dual blade: short-term results. J Cataract Refract Surg. 2017;43:1197–1201.
15. Dorairaj SK, Seibold LK, Radcliffe NM, et al. 12-month outcomes of goniotomy performed using the kahook dual blade combined with cataract surgery in eyes with medically treated glaucoma. Adv Ther. 2018;35:1460–1469.
16. ElMallah MK, Seibold LK, Kahook MY, et al. 12-month retrospective comparison of kahook dual blade excisional goniotomy with istent trabecular bypass device implantation in glaucomatous eyes at the time of cataract surgery. Adv Ther. 2019;36:2515–2527.
17. Le C, Kazaryan S, Hubbell M, Zurakowski D, Ayyala RS. Surgical outcomes of phacoemulsification followed by istent implantation versus goniotomy with the kahook dual blade in patients with mild primary open-angle glaucoma with a minimum of 12-month follow-up. J Glaucoma. 2019;28:411–414.
18. Hirabayashi MT, King JT, Lee D, An JA. Outcome of phacoemulsification combined with excisional goniotomy using the kahook dual blade in severe glaucoma patients at 6 months. Clin Ophthalmol. 2019;13:715–721.
19. Dorairaj SK, Kahook MY, Williamson BK, Seibold LK, ElMallah MK, Singh IP. A multicenter retrospective comparison of goniotomy versus trabecular bypass device implantation in glaucoma patients undergoing cataract extraction. Clin Ophthalmol. 2018;12:791–797.
20. Sieck EG, Epstein RS, Kennedy JB, et al. Outcomes of kahook dual blade goniotomy with and without phacoemulsification cataract extraction. Ophthalmol Glaucoma. 2018;1:75–81.
21. Dorairaj S, Tam MD. Kahook dual blade excisional goniotomy and goniosynechialysis combined with phacoemulsification for angle-closure glaucoma: 6-month results. J Glaucoma. 2019;28:643–646.
22. Dorairaj S, Tam MD, Balasubramani GK. twelve-month outcomes of excisional goniotomy using the kahook dual blade((R)) in eyes with angle-closure glaucoma. Clin Ophthalmol. 2019;13:1779–1785.
23. Berdahl JP, Gallardo MJ, ElMallah MK, et al. Six-month outcomes of goniotomy performed with the Kahook dual blade as a stand-alone glaucoma procedure. Adv Ther. 2018;35:2093–2102.
24. Salinas L, Chaudhary A, Berdahl JP, et al. Goniotomy using the kahook dual blade in severe and refractory glaucoma: six month outcomes. J Glaucoma. 2018.
25. Seibold LK, Soohoo JR, Ammar DA, Kahook MY. Preclinical investigation of ab interno trabeculectomy using a novel dual-blade device. Am J Ophthalmol. 2013;155(524–9):e2.
26. Kaplowitz K, Bussel II, Honkanen R, Schuman JS, Loewen NA. Review and meta-analysis of ab-interno trabeculectomy outcomes. Br J Ophthalmol. 2016;100:594–600.
27. Mizoguchi T, Nishigaki S, Sato T, Wakiyama H, Ogino N. Clinical results of trabectome surgery for open-angle glaucoma. Clin Ophthalmol. 2015;9:1889–1894.
28. Ting JL, Damji KF, Stiles MC. Ab interno trabeculectomy: outcomes in exfoliation versus primary open-angle glaucoma. J Cataract Refract Surg. 2012;38:315–323.
29. Maeda M, Watanabe M, Ichikawa K. Evaluation of trabectome in open-angle glaucoma. J Glaucoma. 2013;22:205–208.
30. Ahuja Y, Ma Khin Pyi S, Malihi M, Hodge DO, Sit AJ. Clinical results of ab interno trabeculotomy using the trabectome for open-angle glaucoma: the mayo clinic series. Am J Ophthalmol. 2013.
31. Minckler D, Mosaed S, Dustin L, Ms BF. Trabectome (trabeculectomy-internal approach): additional experience and extended follow-up. Trans Am Ophthalmol Soc. 2008;106(149–59):discussion 59–60.
32. Grover DS, Godfrey DG, Smith O, Feuer WJ. Montes de Oca I, Fellman RL. Gonioscopy-assisted transluminal trabeculotomy, Ab interno trabeculotomy: technique report and preliminary results. Ophthalmology. 2014;121:855–861.
33. Grover DS, Godfrey DG, Smith O, Shi W, Feuer WJ, Fellman RL. Outcomes of gonioscopy-assisted transluminal trabeculotomy (Gatt) in eyes with prior incisional glaucoma surgery. J Glaucoma. 2017;26:41–45.
34. Rahmatnejad K, Pruzan NL, Amanullah S, et al. surgical outcomes of gonioscopy-assisted transluminal trabeculotomy (Gatt) in patients with open-angle glaucoma. J Glaucoma. 2017;26:1137–1143.
35. Grover DS, Smith O, Fellman RL, et al. gonioscopy-assisted transluminal trabeculotomy: an Ab interno circumferential trabeculotomy: 24 months follow-up. J Glaucoma. 2018;27:393–401.
36. Aktas Z, Ozmen MC, Atalay HT, Ucgul AY. Evaluation of episcleral venous fluid wave during gonioscopy assisted transluminal trabeculotomy in patients with advanced glaucoma. Eye. 2019;33:668–673.
37. Olgun A, Aktas Z, Ucgul AY. Xen gel implant versus gonioscopy-assisted transluminal trabeculotomy for the treatment of open-angle glaucoma. Int Ophthalmol. 2020.
38. Sarkisian S, Allan EJ, Ding K, Dvorak J, Badawi DY. New way for ab interno trabeculotomy: initial results.
39. Sarkisian SR, Mathews B, Ding K, Patel A, Nicek Z. 360 degrees Ab-interno trabeculotomy in refractory primary open-angle glaucoma. Clin Ophthalmol. 2019;13:161–168.
40. Glaukos Inc. Istent and istent inject dfu and MRI information. https://www.glaukos.com/dfu-mri-information/.
41. Ivantis, Inc. Hydrus Microstent Instructions for Use. Irvine; CA2018.
42. Armstrong JJ, Wasiuta T, Kiatos E, Malvankar-Mehta M, Hutnik CM. The effects of phacoemulsification on intraocular pressure and topical medication use in patients with glaucoma: a systematic review and meta-analysis of 3-year data. J Glaucoma. 2017;26:511–522.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
